Invasive fungal infections (IFIs) are becoming more frequent due to the increasing number of patients at risk. Over the last decade, their prognosis has improved with the diagnostic and therapeutic advances, including new antifungals. In the two years, from 2007 to 2009, antifungal consumption increased by 27%, 67 times more than antibacterial consumption, albeit with great differences between hospitals. The scientific evidence of the indications for antifungal prophylaxis and targeted antifungal therapy is strong; however, it is weak for empirical antifungal therapy, which is the most common indication. Antifungals are not harmless, since they are associated with a wide range of adverse effects and drug interactions, favor the development of resistance, contribute to other fungal superinfections and cause significant healthcare spending.
Therefore, the question arises whether this extraordinary increase in consumption is justified, whether the use of antifungals is optimal, or whether it is necessary to reconsider the current treatment of IFIs instead.
Las infecciones fúngicas invasoras (IFIs) son cada vez más frecuentes debido a un aumento pacientes en riesgo. En la última década, su pronóstico ha mejorado con los avances diagnósticos y terapéuticos, incluyendo los nuevos antifúngicos. En dos años, de 2007 a 2009, el consumo antifúngico aumentó un 27%, 67 veces más que el consumo de antibacterianos, aunque con grandes diferencias entre hospitales. La evidencia científica de las indicaciones de profilaxis antifúngica y terapia antifúngica dirigida es fuerte, sin embargo es débil para la terapia antifúngica empírica, que es la indicación más común. Los antifúngicos no son inofensivos ya que se asocian efectos adversos e interacciones medicamentosas, favorecen el desarrollo de resistencias, contribuyen a otras sobreinfecciones micóticas y causan un importante gasto sanitario.
Por lo tanto, la pregunta que surge es si este aumento extraordinario del consumo se justifica, si el uso de antifúngicos es óptimo o si es necesario reconsiderar el tratamiento actual de las infecciones fúngicas invasoras.
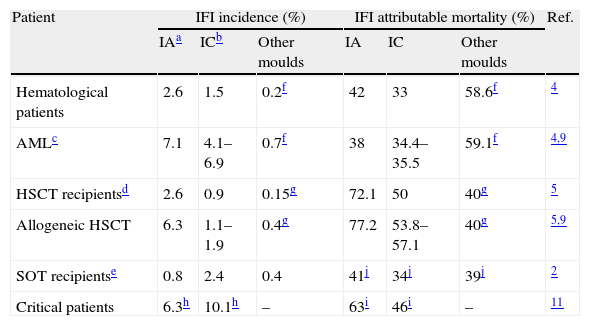
Over the last two decades, the incidence of invasive fungal infection (IFI) has increased due to the advances in medicine resulting in increased life expectancy of immunocompromised and critically ill patients at high risk for IFI.1–3 In hematological patients, invasive aspergillosis (IA) is currently the most frequent IFI. Its incidence is much higher in patients with acute myeloblastic leukemia (AML) and allogeneic hematopoietic stem cell transplantation (HSCT) recipients, reaching 10.9%, and with an attributable mortality as high as 77% in allogeneic HSCT recipients.1,4–9 In other immunocompromised patients as solid-organ transplant (SOT) (other than lung) recipients, invasive candidiasis (IC) is the most frequent fungal infection.2 In critically ill patients IC, mostly presented as candidemia, is the most common IFI with a reported prevalence of 6.9–10.08 episodes/1000 intensive care units (ICUs) admissions and crude mortality rate ranging from 42 to 46%.10,11 The IFI incidence and IFI attributable mortality in the most important risk groups of patients are specified in Table 1.
Incidence of invasive fungal infection (IFI) and IFI attributable mortality in different groups of patients.
| Patient | IFI incidence (%) | IFI attributable mortality (%) | Ref. | ||||
| IAa | ICb | Other moulds | IA | IC | Other moulds | ||
| Hematological patients | 2.6 | 1.5 | 0.2f | 42 | 33 | 58.6f | 4 |
| AMLc | 7.1 | 4.1–6.9 | 0.7f | 38 | 34.4–35.5 | 59.1f | 4,9 |
| HSCT recipientsd | 2.6 | 0.9 | 0.15g | 72.1 | 50 | 40g | 5 |
| Allogeneic HSCT | 6.3 | 1.1–1.9 | 0.4g | 77.2 | 53.8–57.1 | 40g | 5,9 |
| SOT recipientse | 0.8 | 2.4 | 0.4 | 41j | 34j | 39j | 2 |
| Critical patients | 6.3h | 10.1h | – | 63i | 46i | – | 11 |
Facing the need to combat IFI, from 1990s a growing arsenal of new antifungal drugs have been authorized by the European Medicine Agency for prophylaxis, empirical or targeted antifungal therapy contributing to reduce the IFI attributable mortality.8,12 However, antifungal therapy is not exempt from risks as hepatotoxicity or nephrotoxicity, and infusion related reactions are common (up to 45.5%) and may be severe. Discontinuation due to adverse effects reaches up to 18.6% in clinical trials13–15; side effects such as nephrotoxicity of amphotericin and pharmacologic interactions between azoles and immunosuppressants agents are a frequent problem especially in HSCT and SOT recipients13,14,16; and previous azole exposition may lead to emergence of Candida spp. resistance17 or breakthrough mucormicosis.18 Moreover, the growth of antifungal consumption in health care has become an important health spending19,20 turning the optimization of antifungal therapy into a priority for health care systems.
Facing this scenario the question then arises: ‘Are we using antifungal drugs appropriately?’
Antifungal consumption and suitability of the indicationAntifungal consumption has risen continuously in recent years, particularly since the approval of the echinocandins. In a study conducted in five German hospitals between 2001 and 2003, the antifungal consumption increased by 13.4% with great differences among centers.19 This increase in the consumption of antifungal drugs is 67 times higher than the increase of the consumption of antibacterial drugs. In a multicenter study conducted by the Spanish Network for Research in Infectious Diseases (REIPI) during the years 2007–2009, an increase of the antifungal consumption of 27% of defined daily dose/100 occupied bed-days (DDD/100 OBD) was observed, compared to an increase in the antibacterial consumption of 0.4% DDD/100 OBD. Also, the variability of the antifungal consumption among the participating centers was 9-fold higher than variability in antibacterial consumption.21 Other studies in American hospitals confirmed the great increase in the consumption of antifungals and the large variability intercenters.19,22 The burden of antifungal consumption is different for each indication of antifungal prophylaxis and empirical and targeted antifungal therapy. Although there are only limited published data about the distribution of antifungal consumption,23,24 we may infer it from the above mentioned study.21 According to a standard hospital of 1200 beds with 84% occupancy, antifungal consumption represents 5.6 DDD/100 OBD. Considering a reported incidence of 0.14 episode/day of therapy for candidemia and 0.12 episode/day of therapy for IA,25,26 the expected targeted antifungal therapy should be 0.26 DDD/100 OBD for each IFI. The remaining antifungal consumption, approximately 5.0 DDD/100 OBD, must be spent for both antifungal prophylaxis and especially in empirical antifungal therapy (EAT). Although it should be noted that most of the antifungal consumption in the prophylaxis indication is carried out in outpatients, and therefore it is not included in the evaluation of consumption in DDD/100 OBD.
The use of antifungals remains still a room for improvement. The results of a study performed in the ICU and oncohematology department of a French hospital showed that 40% of the antifungal therapies indicated were inappropriate.23 Moreover, 48% of the antifungal indications in a multicenter study carried out in 147 Spanish ICUs were empirical.27
Furthermore, the cost of antifungals significantly impact on the overall antimicrobial budget. Data from the implementation of an antimicrobial stewardship program in a large tertiary hospital, during seven years, indicated that the average rate of the antifungal cost represented 29.5% of the overall antimicrobial expenditure ranging from 47.7% ($3.7 million) to 18.8% ($1.2 million) before and after the program implementation.28
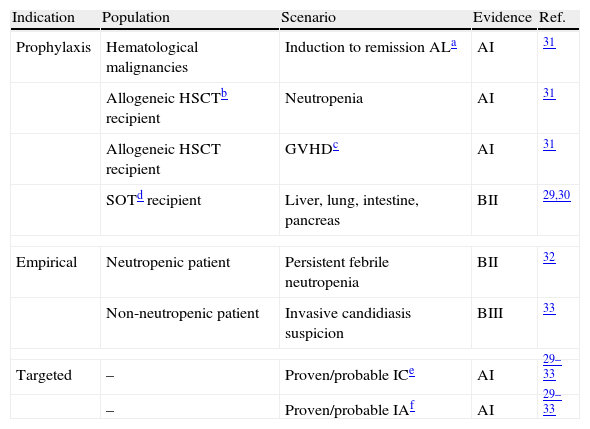
Despite the vast inpatient antifungal consumption used for empirical therapy, this indication lacks of a high level of scientific evidence29–33 (Table 2). We will review below said indications of EAT, in the neutropenic patient and the critically ill patient, and the targeted antifungal therapy.
Scientific evidence for the antifungal therapy indication.
| Indication | Population | Scenario | Evidence | Ref. |
| Prophylaxis | Hematological malignancies | Induction to remission ALa | AI | 31 |
| Allogeneic HSCTb recipient | Neutropenia | AI | 31 | |
| Allogeneic HSCT recipient | GVHDc | AI | 31 | |
| SOTd recipient | Liver, lung, intestine, pancreas | BII | 29,30 | |
| Empirical | Neutropenic patient | Persistent febrile neutropenia | BII | 32 |
| Non-neutropenic patient | Invasive candidiasis suspicion | BIII | 33 | |
| Targeted | – | Proven/probable ICe | AI | 29–33 |
| – | Proven/probable IAf | AI | 29–33 | |
AI, BII, BIII are referred to the scientific evidence and degree of standard recommendation.
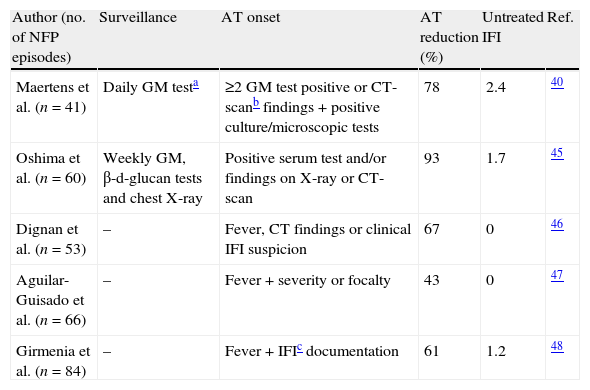
The main risk factor for IFI in neutropenic hematological patients is profound and prolonged neutropenia, and the most common clinical presentation is neutropenic fever. Since the main objective of the EAT is to improve IFI prognosis with prompt therapy, universal EAT in neutropenic patients after 5–7 days of persistent fever has been traditionally recommended by the main scientific societies. Scientific evidence supporting this recommendation is weak and based on two clinical trials with small sample size and questionable methodology that compared the administration of amphotericin vs. placebo and failed to demonstrate significantly reduced IFI incidence or IFI related mortality.34,35 Subsequently there have been several large comparative studies to identify which is the best drug for EAT,13–15,36–39 but no further studies have been done demonstrating that EAT reduces IFI incidence or IFI related mortality. Also, the advances in early diagnosis and the development of new drugs have improved the outcome of IFI.40–44 Thus, in recent years different approaches have been proposed with the aim of selecting patients for antifungal therapy (AT) guided by clinical criteria and risk profiles.38–47 In these approaches, antifungal therapy is given only when evidence of IFI is suggested by serum tests for fungal antigens or DNA, high-resolution chest CT, and/or clinical criteria as shown in Table 3.40,45–48 The only randomized study comparing universal EAT with pre-emptive therapy showed a 35% reduction of EAT without increasing IFI mortality.49
Empirical antifungal therapy in hematological patients with persistent fever and neutropenia.
| Author (no. of NFP episodes) | Surveillance | AT onset | AT reduction (%) | Untreated IFI | Ref. |
| Maertens et al. (n=41) | Daily GM testa | ≥2 GM test positive or CT-scanb findings+positive culture/microscopic tests | 78 | 2.4 | 40 |
| Oshima et al. (n=60) | Weekly GM, β-d-glucan tests and chest X-ray | Positive serum test and/or findings on X-ray or CT-scan | 93 | 1.7 | 45 |
| Dignan et al. (n=53) | – | Fever, CT findings or clinical IFI suspicion | 67 | 0 | 46 |
| Aguilar-Guisado et al. (n=66) | – | Fever+severity or focalty | 43 | 0 | 47 |
| Girmenia et al. (n=84) | – | Fever+IFIc documentation | 61 | 1.2 | 48 |
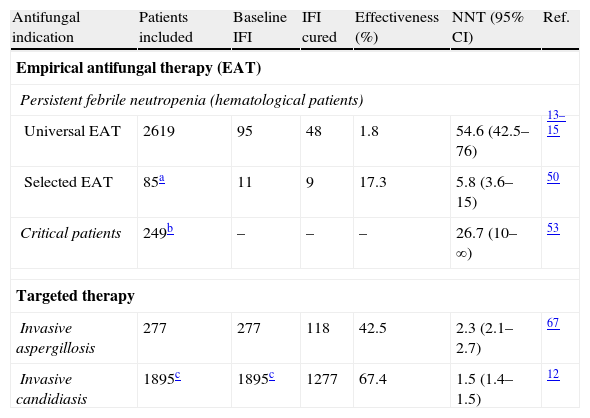
Our group has developed a tailored diagnostic and therapeutic approach to select those patients without the need for EAT based on two main steps: first, to evaluate the severity of the episode (severe sepsis or septic shock) and second, to identify any clinical infectious foci susceptible to be caused by fungi.41,47 For patients who had neither severe signs nor infectious foci, AT was not initially indicated and further diagnostic evaluations are performed. This tailored approach has been validated in a fairly large series of patients with hematologic malignancies, including IFI high-risk patients, with a negative predictive value of 100% and a reduction of 38.8% in the indication of EAT.50 The effectiveness of this approach is similar to that of universal EAT reported in strategies for controlled trials according to the five-component end-point criteria (successful treatment of baseline IFI, absence of breakthrough IFI, survival for seven days after completion of therapy, no premature discontinuation of antifungal therapy and resolution of fever during neutropenia). On the other hand, when we compare the proportion of baseline IFI curation, the number needed to treat (NNT) is 5.8 in our said approach vs. 54.6 in universal EAT taking as reference the three major Walsh clinical trials13–15 as shown in Table 4.
Results on effectiveness and number of patients needed to treat (NNT) to avoid a failure response of invasive fungal infections (IFI) for the different indications of the antifungal therapy.
| Antifungal indication | Patients included | Baseline IFI | IFI cured | Effectiveness (%) | NNT (95% CI) | Ref. |
| Empirical antifungal therapy (EAT) | ||||||
| Persistent febrile neutropenia (hematological patients) | ||||||
| Universal EAT | 2619 | 95 | 48 | 1.8 | 54.6 (42.5–76) | 13–15 |
| Selected EAT | 85a | 11 | 9 | 17.3 | 5.8 (3.6–15) | 50 |
| Critical patients | 249b | – | – | – | 26.7 (10–∞) | 53 |
| Targeted therapy | ||||||
| Invasive aspergillosis | 277 | 277 | 118 | 42.5 | 2.3 (2.1–2.7) | 67 |
| Invasive candidiasis | 1895c | 1895c | 1277 | 67.4 | 1.5 (1.4–1.5) | 12 |
The effectiveness has been calculated based on documented IFI/patients receiving antifungal therapy.
The second main indication of antifungal therapy is EAT in non-neutropenic critically ill patients with risk factors for IC and no other known cause of fever.33 Criteria for starting EAT in non-neutropenic patients remain poorly defined and were established in two retrospective studies that showed an association between the delay of appropriate antifungal therapy and hospital mortality.51,52 Of them, the largest and multicenter study excluded those patients who receiving antifungal treatment died early after blood culture extractions.52 Thereafter, the only clinical trial designed to asses the efficacy of EAT in critically ill patients, including 270 patients fever despite the administration of broad-spectrum antibiotics, concluded that the indication of empirical fluconazole does not improve the outcome when it is compared with placebo.53 In these studies, authors recognize the need for new strategies such as rapid diagnostic tests or the identification of specific risk factors for Candida infection to avoid massive EAT in patients without IC.52,53 However, most of the new surrogate markers of IFI are under investigation.54–56 In an attempt to select more accurately those ICU patients with suspected candidiasis for EAT, several prediction rules have been developed such as the Pittet corrected colonization index for surgical ICU patients57,58 or the “Candida score” for critically ill ICU patients.59 These rules are based on Candida spp. colonization, risk factors for IFI and infection signs, indicating EAT when a concrete value is reached. In spite of its high sensitivity, these rules have a low specificity and low positive predictive value.57,59,60 In particular, it is unknown if the outcome is modified when these scores are applied to decide the initiation of EAT in comparison to EAT indication following individualized clinical criteria in patients with risk factors for IC and no other known cause of fever.
In conclusion, currently available scientific evidence does not justify the universal empirical antifungal treatment in most neutropenic and critically ill patients (Table 2) and the widespread use of EAT entails a risk of over treatment and side effects when compared with the real incidence of IFI in these populations.
Targeted therapy in invasive candidiasisTargeted antifungal therapy in IC with established etiology has the greatest degree of scientific evidence (Table 2). International guidelines recommend several antifungal drugs for primary and alternative AT of IC depending on previous azole use, local susceptibility patterns, severity signs, comorbidities, potential adverse effects and causative yeast. In non-neutropenic patients, most international guidelines recommend primary AT with fluconazole or an echinocandin in patients with moderately severe illness or recent azole exposure. Amphotericin B is recommended as alternative in case of intolerance to or limited availability of other antifungals and voriconazole to switch to oral therapy when possible.29,33,61
Recently, some authors have suggested that the use of an echinocandin as primary therapy should be extended to most patients with IC. The basis for this recommendation is an individual patient-level quantitative review of 7 randomized trials including 1915 patients for the treatment of candidemia and invasive candidiasis; and it concluded that ‘two treatment-related factors were associated with improved survival and greater clinical success: the use of an echinocandin and removal of the CVC’. Mortality using an echinocandin was 27% compared with 36% when other antifungal drugs were used.12 However, a critical review of the study finds methodological concerns that may bias the final conclusion. Firstly, it includes studies that took place between 1989 and 2006, covering a total of 17 years, with the echinocandin trials being conducted between 1997 and 2006, the last period. Secondly, the 7 trials included are heterogeneous in design as only 3 of them compare the use of an echinocandin with other antifungal drugs. This selection bias could have been avoided, if only the 3 studies comparing echinocandins with other antifungals (amphotericin deoxycholate, liposomal amphotericin, and fluconazole), including a total of 1000 patients, were compared.62–64 However, in that case conclusions would have been different, as the mortality rate of patients who received echinocandins in these 3 studies (23% [115 out of 500 patients]) was similar to that of patients who received other antifungal treatment (24.2% [121 out of 500 patients]).65
In conclusion, echinocandins are effective and expensive antifungals with specific indications in empirical and targeted therapy; an expansion of these specific recommendations to most patients with IC is not supported by scientific evidence, and would result in increased costs. A more rational prescription of targeted AT may be encouraged.
Targeted therapy in invasive aspergillosisThe current recommendations for treatment of IA is the use of voriconazole as elective primary therapy and liposomal amphotericin, with similar efficacy but higher toxicity, as alternative therapy.30,66,67 For salvage therapy, a change of the antifungal class to lipid formulations of amphotericin, posaconazole, itraconazole, caspofungin should be considered. Primary combination therapy is not routinely recommended based on lack of clinical data, but the addition or combination of antifungal drugs of different classes other than those in the initial regimen might be used as salvage therapy.66,68 Despite the fact that these recommendations have improved the outcome of IA, the attributable mortality remains high (Table 1). Since the clinical management of IA is hampered by the difficulty on its diagnosis, further research on optimizing the antifungal resources are required such as AT guided by levels or combined therapy. In this regard, preliminary unpublished data from a multicenter randomized double-blind study, comparing combined AT with voriconazole and anidulafungin vs. voriconazole monotherapy for primary treatment of IA in 277 patients, seem to improve the mortality rate (19.3% vs. 27.5%, respectively) without differences in safety.69 If these data were confirmed, then the combined therapy with both antifungals would become the treatment of choice for IA, rather than monotherapy with voriconazole.
Final considerationsThe extraordinary increase in the consumption of antifungals in recent years has taken place mainly in the empirical treatment, which is the indication with the weakest level of scientific evidence, both in neutropenic patients and in critically ill patients. Besides, the use of antifungals is very often inappropriate, exposing patients to adverse effects, drug interactions, development of resistance and superinfections by other fungi, reducing their effectiveness and resulting in a significant health spending. To improve this poor use of antifungals, it is necessary to consider new strategies such as the empirical antifungal therapy in selected patients with persistent febrile neutropenia post-chemotherapy, and conduct an independent continuous training, within the framework of the Antimicrobial Stewardship Programs, geared to make less empirical therapy and more targeted therapy.
FundingThis work was supported by public research funds from the Regional Health Ministry of Andalucía [PI-0361-2010].
Conflict of interestJ.M.C. has received speaking honoraria from Merck, Astellas and Pfizer. All other authors do not report potential conflicts of interests.