To perform epidemiological surveillance of Legionella pneumophila in recreational swimming pools in the city of Valladolid (Spain), an area with a continental climate and low incidence of legionella-associated infections. Additionally, wild-type minimum inhibitory concentration (MIC) distributions for eight antibiotics commonly used for the treatment of legionellosis were calculated from the isolates obtained.
MethodsTwelve recreational pools were enrolled between June 2003 and December 2016 and 7221 water samples were taken from three different points of the water network (tank, tap and shower). Legionella culture was performed according to ISO 11731 and 11731-2 standards. MICs of antibiotics were obtained by a gradient test.
Results1.44% of the water samples were positive for L. pneumophila. 60 strains (57.69%) were isolated from showers, 26 (25.00%) from tanks and 18 (17.31%) from taps. L. pneumophila counts were <100CFU/L in 75 samples (72.12%), 100–1000CFU/L in 17 (16.35%) and >1000CFU/L in 12 (11.54%). The MIC90 values obtained were for Rifampicin 0.125mg/L; Trimethoprim-Sulfamethoxazole 0.25mg/L; Azithromycin and Levofloxacin 0.5mg/L; Clarithromycin and Ciprofloxacin 1.0mg/L; Doxycycline and Tigecycline 4.0mg/L.
ConclusionsThe use of showers in recreational pools can become a potential pathway for exposure to L. pneumophila, even in cold climates. The wild-type MIC distributions presented in this article may be useful for a better detection of antibiotic resistance and can contribute to improvements in the choice of the antibiotic treatment of legionellosis.
Realizar la vigilancia epidemiológica de Legionella pneumophila en piscinas recreacionales de Valladolid (España), un área con clima continental y baja incidencia de legionelosis. La distribución de las CMIs de ocho antibióticos usados en la legionelosis fue calculada a partir de los aislados obtenidos.
MétodosSe incluyeron doce piscinas recreacionales entre junio 2003-diciembre 2016. 7.221 muestras de agua fueron tomadas en tres puntos de la red (vaso, grifo y ducha). El cultivo de legionela se realizó acorde a las normas ISO 11731 y 11731-2. Las CMIs de los antibióticos se obtuvieron mediante un método en gradiente.
Resultados1,44% de las muestras proporcionaron crecimiento de L. pneumophila. 60 cepas (57,69%) se aislaron en duchas, 26 (25,00%) en vasos y 18 (17,31%) en grifos. Los recuentos de L. pneumophila fueron < 100UFC/L en 75 muestras (72,12%), 100-1.000UFC/L en 17 (16,35%) y > 1.000UFC/L en 12 (11,54%). Las CMI90 obtenidas fueron para rifampicina 0,125mg/L; trimetoprim-sulfametoxazol 0,25mg/L; azitromicina y levofloxacino 0,5mg/L; clarithromicina y ciprofloxacino 1,0mg/L; doxiciclina y tigeciclina 4,0mg/L.
ConclusionesEl uso de las duchas en piscinas recreacionales puede convertirse en una vía potencial para la exposición a L. pneumophila, incluso en climas fríos. Las CMIs presentadas en este artículo son útiles para la detección de la resistencia a antibióticos y pueden mejorar la elección del tratamiento antibiótico de la legionelosis.
Legionella pneumophila is a gramnegative rod present in natural aquatic environments and soil, in association with amoebae and other protozoa, and in biofilms.1 From these reservoirs, L. pneumophila can reach different human-made water distribution systems, where aerosols loaded with bacteria can be formed and subsequently inhaled by humans, causing sporadic cases or outbreaks of legionellosis.2 In this way, it can be assumed that L. pneumophila environmental isolates are the source of the clinical cases.
With the aim of minimizing and limiting opportunistic infections in humans, environmental surveillance of L. pneumophila is a key component for establishing control measures to ensure water safety and quality. However, despite this surveillance, incidence of L. pneumophila has increased in the United States of America and Europe over the last few years.3,4 As consequence, current empiric antibiotic treatment of community-acquired pneumonia, based on Quinolones and Macrolides, is active against L. pneumophila.5
The in vitro Antibiotic Susceptibility Test (AST) of L. pneumophila presents some drawbacks. The European Committee of Antimicrobial Susceptibility Testing (EUCAST) has not established MIC clinical breakpoints and, also, has published epidemiological cut-off values (ECOFFs) only for the antibiotics Chloramphenicol, Clarithromycin, Erythromycin and Trimethoprim-Sulfamethoxazole.6 Nevertheless, EUCAST published in 2017 a guidance document entitled Antibiotic susceptibility testing of L. pneumophila. In this document, the procedure and control of AST, and the tentative highest MIC for wild-type are defined.7
First and second-line antibiotic therapy for legionellosis include eight antibiotics, namely: Azithromycin, Clarithromycin, Ciprofloxacin, Levofloxacin, Rifampicin, Trimethoprim-Sulfamethoxazole, Doxycycline and Tigecycline.8,9 Taking into account the aforementioned drawbacks of the AST for L. pneumophila, knowing wild-type MIC distributions for all antibiotics can be useful for a better detection of antibiotic resistance and can contribute to improvements in the choice of the antibiotic treatment of legionellosis.
In view of the foregoing, this work was aimed to describe the presence and distribution of L. pneumophila in recreational swimming pools in Valladolid (Spain), as well as define wild-type MIC distribution of the antibiotics used in clinical practice for legionellosis treatment.
Material and methodsEnvironmental surveillance of L. pneumophilaDuring the period June 2003 to December 2016, environmental surveillance was performed on water sampling coming from 12 recreational swimming pools of Valladolid (Spain). Valladolid is a city of 300,000 inhabitants located in northwestern Spain with a continental climate. Temperature ranges are extreme; winters are long with minimums temperatures as low as −8°C while summers are short with high temperatures up to 37°C.
Six indoor pools, coded A to F, were enrolled during the whole period; from July 2004, an indoor pool, coded G, was added. Five outdoor pools, coded H to L, were enrolled until December 2011; from January 2012 the pool L was eliminated from the surveillance programme.
The water sampling from indoor pools was carried out once a month throughout the whole period while for outdoor pools it was performed monthly between June and August every year (warmest months). The sampling encompassed three water samples of 1 litre taken at three different points of the water network: tank water, tap water and inside their nozzles using swabs, and shower water and inside their nozzles with swabs. Sterile containers containing sodium thiosulfate to neutralize any disinfectant present in the water were used to collect the samples. Globally, 7221 water samples were obtained during the period of study.
During transport to the laboratory, water samples were kept at room temperature and protected from light. Legionella culturing was performed following ISO standard 11731 (from June 2003 to September 2007) and ISO standard 11731-2 (from October 2007 to December 2016) using buffered charcoal yeast extract agar supplemented with α-Ketoglutarate, Glycine, Vancomycin, Polymyxin B and Cycloheximide (GVPC agar) (Oxoid, Basingstoke, UK). After incubation of plates at 35°C for 15 days in humidified atmosphere, suspected colonies of L. pneumophila were identified by direct immunofluorescence with MonoFluo™ L. pneumophila IFA Test (BIO-RAD, Hercules, CA, USA). If positive, colonies were categorized in three groups: <100CFU/L, 100–1000CFU/L and >1000CFU/L.
Characterization of L. pneumophila serogroup was performed using specific antibodies (L. pneumophila serogroup 1 Test Reagent and L. pneumophila serogroup 2-14 Test Reagent, Oxoid). Finally, strains were stored at −80°C.
Wild-type MIC distribution of L. pneumophila strainsOne hundred of 104 strains were recovered from stock cultures using buffered charcoal yeast extract agar supplemented with α-Ketoglutarate (BCYEα agar) (Oxoid) and, before performing AST, two passages were performed. The antibiotics Azithromycin, Clarithromycin, Ciprofloxacin, Levofloxacin, Rifampicin, Trimethoprim-Sulfamethoxazole, Doxycycline and Tigecycline (BioMérieux, Marcy l’Etoile, France) were tested by a gradient test. A 0.5 McFarland standard bacterial suspension was prepared from colonies grown in BCYEα agar (Oxoid). The swab was dipped into the suspension to inoculate the entire plate. Strips with a predefined gradient of the above-mentioned antibiotics were placed onto the inoculated plates; one strip of each antibiotic was applied onto one inoculated plate containing BCYEα agar (Oxoid). According to EUCAST, minimum inhibitory concentrations (MICs) were obtained from the scale on the strip at the point where the ellipsoid of growth inhibition intercepted the strip after 48h of incubation at 35°C in humidified atmosphere.7 Finally, MICs were expressed as a cumulative distribution in mg/L.
In order to obtain wild-type MIC distribution of L. pneumophila strains, MIC50 and MIC90 were calculated from previously obtained MICs. MIC50 and MIC90 are the values at which 50% and 90% of the isolates are inhibited respectively.
Besides, in order to determine the influence of BCYEα agar in the obtained MICs, Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 (with known susceptibility) were tested as controls against the same antibiotics using Mueller-Hinton (M-H) agar (Oxoid) and BCYEα agar (Oxoid).
Control strainL. pneumophila serogroup 1 ATCC 33152 was tested against the antibodies and antibiotics used for environmental strains.
Statistical analysisData were analyzed by Epidat 3.1. Associations were evaluated using the chi-square and Fisher exact test for those cases where more than 25% of the samples were less than 5. For all tests, a significance level of 5% was accepted.
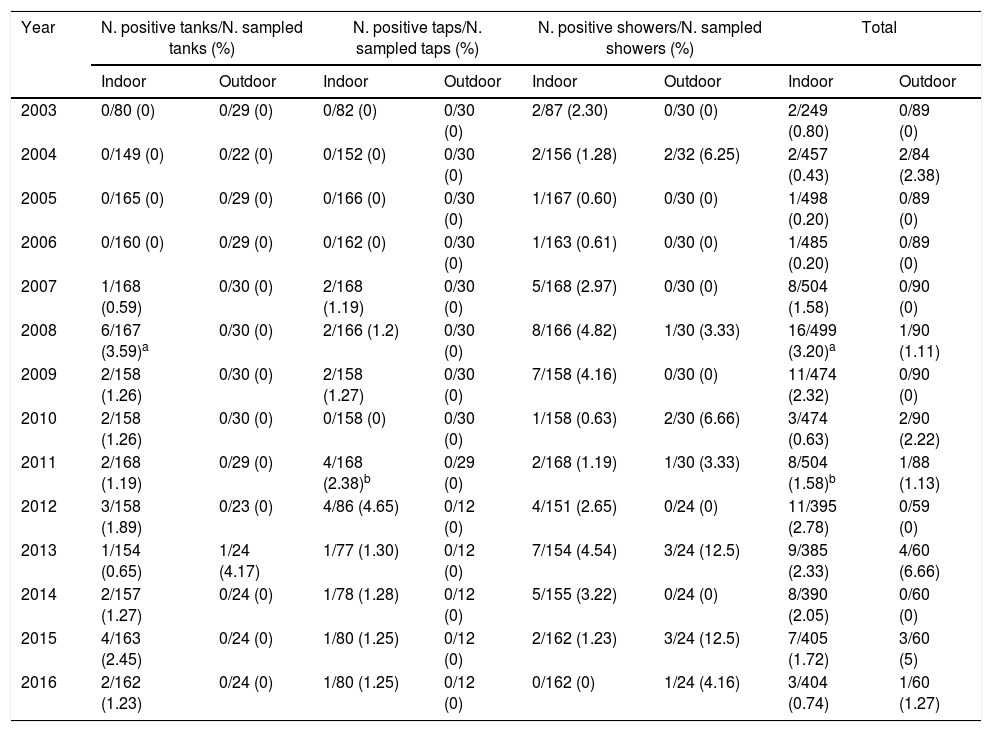
ResultsEnvironmental surveillance of L. pneumophilaL. pneumophila was isolated from all indoor pools and from three out of five outdoor pools enrolled. Table 1 shows the year distribution of legionella positive samples. For indoor pools the percentages of samples testing positive were from tanks (1.15%), taps (1.01%), and showers (2.16%). For outdoor pools, those percentages were from tanks (0.27%), taps (0%), and showers (3.31%). Regarding the whole survey, L. pneumophila was isolated in 1.47% of the indoor pools samples and in 1.28% of the outdoor pools samples; globally, 1.44% samples were positive for L. pneumophila.
Year distribution of water samples tested positive for L. pneumophila from swimming pools.
| Year | N. positive tanks/N. sampled tanks (%) | N. positive taps/N. sampled taps (%) | N. positive showers/N. sampled showers (%) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | Indoor | Outdoor | |
| 2003 | 0/80 (0) | 0/29 (0) | 0/82 (0) | 0/30 (0) | 2/87 (2.30) | 0/30 (0) | 2/249 (0.80) | 0/89 (0) |
| 2004 | 0/149 (0) | 0/22 (0) | 0/152 (0) | 0/30 (0) | 2/156 (1.28) | 2/32 (6.25) | 2/457 (0.43) | 2/84 (2.38) |
| 2005 | 0/165 (0) | 0/29 (0) | 0/166 (0) | 0/30 (0) | 1/167 (0.60) | 0/30 (0) | 1/498 (0.20) | 0/89 (0) |
| 2006 | 0/160 (0) | 0/29 (0) | 0/162 (0) | 0/30 (0) | 1/163 (0.61) | 0/30 (0) | 1/485 (0.20) | 0/89 (0) |
| 2007 | 1/168 (0.59) | 0/30 (0) | 2/168 (1.19) | 0/30 (0) | 5/168 (2.97) | 0/30 (0) | 8/504 (1.58) | 0/90 (0) |
| 2008 | 6/167 (3.59)a | 0/30 (0) | 2/166 (1.2) | 0/30 (0) | 8/166 (4.82) | 1/30 (3.33) | 16/499 (3.20)a | 1/90 (1.11) |
| 2009 | 2/158 (1.26) | 0/30 (0) | 2/158 (1.27) | 0/30 (0) | 7/158 (4.16) | 0/30 (0) | 11/474 (2.32) | 0/90 (0) |
| 2010 | 2/158 (1.26) | 0/30 (0) | 0/158 (0) | 0/30 (0) | 1/158 (0.63) | 2/30 (6.66) | 3/474 (0.63) | 2/90 (2.22) |
| 2011 | 2/168 (1.19) | 0/29 (0) | 4/168 (2.38)b | 0/29 (0) | 2/168 (1.19) | 1/30 (3.33) | 8/504 (1.58)b | 1/88 (1.13) |
| 2012 | 3/158 (1.89) | 0/23 (0) | 4/86 (4.65) | 0/12 (0) | 4/151 (2.65) | 0/24 (0) | 11/395 (2.78) | 0/59 (0) |
| 2013 | 1/154 (0.65) | 1/24 (4.17) | 1/77 (1.30) | 0/12 (0) | 7/154 (4.54) | 3/24 (12.5) | 9/385 (2.33) | 4/60 (6.66) |
| 2014 | 2/157 (1.27) | 0/24 (0) | 1/78 (1.28) | 0/12 (0) | 5/155 (3.22) | 0/24 (0) | 8/390 (2.05) | 0/60 (0) |
| 2015 | 4/163 (2.45) | 0/24 (0) | 1/80 (1.25) | 0/12 (0) | 2/162 (1.23) | 3/24 (12.5) | 7/405 (1.72) | 3/60 (5) |
| 2016 | 2/162 (1.23) | 0/24 (0) | 1/80 (1.25) | 0/12 (0) | 0/162 (0) | 1/24 (4.16) | 3/404 (0.74) | 1/60 (1.27) |
Table 1E (see material supplementary) shows epidemiological data of the 104 isolates of L. pneumophila obtained during the study period. From indoor pools, 90 strains were isolated: 48 (53.33%) from showers, 24 (26.67%) from tanks and 18 (20.00%) from taps. Its bacterial counts were <100CFU/L in 67 samples (74.44%), 100–1000CFU/L in 16 (17.78%) and >1000CFU/L in 7 (7.78%). From outdoor pools, 14 isolates were obtained: 13 (92.85%) from showers and 1 (7.14%) from tanks. L. pneumophila counts were <100CFU/L in 8 samples (57.14%), 100–1000CFU/L in 1 (7.14%) and >1000CFU/L in 5 (35.71%). Globally, 60 (57.69%) strains were isolated from showers, 26 (25.00%) from tanks and 18 (17.31%) from taps; L. pneumophila counts were <100CFU/L in 75 samples (72.12%), 100–1000CFU/L in 17 (16.35%) and >1000CFU/L in 12 (11.54%).
Distribution by serogroups of the 104 L. pneumophila strains isolated showed that 102 strains (98.08%) belonged to serogroup 1 and two strains (1.92%) belonged to any of the serogroups 2 to 14. As expected, L. pneumophila ATCC 33152 belonged to serogroup 1.
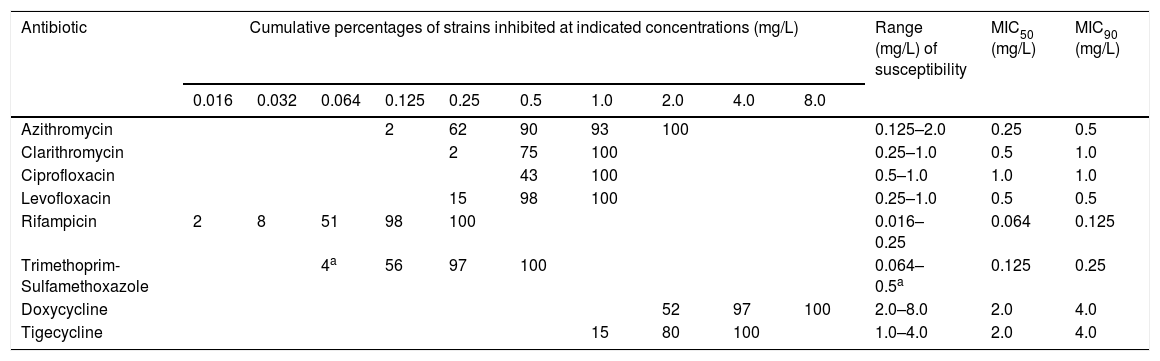
Wild-type MIC distribution of L. pneumophila strainsTable 2 shows the cumulative percentages of 100 isolates of L. pneumophila inhibited by different antibacterial concentrations and the wild-type MIC distribution (formulated as susceptibility range, MIC50 and MIC90). The AST in all 100 environmental strains showed similar results in MICs during the whole period, regardless the pool and the point of the water network tested positive; as consequence, no tendency towards antibiotic resistance was observed. Tetracyclines (Doxycycline and Tigecycline) were the least active drugs (range of susceptibility: 1.0–8.0mg/L). Rifampicin and Trimethoprim-Sulfamethoxazole were the most active drugs (range of susceptibility: 0.016–0.5mg/L). The activity of Macrolides and Quinolones was between the previous ones (range of susceptibility 0.125–2.0mg/L). Within Macrolides, Azithromycin with MIC90 0.5mg/L, was more active than Clarithromycin with MIC90 1.0mg/L. Within Quinolones, Levofloxacin with MIC90 0.5 0.5mg/L, was more active than Ciprofloxacin with MIC90 1.0mg/L.
Cumulative distribution of MICs and wild-type MIC distribution of environmental L. pneumophila isolates (n=100).
| Antibiotic | Cumulative percentages of strains inhibited at indicated concentrations (mg/L) | Range (mg/L) of susceptibility | MIC50 (mg/L) | MIC90 (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.016 | 0.032 | 0.064 | 0.125 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | ||||
| Azithromycin | 2 | 62 | 90 | 93 | 100 | 0.125–2.0 | 0.25 | 0.5 | |||||
| Clarithromycin | 2 | 75 | 100 | 0.25–1.0 | 0.5 | 1.0 | |||||||
| Ciprofloxacin | 43 | 100 | 0.5–1.0 | 1.0 | 1.0 | ||||||||
| Levofloxacin | 15 | 98 | 100 | 0.25–1.0 | 0.5 | 0.5 | |||||||
| Rifampicin | 2 | 8 | 51 | 98 | 100 | 0.016–0.25 | 0.064 | 0.125 | |||||
| Trimethoprim-Sulfamethoxazole | 4a | 56 | 97 | 100 | 0.064–0.5a | 0.125 | 0.25 | ||||||
| Doxycycline | 52 | 97 | 100 | 2.0–8.0 | 2.0 | 4.0 | |||||||
| Tigecycline | 15 | 80 | 100 | 1.0–4.0 | 2.0 | 4.0 | |||||||
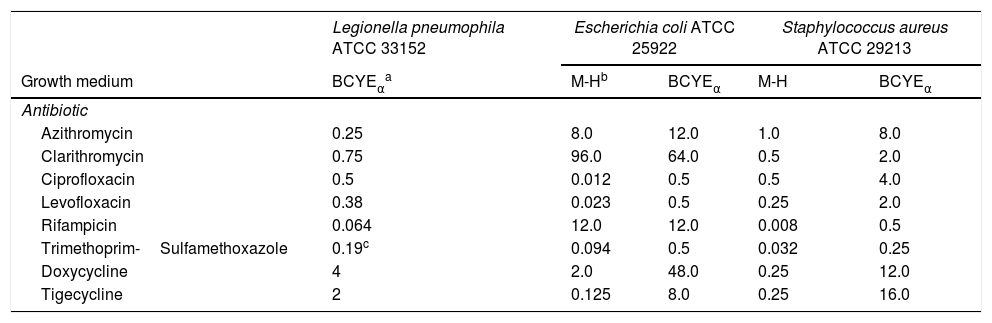
Regarding the control strains, L. pneumophila ATCC 33152 generally showed MICs similar to the values of the environmental strains (Table 3). The MICs obtained from control strains of E. coli ATCC 25922 and S. aureus ATCC 29213 on M-H agar were within the range of accepted values (Table 3). Depending on growth medium, BCYEα agar provided higher MICs in 86.67% of the AST performed.
MICs (mg/L) against three reference strains depending on the growth medium.
| Legionella pneumophila ATCC 33152 | Escherichia coli ATCC 25922 | Staphylococcus aureus ATCC 29213 | |||
|---|---|---|---|---|---|
| Growth medium | BCYEαa | M-Hb | BCYEα | M-H | BCYEα |
| Antibiotic | |||||
| Azithromycin | 0.25 | 8.0 | 12.0 | 1.0 | 8.0 |
| Clarithromycin | 0.75 | 96.0 | 64.0 | 0.5 | 2.0 |
| Ciprofloxacin | 0.5 | 0.012 | 0.5 | 0.5 | 4.0 |
| Levofloxacin | 0.38 | 0.023 | 0.5 | 0.25 | 2.0 |
| Rifampicin | 0.064 | 12.0 | 12.0 | 0.008 | 0.5 |
| Trimethoprim-Sulfamethoxazole | 0.19c | 0.094 | 0.5 | 0.032 | 0.25 |
| Doxycycline | 4 | 2.0 | 48.0 | 0.25 | 12.0 |
| Tigecycline | 2 | 0.125 | 8.0 | 0.25 | 16.0 |
This paper describes, for the first time, two important issues: (a) surveillance of L. pneumophila from indoor and outdoor swimming recreational pools for a long period of time in a cold area of Spain, and (b) wild-type MIC distribution for all antibiotics used in clinical practice for legionellosis treatment.
Environmental surveillance of L. pneumophilaSurveying and monitoring of L. pneumophila in different human-made water distribution systems is necessary in order to prevent and control cases and outbreaks of legionellosis.10 This work was focussed on indoor and outdoor recreational pools in Valladolid, Spain. Water temperature of indoor pools is higher than outdoor pools. As consequence, given that L. pneumophila grows better within a temperature range of 35–45°C11, we expected to find a higher percentage of isolations in indoor pools than in outdoor pools. In fact, L. pneumophila was isolated in all indoor pools and only in three out of five outdoor pools enrolled. However, it is worth to mention that the percentage of positive samples found in indoor pools was practically the same as in outdoor pools (1.47% and 1.28% respectively), with no statistically significant difference (p=0.617) and, moreover, the percentage of isolates with >1000CFU/L was significantly higher in outdoor pools than indoor pools (35.71% versus 7.78%) (p=0.009). This is probably due to that cleaning and disinfection measures in outdoor pools are applied only in warmest months (from June to August). Hence, during the rest of the year, L. pneumophila can grow until reaching high concentrations in water distribution systems. In order to avoid counts >1000CFU/L in outdoor pools, methods for disinfection could be systematically applied before opening.
Several authors12–14 performed an environmental surveillance of L. pneumophila from pools and reported a percentage of positivity of 5.90%, 25.60% and 39.60% respectively. Our percentage of isolation of L. pneumophila was lower (1.44%), with statistically significant differences compared to the percentage founded by De Filippis et al.13 and Leoni et al.14 (p<0.001). The low prevalence of L. pneumophila isolations in pools of Valladolid could be extrapolated to the city's water distribution system given that an outbreak has never been detected in the city. Furthermore, the incidence of legionellosis in Valladolid is approximately 0.4 cases/100,000 inhabitants/year. This value is lower than in other regions of Spain, such as Comunitat Valenciana, where the rate of legionellosis is about 5.0 cases/100,000 inhabitants/year and several outbreaks of legionellosis have been documented over time.15
L. pneumophila concentrations in environmental sites obtained from the surveillance may be used as a predictive risk factor for the acquisition of the infection.16 As a consequence, the Spanish law sets out that disinfection measures for prevention and control of legionellosis must be applied when >1000UFC/L are detected. Those measures consisted mainly of repeated superheating and flushing of the water system because L. pneumophila cannot survive at >60°C.17 However, it is worth to mention that L. pneumophila concentration in water distribution systems can vary over time;18 for this reason, when L. pneumophila was detected in a pool, regardless its bacterial concentration, disinfection measures were applied, providing the following results: in five pools (4 indoor: B, C, E and F, and 1 outdoor: L) only one L. pneumophila isolation was detected. In the outdoor pool K, a second isolation was obtained in 2015, two years after the first one. In the indoor pool G, the first isolation took place in 2012; later, one isolate was obtained in 2015 and two in 2016. From the indoor pools A and D and the outdoor pool I, 93 isolations were obtained, which represented 89.42% of the total. Interestingly, isolations from indoor pools A and D were obtained all over the months of the study period, with no trend or seasonality and alternating high counts with low counts. These data suggest that, despite control measures in certain settings, L. pneumophila cannot be completely eradicated. Nevertheless, disinfection measures help reduce possible infection in some cases.
Regarding the point of the water network analyzed, showers provided the highest rate of positivity with statistically significant differences compared to isolations from tanks and taps (p<0.001). In this way, 60 L. pneumophila isolations (57.69% of the total) were obtained from showers; moreover, 11 (18.33%) of these 60 provided >1000CFU/L, accounting for 91.67% of the positive samples with these counts. These results matched with previous studies,13,14 where shower of pools were identified as the main point of water network for legionellosis development. This fact is very important given that during the use of showers, which is mandatory in Spain before swimming, aerosol loaded with L. pneumophila can be formed. In contrast, 26 isolations (25.00% of the total) were obtained from water tanks with <100CFU/L and 18 (17.31% of the total) were from water taps with only one with >1000CFU/L.
It is well known that L. pneumophila serogroup 1 accounts for ≈85% of cases of legionellosis.19 Studies carried out in France, Germany and Italy reported a 28.20%, 22.00% and 69.40% of environmental strains belonging to serogroup 1 respectively.19–21 The percentage found in our work was 98.08%, which is higher than those mentioned above. This fact deserves more attention and additional research in light of low rates of human infections observed in Valladolid during the period of study.
Wild-type MIC distribution of L. pneumophila strainsThe growth medium BCYEα contains charcoal and its surface area is available for adsorption of toxic substances for L. pneumophila formed during its growth.22 In the same way, when an AST is performed, charcoal can also adsorb the spreaded antibiotic in the culture medium, thus resulting in elevated MICs for most antibiotics tested. In order to determine the influence of medium growth in the obtained MICs, two reference strains (E. coli ATCC 25922 and S. aureus ATCC 29213 with a known susceptibility) were used as controls. MICs obtained from BCYEα in this study were higher than that from agar M-H in 14 out of 16 AST performed (86,67%); it is worth to mention that Tigecycline and Quinolones were the most influenced antibiotics by BCYEα in both reference strains. In a similar manner, several authors23,24 obtained higher MICs in BCYEα than in M-H agar in 70% of AST performed. Thus, the use of BCYEα seems to be an important step for the correct interpretation of susceptibility of L. pneumophila to antibiotics. In order to overcome this problem, several authors proposed the use of BYEα (without carchoal) instead of BCYEα.25,26 However, EUCAST, in its most recent document, establishes that BCYEα must be used for L. pneumophila AST.7
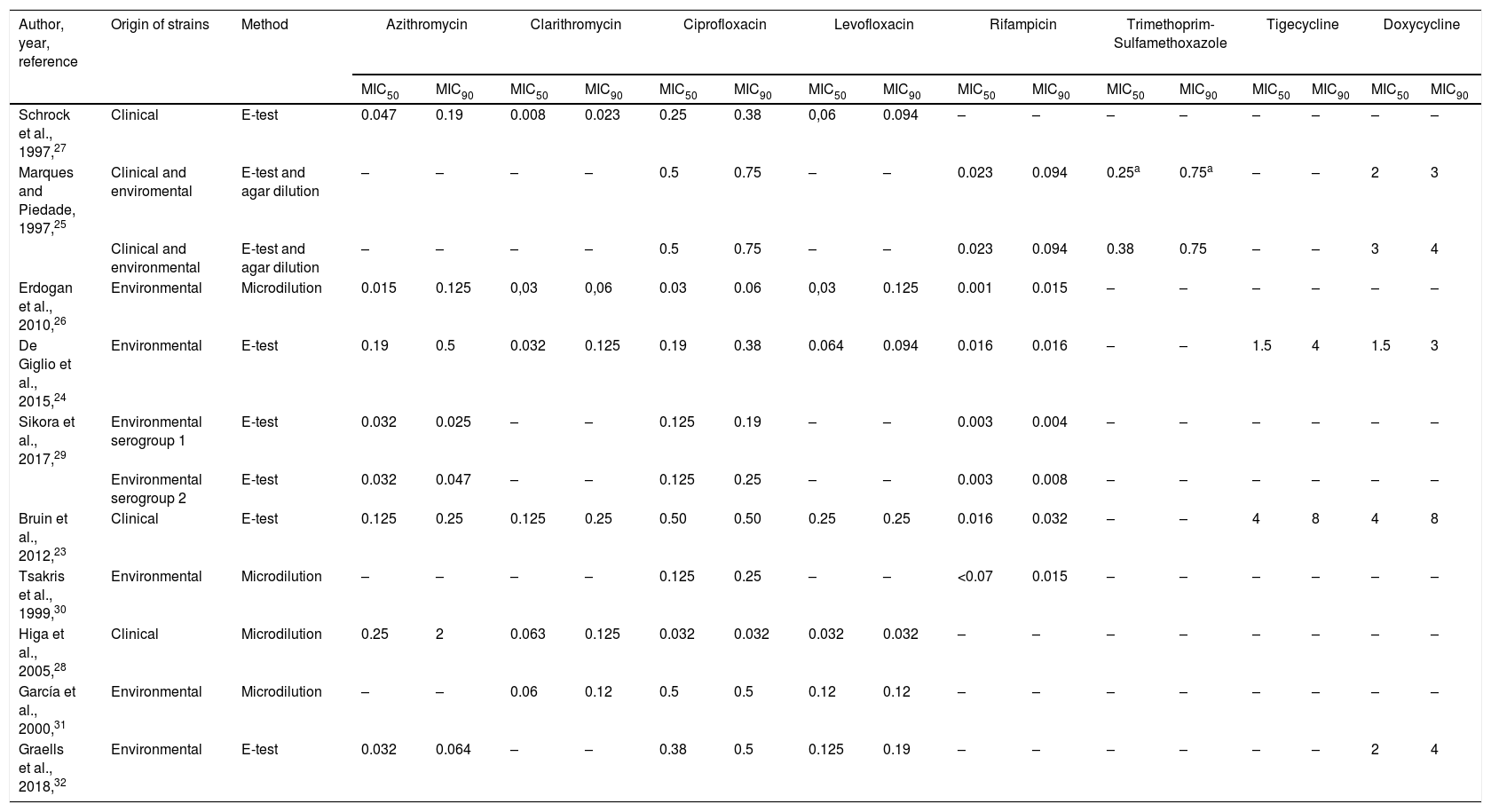
MICs of several antibiotics against clinical,23,27,28 environmental,24,26,29–32 or both strains25 of L. pneumophila have been determined (Table 4). By comparing these MICs, a wide disparity between MIC50 and MIC90 was observed. However, according to these studies and our results, Rifampicin provided the lowest MICs and Tetracyclines (Doxycycline and Tigecycline) the highest one. It is worth to mention that MIC50 and MIC90 of Clarithromycin, Quinolones and Rifampicin obtained in this study were higher than those previously published (Table 4), including one study also carried out in Spain.32 So, it can be assumed that geographical location is not a key factor in MICs values against L. pneumophila. Moreover, our MIC90 of Clarithromycin and Rifampicin obtained against environmental strain were above the tentative highest MIC for wild-type L. pneumophila strains reported by EUCAST.7 And, surprisingly, our MICs of Clarithromycin and Rifampicin, and MIC of Rifampicin reported by Bruin et al.,23 obtained against the control strain L. pneumophila ATCC 33152, were also above the tentative highest MIC for wild-type.7 These disparities on MIC values can be due to differences in the amount of charcoal in the used mediums and the AST methodology.
MIC50 and MIC90 of different antibiotics tested against L. pneumophila.
| Author, year, reference | Origin of strains | Method | Azithromycin | Clarithromycin | Ciprofloxacin | Levofloxacin | Rifampicin | Trimethoprim-Sulfamethoxazole | Tigecycline | Doxycycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |||
| Schrock et al., 1997,27 | Clinical | E-test | 0.047 | 0.19 | 0.008 | 0.023 | 0.25 | 0.38 | 0,06 | 0.094 | – | – | – | – | – | – | – | – |
| Marques and Piedade, 1997,25 | Clinical and enviromental | E-test and agar dilution | – | – | – | – | 0.5 | 0.75 | – | – | 0.023 | 0.094 | 0.25a | 0.75a | – | – | 2 | 3 |
| Clinical and environmental | E-test and agar dilution | – | – | – | – | 0.5 | 0.75 | – | – | 0.023 | 0.094 | 0.38 | 0.75 | – | – | 3 | 4 | |
| Erdogan et al., 2010,26 | Environmental | Microdilution | 0.015 | 0.125 | 0,03 | 0,06 | 0.03 | 0.06 | 0,03 | 0.125 | 0.001 | 0.015 | – | – | – | – | – | – |
| De Giglio et al., 2015,24 | Environmental | E-test | 0.19 | 0.5 | 0.032 | 0.125 | 0.19 | 0.38 | 0.064 | 0.094 | 0.016 | 0.016 | – | – | 1.5 | 4 | 1.5 | 3 |
| Sikora et al., 2017,29 | Environmental serogroup 1 | E-test | 0.032 | 0.025 | – | – | 0.125 | 0.19 | – | – | 0.003 | 0.004 | – | – | – | – | – | – |
| Environmental serogroup 2 | E-test | 0.032 | 0.047 | – | – | 0.125 | 0.25 | – | – | 0.003 | 0.008 | – | – | – | – | – | – | |
| Bruin et al., 2012,23 | Clinical | E-test | 0.125 | 0.25 | 0.125 | 0.25 | 0.50 | 0.50 | 0.25 | 0.25 | 0.016 | 0.032 | – | – | 4 | 8 | 4 | 8 |
| Tsakris et al., 1999,30 | Environmental | Microdilution | – | – | – | – | 0.125 | 0.25 | – | – | <0.07 | 0.015 | – | – | – | – | – | – |
| Higa et al., 2005,28 | Clinical | Microdilution | 0.25 | 2 | 0.063 | 0.125 | 0.032 | 0.032 | 0.032 | 0.032 | – | – | – | – | – | – | – | – |
| García et al., 2000,31 | Environmental | Microdilution | – | – | 0.06 | 0.12 | 0.5 | 0.5 | 0.12 | 0.12 | – | – | – | – | – | – | – | – |
| Graells et al., 2018,32 | Environmental | E-test | 0.032 | 0.064 | – | – | 0.38 | 0.5 | 0.125 | 0.19 | – | – | – | – | – | – | 2 | 4 |
In conclusion, among the points of the water network of recreational swimming pools analyzed for L. pneumophila investigation, showers provided the highest rate of positivity with statistically differences compared to isolations from tanks and taps; moreover, isolations from showers accounted nearly 92% of the positive samples with >1000CFU/L. As consequence, the use of showers in recreational swimming pools can become a potential pathway for exposure to L. pneumophila. In some cases, disinfection measures, consisting of repeated superheating and flushing of the water system may be helpful in providing a safe environment and help reduce possible L. pneumophila infection. In other cases, L. pneumophila cannot be completely eradicated and other disinfection measures have to be explored. For the first time, wild-type MIC distribution here presented can be useful for a better detection of antibiotic resistance and can contribute to improvements in the choice of the antibiotic treatment of legionellosis.
FundingThis research was supported by a grant from the Consejería de Sanidad de la Junta de Castilla y León, Spain, reference GRS 1370/A/16.
Conflict of interestThe authors declare they have no actual or potential competing financial or non-financial interests.
The authors thank Roberto Cantalapiedra and Raquel Carretero for their technical assistance.