Because hepatitis C virus (HCV) infection is curable in the majority of cases, the diagnosis of all infected patients has become a priority. In difficult-to-diagnose populations, simpler diagnostic methods are required such as the use of dried blood spots (DBS) as an alternative to blood drawn by venipuncture (VP). Before being able to include it as a HCV diagnostic detection method within the Spanish National Health System, the diagnostic accuracy of standard hospital equipment must be evaluated.
MethodologyDBS samples were evaluated in vitro and in a field test after being processed in the Cobas 6800 system, establishing a correlation with the result by VP. Performance with different viral loads and intra-assay variability was compared.
ResultsIn samples with a viral load of >3 log10IU/ml, viraemia was detected in all cases when at least two blood spot elutions were used (94 detections out of 95 spot elutions). The performance with 2 spots was lower in samples with <3 log10IU/ml (7/20). Correlation between VP and DBS viraemia was excellent (maximum with 2 spots, r2=0.906, P<.001) with a coefficient of variation of 0.05%. In routine clinical practice with specimens from screened subjects (n=61), excellent diagnostic accuracy was also observed.
ConclusionViral load detection using DBS of at least two spots is a reliable method for HCV diagnosis. The standardisation of the method is feasible and our results support the incorporation of this diagnostic tool in Spain's Public Health System.
La infección por el virus de la hepatitis C (VHC) es curable en la mayoría de los casos tratados, siendo actualmente una prioridad diagnosticar a todos los infectados. Para ello se necesitan, especialmente en poblaciones de difícil diagnóstico, métodos diagnósticos simples, como es el uso de muestras de gotas de sangre seca (GSS), como alternativa a la extracción de sangre mediante venopunción. Como paso previo para poder implantarlo como método diagnóstico de detección de pacientes con VHC dentro del Sistema Nacional de Salud se precisa evaluar la precisión diagnóstica en equipos de uso hospitalario habitual.
MetodologíaSe evaluaron in vitro y en ensayo de campo muestras de GSS tras ser procesadas en el equipo Cobas 6800, estableciendo una correlación con el resultado obtenido con sangre completa. Se realizaron pruebas de correlación y de variabilidad intraensayo de la determinación con sangre completa y GSS para cuantificar la carga viral del VHC.
ResultadosEn muestras de sangre completa, con una carga viral≥3 log10UI/ml, se detectó viremia en todos los casos cuando se utilizaron eluciones de 2 gotas (94 detecciones de 95 eluciones de círculos). El rendimiento con 2 gotas fue menor en muestras con <3 log10UI/ml (7/20). La correlación entre la viremia determinada con sangre completa y con GSS fue excelente (máximo con 2 gotas, r2=0,906; p<0,001), con un coeficiente de variación del 0,05%. En práctica clínica habitual con muestras de pacientes analizados (n=61) se obtuvo igualmente una excelente precisión diagnóstica.
ConclusiónLa determinación de la carga viral mediante GSS, procesando al menos 2 gotas, es un método fiable para el diagnóstico de infección por el VHC. La estandarización del método es factible en nuestro equipo Cobas 6800 local, y nuestros resultados respaldan la incorporación de esta herramienta diagnóstica al Sistema Nacional de Salud para facilitar planes de microeliminación.
Infection with the hepatitis C virus (HCV) is one of the main causes of morbidity and mortality of liver aetiology worldwide.1 The long-term repercussions are highly variable, ranging from minimal alterations to the onset of decompensated cirrhosis and hepatocellular carcinoma.2
Clinical care for patients with HCV infection has improved considerably in recent years, mainly due to advances in treatment. These allow for a sustained virologic response and cure of the infection in practically all patients, preventing disease progression and the onset of complications.3 However, one of the obstacles for patients to benefit from treatment is the lack of diagnosis of the infection and referral to the specialist.4 In groups such as intravenous drug users, another of the limitations for access by patients to treatment and, therefore, to cure, is the refusal to undergo a venipuncture (VP) for blood extraction and the diagnosis due to poor venous access, difficulties in being transferred to the hospital centre due to financial problems or stigmatisation that complicate the diagnosis.5
Antibodies to HCV and viraemia can currently be detected from a dried blood spot (DBS) sample on paper after finger-prick blood draw. This test has shown, with different commercial kits, a high sensitivity and specificity.6–8 The great advantage of this procedure as a diagnostic method is the low volume of blood it requires (a few spots deposited on paper and dried in the air), the ease of transport and storage of the samples until they are processed in the laboratory. In addition, it does not require personnel with specific training, is less invasive and is better accepted, especially in those patients with little venous accessibility, such as intravenous drug users with a history of phlebitis.9
A diagnostic test of this type not only facilitates the diagnosis, but it is also more acceptable for the subject to be screened, two fundamental strategies to identify infected patients and facilitate their subsequent therapeutic evaluation and cure.
Control of HCV infection within the Spanish National Health System, according to the current National Plan,10 necessarily involves diagnosing and treating groups which are traditionally difficult to link to health care. Decentralised or out-of-hospital diagnosis with sample collection for DBS testing at the place where the individual to be diagnosed is located, but with hospital management through processing in the reference centre, has proven to be useful as an opportunistic screening method in one-off initiatives.11,12 However, in order to facilitate and establish a systematic screening in these groups, it is necessary to implement this procedure in the Spanish National Health System, as a tool equivalent to the determination of plasma viraemia by VP. In the absence of a protocol by the manufacturer, the World Health Organisation recommends locally validating and standardising this procedure. In order to be able to implement this diagnostic method in the routine healthcare circuit, and through it to diagnose HCV, especially in those in whom VP is an obstacle, this study evaluates the diagnostic accuracy of a protocol applied to a DBS sample on paper in the cobas 6800 equipment of our hospital centre affiliated to the Spanish National Health System.
Material and methodsIncluded samplesThis prospective study consisted of an in vitro evaluation from DBS samples from the blood of HCV-infected subjects, and an evaluation in the field trial from samples obtained from enrolled subjects during the period of July–September 2018, in a study which started in January 2017 that evaluated the efficacy of voluntary screening for HCV in drug addiction care centres in the north of the island of Tenerife, with determination of antibodies to HCV using DBS, collected by pharmacists and pharmacy assistants.
For the two evaluations, whole blood samples were available by VP in tripotassium EDTA tubes to obtain reference values, and the same sample was used to load the circles in the in vitro evaluation to obtain DBS results. Both samples were processed in the cobas 6800 equipment, which works with the same sample volume in both cases.
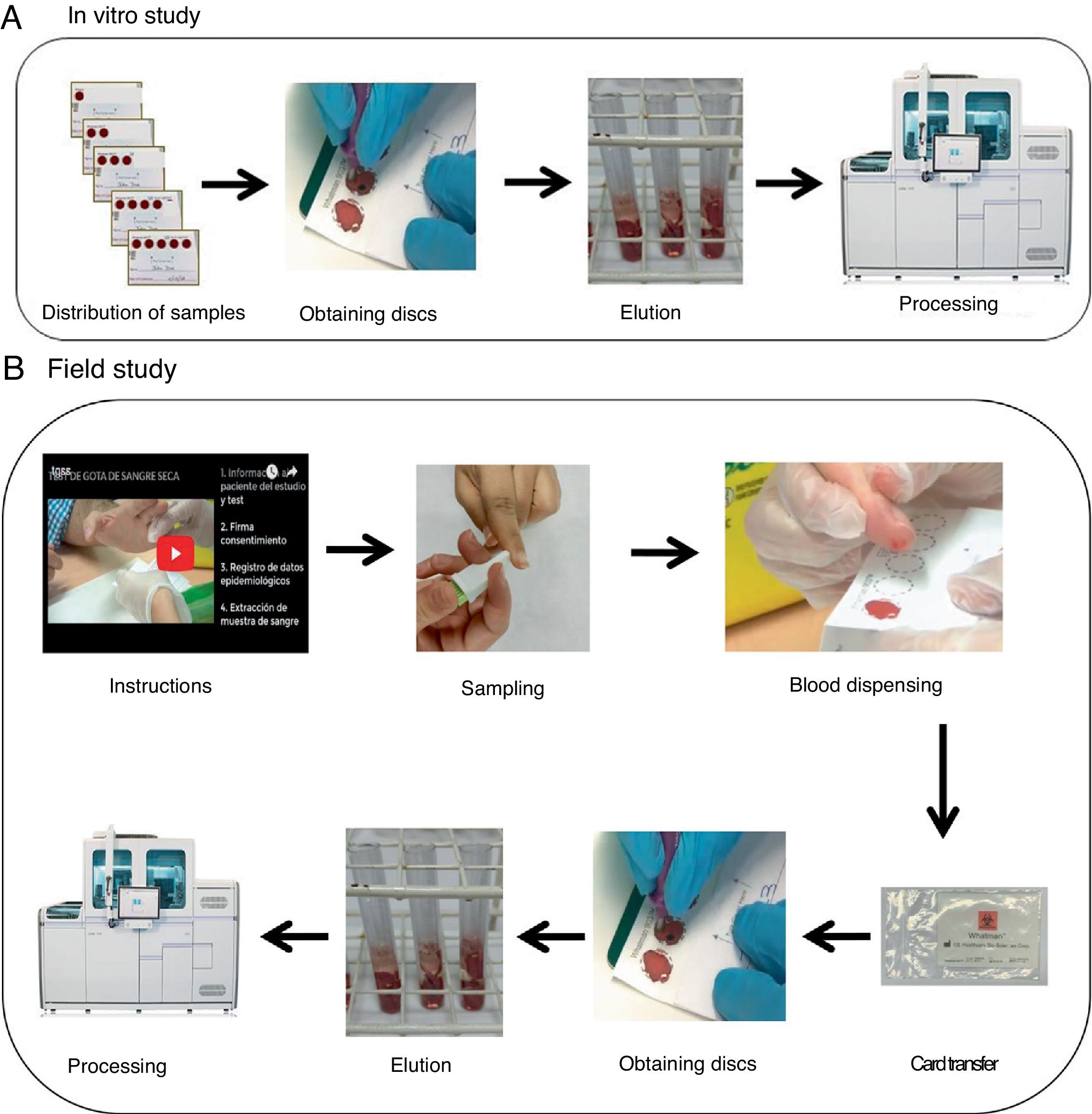
In vitro studyFor the in vitro validation process, diagnostic performance and intra-assay variability were compared on a total of 300 circles loaded with samples from 20 HCV-infected subjects for whom viral load data were available.
Cards with five-circle Whatman 903© (Merck, Germany) paper were used to collect the samples (Fig. 1A). A total of five cards per patient (100 cards in total) were used which were loaded from one to five circles (i.e. 15 circles per patient, and a total of 300 circles) with 80μl of blood anticoagulated with tripotassium EDTA. The results were expressed quantitatively (log10IU/ml) and qualitatively as positive if viraemia was detected, or negative stratifying into four groups: very low viral load (<3log10IU/ml); low viral load (≥3–4log10IU/ml); medium viral load (>4–6log10IU/ml) and high viral load (>6log10IU/ml).
As the incidence of patients with very low viral loads (<3log10IU/ml) is very small, in order to assess the accuracy
of the DBS test in these types of patients 1/10 or 1/100 blood samples were diluted from three additional subjects (with known viral loads) in physiological serum, until values were obtained in the sample of <3log10IU/ml, for subsequent dispensing in paper circles.
Field studyThe personnel in charge of obtaining the sample for the DBS test were trained in obtaining it through a previous interview and with the delivery of material, including the viewing of an online video (https://www.youtube.com/watch?v=Jagy918AXTc) with the intention of collecting, after the puncture with a lancet (MenaLancetPro©, 23G, Menarini), a sufficient sample to complete up to five circles on each card. The cards were transferred at room temperature (between 22–23°C) from extraction to processing with a median of 17.5 days (range 14–21 days) (Fig. 1B).
Sample processing and equipmentSamples from the paper were obtained manually from each outlined circle with the aid of a small disc punch, which were placed in tubes of the Roche Diagnostics equipment (tube 5ml PS 12×75mm without marks, reference: 30080.1, Deltalab, Barcelona), 1500μl of lysis reagent was added for use in cobas equipments 6800/8800 (REF P/N: 06997538190) in tubes with four circles or less and 2000μl in those in which five circles could be obtained. The tubes were placed in a water bath for 15min, stirring them in a vortex after 7–8min. The supernatant was separated into a new tube and they were introduced into the cobas 6800 equipment (Roche Molecular Systems, Pleasanton, CA) for the subsequent determination of viral load (log10IU/ml).
Statistical analysisThe results were expressed as absolute, percentage, mean values and their standard deviation. Student's t-test was used for quantitative variables that followed normality or the Mann Whitney U-test for those that did not follow a normal distribution, while qualitative variables were compared using the Chi-square test. Correlations were studied using Pearson's coefficient and analysis of coefficients of variation. The results were expressed as mean±standard deviation. Data analysis was performed with SPSS 15. Differences with p-values <0.05 were considered statistically significant.
Ethical aspectsThis study, in accordance with the principles of the Declaration of Helsinki, adopted by the 18th World Medical Assembly, Helsinki, Finland, in 1964 and amended in Tokyo (1975), Venice (1983), Hong Kong (1989), South Africa (1996), Edinburgh (2000), Washington (2002), Tokyo (2004), Seoul (2008) and Brazil (2013); and the Laws and Regulations in force in Europe and Spain, was approved by the Ethics Committee of the Hospital Universitario de Canarias [University Hospital of the Canary Islands], and patients were given informed consent forms and they signed them for the collection of samples.
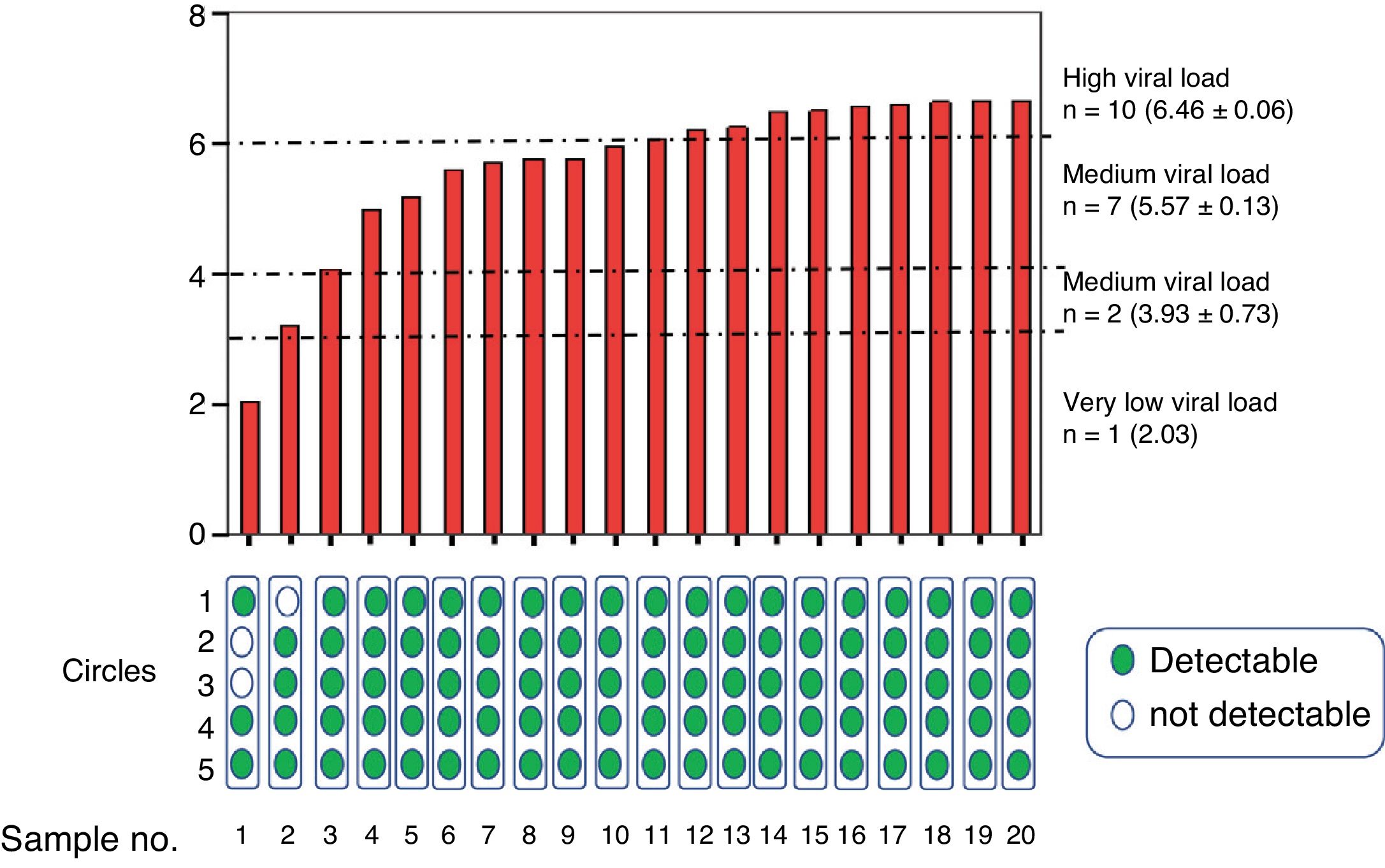
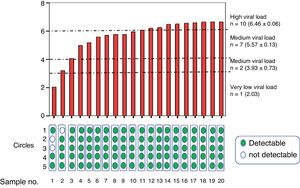
ResultsIn vitro studyOnce processed, the samples were grouped into a group with a high viral load (n=10, 6.4±0.06log10IU/ml), medium (n=7, 5.5±0.3log10IU/ml), low (n=2, 3.9±0.7log10IU/ml) and very low (n=1, 2.03log10IU/ml) (Fig. 2).
Average viraemia determined in venipuncture samples from patients grouped according to their very low, low, medium or high viral load. The results of detectable (green) or undetectable (white) viraemia are shown at the bottom, according to the number of circles used in the determination after elution.
Viraemia was detected in 100% of the circles and evaluated averages of samples with at least medium viral load. In samples from a patient with low viraemia (3.21log10IU/ml) the average of at least two circles was required to be detectable, and in samples from another patient with very low viraemia (2.03log10IU/ml) more circles were needed in order to reliably detect viraemia. In additional diluted samples with very low viraemia (n=3, 2.1±0.2log10IU/ml) only viraemia was detected (and in range <20IU/ml) in four of the 15 DBS dilutions, so overall viral load was detected in seven out of 20 dilutions (Table 1).
Distribution of results of the diluted samples.
| Sample | Applied dilution | VL (IU/ml) | Sample, no. of circles | No. of circles | VL IU/ml) on card |
|---|---|---|---|---|---|
| Sample 1 | 1/10 | 108 | 3, 1 | 1 circle | <12 |
| 3, 2 | 2 circles | Not detectable | |||
| 3, 3 | 3 circles | Not detectable | |||
| 3, 4 | 4 circles | 33 | |||
| 3, 5 | 5 circles | <12 | |||
| Additional sample 1 | 1/10 | 213 | 1, 1 | 1 circle | Not detectable |
| 1, 2 | 2 circles | Not detectable | |||
| 1, 3 | 3 circles | Not detectable | |||
| 1, 4 | 4 circles | Not detectable | |||
| 1, 5 | 5 circles | Not detectable | |||
| Additional sample 2 | 1/10 | 304 | 2, 1 | 1 circle | Not detectable |
| 2, 2 | 2 circles | <20 | |||
| 2, 3 | 3 circles | <20 | |||
| 2, 4 | 4 circles | <20 | |||
| 2, 5 | 5 circles | Not detectable | |||
| Additional sample 3 | 1/10 | 36 | 3, 1 | 1 circle | Not detectable |
| 3, 2 | 2 circles | Not detectable | |||
| 3, 3 | 3 circles | Not detectable | |||
| 3, 4 | 4 circles | Not detectable | |||
| 3, 5 | 5 circles | <20 | |||
VL: viral load.
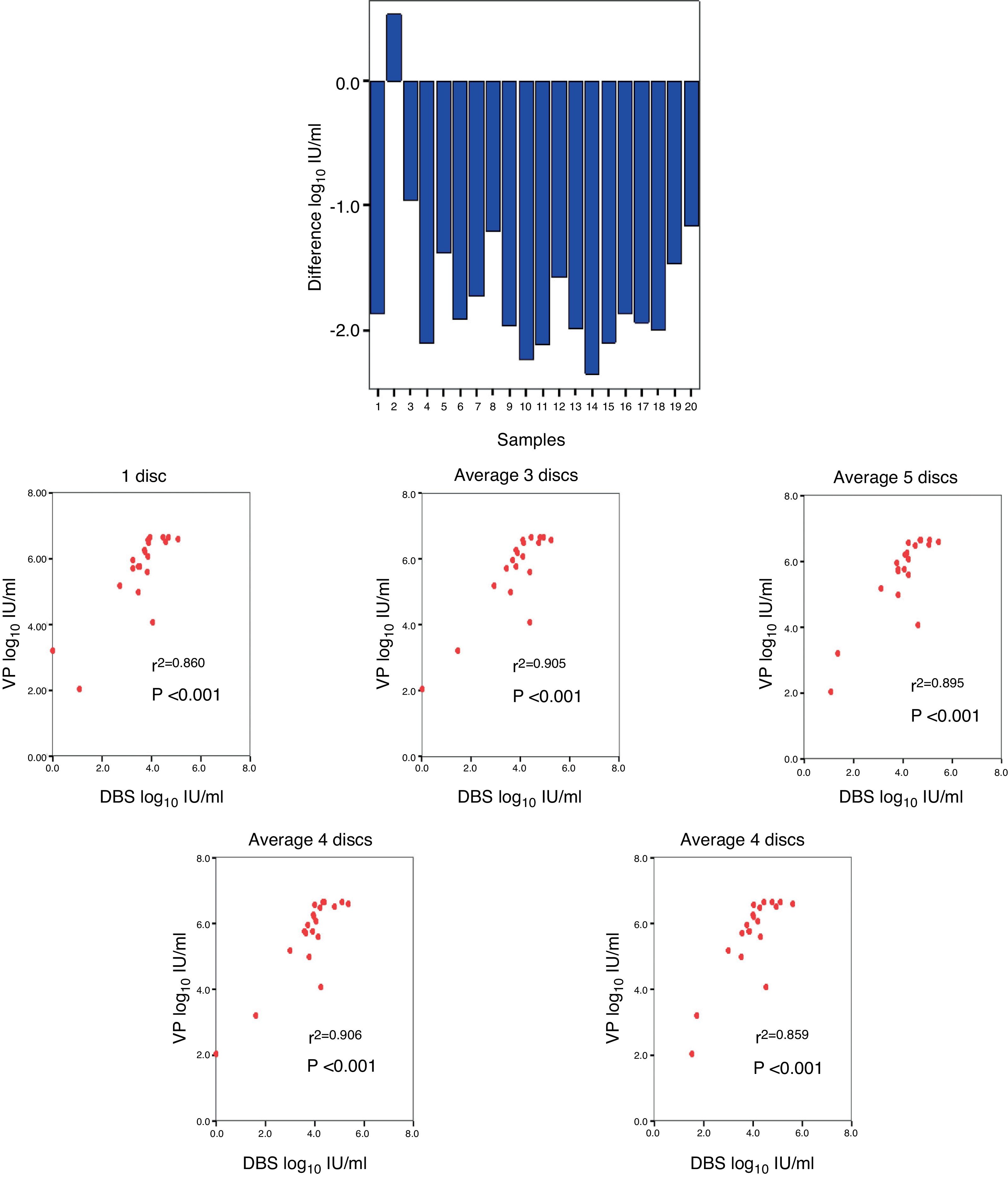
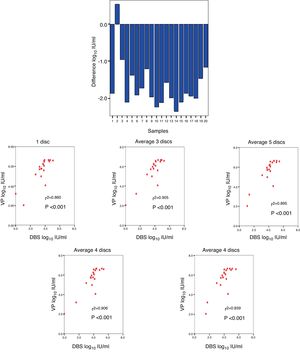
A comparison was made between the viraemia detected by VP with respect to that obtained from DBS, obtaining a mean difference of −1.66log10IU/ml (Fig. 3A). The correlation between the viraemia detected in VP and in DBS was high for any average of circles used per card. The maximum correlation was obtained when two circles were used (r2=0.906; p<0.001) (Fig. 3B).
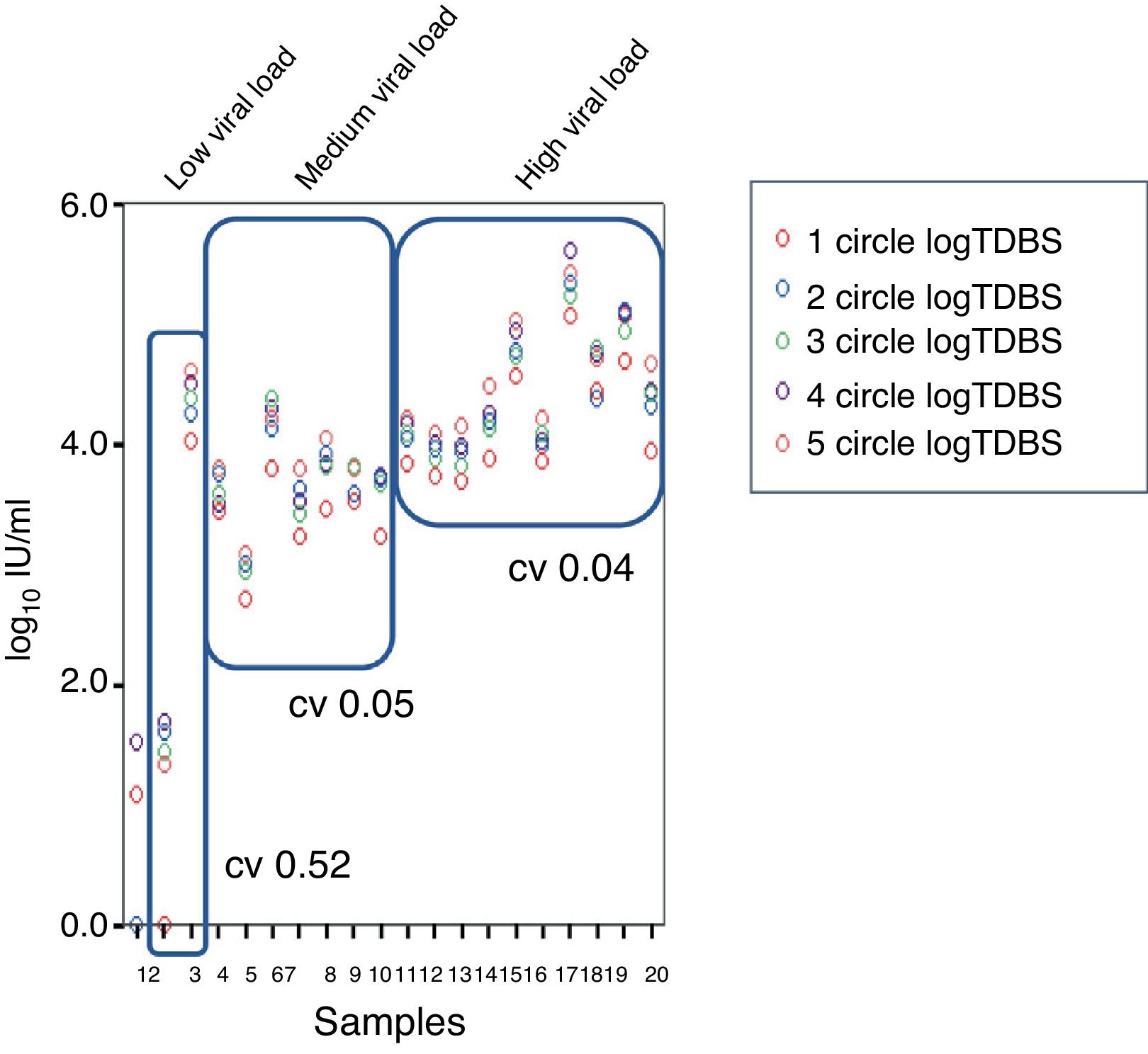
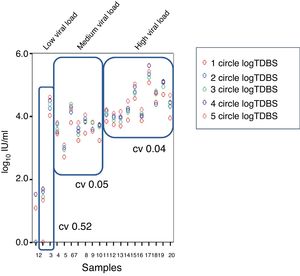
Regarding the coefficients of variation (CV), a CV of 0.57%, 0.05% and 0.04% was obtained for low-, medium- and high-level viraemia, respectively (Fig. 4).
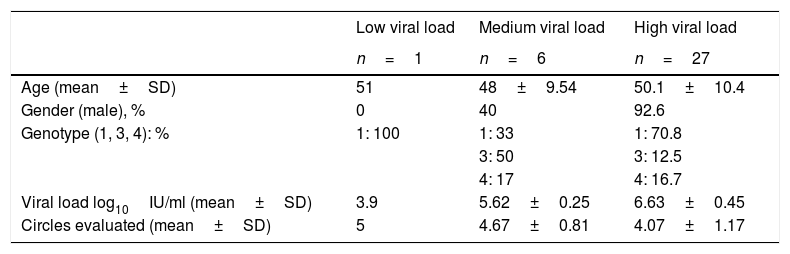
Field studyFor the validation of the samples in real clinical practice, a total of 61 subjects were tested, 34 subjects with positive viral load (median 6.41log10IU/ml, range 3.9–7.81log10IU/ml), 79.4% had a high viral load and 27 had an undetectable viral load. In the group with detectable viral load, the majority were men, with an average age of around 50 (Table 2). In all of them, at least four circles could be obtained during the DBS sample collection. The cards were transferred at room temperature with a median of 17.5 days (range 14–21 days) from extraction to processing.
Distribution of results according to viral loads in subjects with positive results and characteristics in the trial in real clinical practice.
| Low viral load | Medium viral load | High viral load | |
|---|---|---|---|
| n=1 | n=6 | n=27 | |
| Age (mean±SD) | 51 | 48±9.54 | 50.1±10.4 |
| Gender (male), % | 0 | 40 | 92.6 |
| Genotype (1, 3, 4): % | 1: 100 | 1: 33 | 1: 70.8 |
| 3: 50 | 3: 12.5 | ||
| 4: 17 | 4: 16.7 | ||
| Viral load log10IU/ml (mean±SD) | 3.9 | 5.62±0.25 | 6.63±0.45 |
| Circles evaluated (mean±SD) | 5 | 4.67±0.81 | 4.07±1.17 |
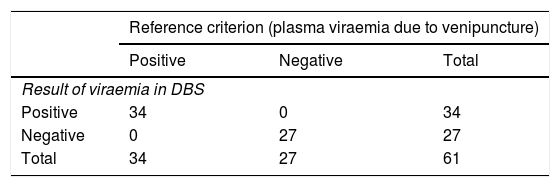
The diagnostic accuracy obtained demonstrates a sensitivity, specificity, positive predictive value and negative predictive value of 100% in our real clinical practice sample (Table 3).
Diagnostic accuracy obtained with dry blood spot (DBS) test.
| Reference criterion (plasma viraemia due to venipuncture) | |||
|---|---|---|---|
| Positive | Negative | Total | |
| Result of viraemia in DBS | |||
| Positive | 34 | 0 | 34 |
| Negative | 0 | 27 | 27 |
| Total | 34 | 27 | 61 |
| 95% CI | |||
|---|---|---|---|
| Lower limit | Upper limit | ||
| Disease prevalence | 55% | 42.51% | 68.24% |
| Correctly diagnosed patients | 100.00% | 92.62% | 99.85% |
| Sensitivity | 100.00% | 87.36% | 99.73% |
| Specificity | 100.00% | 84.50% | 99.66% |
| Positive predictive value | 100.00% | 87.36% | 99.73% |
| Negative predictive value | 100.00% | 84.50% | 99.66% |
Our results indicate that, based on the described protocol, and in the cobas 6800 equipment used, the determination of viral load from a DBS sample, processing at least two circles, is a reliable qualitative expression method in the detection of the majority of patients with active HCV infection.
In addition to correctly detecting the presence of viraemia with the protocol used, the observed correlation between viraemia obtained by VP and in DBS was excellent. In a published study using a single disc impregnated with similar quantities of sample (50μl of blood) and in a card of equivalent characteristics to those used in this study, a similar correlation was found.7 Despite this observation, in the era of direct-acting antivirals, in which, due to their highly pangenotypic efficacy characteristics, it would not be necessary to quantify or genotype, since it does not condition either the type of therapy or its duration, only the presence or not of the virus, the value of establishing an excellent correlation between both methods, even with the described quantitative difference or the excellent correlation coefficient between observed samples, seems otherwise irrelevant.13 In addition, viral load determination tests throughout treatment are currently in disuse and limited to the practice to verify therapeutic compliance.3 In fact, almost all subjects obtain undetectable viral loads at an early stage due to the direct effects of new treatments on viral replication. That is why, in the monitoring of new treatments, the quantification of RNA could be dispensed with, and it could begin to be substituted with qualitative tests that confirm the infection at the beginning of the treatment and the sustained virologic response after its completion.13 In this sense, HCV core antigen determination has been used again in recent years as it is cheaper and easier than determining viraemia.14,15
Our results show that, under the conditions analysed, for patients with medium- and high-level viraemia, only one circle is sufficient to obtain a positive result; however, in those patients with low-level viraemia, two circles are required. We postulate that this may be determined by the storage of the sample with a degradation of the few copies of RNA present in the DBS.16 In fact, in a study that compared samples stored at room temperature to storage at −20°C, a difference of up to three times in the RNA levels was observed, six days after their extraction.7 However, another study did not observe differences in detectable RNA value after about one year, but it was not quantified to determine if there was a decrease in viral load levels.17 Finally, a more recent study found no variation in HCV stability in DBS samples over a one-year period.18 However, this limitation would be reduced by taking advantage of the ease of obtaining the sample with the appropriate lancet and using the total number of circles available on the card, which are up to five, to determine viraemia.
After the validation of the in vitro method, a subsequent validation study was carried out in real clinical practice in a screening programme in drug addiction care centres, a group with a high prevalence of infection and difficult to link with medical care for diagnosis.5 These centres or units in which patients are treated by medical practitioners, psychologists, social workers and pharmacists, are key to the success of the plans put in place to diagnose, and even treat, these patients without, until now, care for HCV. Without a doubt, the implementation of this diagnostic tool will require organisational adjustments and structural allowance, which, however, given the simplicity of the test, will be minor. Furthermore, the simplicity allows the test to be carried out by different members of the centre. In fact, in our case, it was the pharmacists who collaborated in the collection of the samples, as occurs in other places where screening using DBS has been implemented.19 It is even likely that in the future, as has been evaluated in patients with human immunodeficiency virus infection, it will be the patient him or herself who collects the sample.20,21
The DBS test proved to be a sensitive and specific method for the quantification of HCV RNA compared to the results obtained after VP. From the results obtained in our study we can observe a high diagnostic accuracy. These results are even superior to those published in other works in which a sensitivity of 100% and a specificity of 95.8% were obtained,18 and in another study that showed a sensitivity of 98% and a specificity of 94.3%.22 This greater accuracy that we have reported with our protocol probably comes from the identification methodology. The aforementioned works used the extraction platform of Abbott m2000 and RT-PCR,18 and a qPCR22 that has a detection limit of viral RNA of 50IU/ml, while our equipment has a limit of 12IU/ml.
The fact that the test has a very high negative predictive value is especially important in this group of patients and in populations at risk of infection, since it could be considered as an alternative tool for diagnosis and screening in populations with difficult access to conventional diagnosis by VP, despite presenting low sensitivity in those patients with very low viral loads, since this could establish a diagnosis in a very high number of patients (although not in their entirety) who would not be diagnosed in any other way in our health system as it does not happen in others either,23 in order to achieve the objectives proposed by the World Health Organisation for 2030 of raising the number of diagnosed to 90% and treating at least 80% in the global strategy of eliminating hepatitis C.24 This should be focused on programmes based on the elimination of infection in patients from drug addiction care centres, prisons or social integration centres, among others. Recently, our group evaluated the acceptance of this method for the diagnosis of HCV infection through the collection of fingerprick samples, aiming to be very well accepted not only in the general population, but also in individuals at risk of social exclusion. This is particularly interesting in this group of high prevalence in viraemia, in which infection detection is essential and in which this method would bring the diagnosis closer to these patients, also reluctant to use the health system for the diagnosis of diseases by drawing blood.12 It should be noted that the DBS test allows, as in our case, to determine RNA directly in a population with high seroprevalence and active HCV infection (30–60%). However, in the general population with a lower prevalence figure (0.5–3%),25,26 even though the technique to determine antibodies from a DBS sample can be developed and the diagnosis can be carried out in one step (in the case of the presence of HCV-antibody, RNA would be performed), since the use of DBS in this general population is less beneficial, the use of conventional VP blood collection would prevail, which also allows for one-step diagnosis, saving costs and losses of patients.27,28
This diagnostic method of DBS on paper is not exclusive of other decentralised diagnostic methods, which due to their speed in obtaining results are an interesting tool applied in point-of-care health centres or sites when immediate diagnosis and care are especially interesting, particularly in groups that are difficult to link with conventional healthcare.29–31 However, the DBS test, integrated into the routine of analysis of samples in a central laboratory, allows for the centralised reading and management of the result, which is undoubtedly advantageous from the point of view of public health for the purposes of records and epidemiology studies.32
This study is not without limitations. The samples for the field study were obtained from a population with a high prevalence of HCV, such as patients who go to drug addiction centres, and who are not representative of the general population. However, the objective of this section of the study was to validate in real clinical practice the viability of the DBS test, and in this sense this study has more value, since it is in this population where this test has greater applicability for the reasons discussed. Furthermore, viral loads were evaluated in samples with genotypes mainly of type 1, which are the most prevalent in the general population, but which contrasts with the highest percentages of genotype 3 in patients treated by drug addiction care centres. However, genotype type is not expected to influence viral load detection performance.23,33 Regarding the correlation between samples by VP and DBS, although the correlation was very high, it would be expected that the highest correlation was when the five circles were used. However, in our samples the maximum was with two circles. This could be due to the fact that perhaps the amount of lysis reagent necessary to allow for the complete elution of the viral RNA from the totality of the five circles was not used, or to a physical impediment, since perhaps with a greater number of circles, and as a result of discs in the tube, less surface area of the disc comes into contact with the lysis reagent and less elution occurs. Finally, in those patients with very low-level viraemias (<0.01% of cases in clinical practice)13 more studies would be required to confirm that the use of this test for the determination of viraemia is reliable. In these types of patients, our protocol is ineffective and the presence of false negatives would be expected, especially in a population with coinfection by human immunodeficiency virus in which a higher frequency of patients with very low viral loads has been reported.34,35 In this sense, we must not forget that in all screening programmes a false negative rate is assumed, which in this case would be acceptable, given the low frequency of individuals with viral loads of less than 3 logarithms. Furthermore, in this and other groups with difficult healthcare access, this diagnostic alternative is the only feasible diagnostic route. Finally, it remains to be evaluated whether the determination of antibodies or core antigen,16 instead of viraemia in these cases, would improve performance.
In conclusion, the use of a DBS sample to determine HCV viraemia seems to be an alternative to diagnosis based on a sample by VP with high diagnostic accuracy. Due to its advantages, especially in groups with a high prevalence of infection and complicated diagnostic access, the implementation of this method and its standardisation could allow this tool to be incorporated into the Spanish National Health System to facilitate the implementation of micro-elimination plans in Spain.
Conflicts of interestThe authors have no conflicts of interest to declare.
We are grateful for the work and dedication of the laboratory technicians of the Central Laboratory of the Hospital Universitario de Canarias, the researchers of FUNCANIS Verónica Casañas, Sara García and Pablo Yanes, as well as the staff of the ANTAD and San Miguel Adicciones Drug Addiction Care Centres for their active participation in the project.
Please cite this article as: Gómez L, Reygosa C, Morales-Arráez DE, Ramos R, Pérez A, Hernández A, et al. Evaluación de la precisión diagnóstica del sistema Cobas 6800 para la detección de los niveles de viremia del virus de la hepatitis C a partir de muestras de gotas de sangre seca en papel de filtro. Enferm Infecc Microbiol Clin. 2020;38:267–274.