Recent infection testing algorithms (RITAs) are used in public health surveillance to estimate the incidence of recently acquired HIV-1 infection.
ObjectivesOur aims were (i) to evaluate the precision of the VITROS® Anti-HIV 1+2 automated antibody avidity assay for qualitative detection of antibodies to HIV 1+2 virus; (ii) to validate the accuracy of an automated guanidine-based antibody avidity assay to discriminate between recent and long standing infections using the VITROS 3600 platform; (iii) to compare this method with BED-CEIA assay; and (iv) to evaluate the occurrence of false recent misclassifications by the VITROS antibody avidity assay in patients with a CD4 count <200cells/μL and in patients on combination antiretroviral therapy (cART).
ResultsThe VITROS® antibody avidity assay is highly reproducible. The ROC curve analysis of the accuracy of this assay, optimized for sensitivity and specificity, had an AI cut off of ≤0.51, with sensitivity and specificity values of 86.67% (95% CI: 72.51–94.46) and 86.24% (95% CI: 78.00–91.84), respectively. The agreement between VITROS antibody avidity and BED-CEIA assays was good. Misclassifications of long standing infections as recent infection occurred in 8.2% of patients with CD4 <200cell/μL and 8.7% in patients on combination antiretroviral therapy.
ConclusionsThe VITROS antibody avidity assay is a reliable serological method to detect recent HIV-1 infections and it could be incorporated into a RITA to estimate HIV incidence.
El algoritmo RITA (recent infection testing algorithm) es utilizado en los sistemas de vigilancia epidemiológica de Salud Pública para estimar la incidencia de infección por VIH-1 en nuestro medio.
ObjetivosLos objetivos de nuestro estudio fueron: (i) Evaluar la precisión del ensayo de avidez automatizado VITROS® Anti-HIV1+2 assay para la detección cualitativa de anticuerpos frente al VIH-1 y el VIH-2; (ii) Validar la precisión de un ensayo de avidez automatizado para discriminar entre infección reciente y crónica por el VIH-1 utilizando la plataforma VITROS 3600; (iii) Comparar este método con el ensayo BED-CEIA; y (iv) Evaluar la tasa de infecciones crónicas por VIH-1 clasificadas incorrectamente como recientes en los pacientes en tratamiento antirretrovírico combinado y en pacientes con un recuento de CD4<200 céls/μL.
ResultadosEl ensayo de avidez de VITROS es altamente reproducible. El análisis de curvas ROC reveló que un valor de punto de corte ≤0,51 con una sensibilidad y especificidad del 86,7% (IC 95%: 72,5-94,5) y del 86,2% (IC 95%: 78-91,8) respectivamente, es óptimo para identificar infecciones recientes por VIH-1. La correlación entre el ensayo de VITROS® avidez y BED-CEIA fue buena (κ=0,77; IC 95%: 0,67-0,86). La tasa de infecciones crónicas por VIH-1 clasificadas incorrectamente como recientes por el ensayo de avidez fueron del 8,2% en los pacientes con CD4<200 céls/μL y del 8,7% en los pacientes en tratamiento antirretrovírico combinado.
ConclusionesEl ensayo de avidez evaluado es un método fiable para detectar infecciones recientes por VIH-1 y podría ser utilizado dentro de un algoritmo RITA para estimar la incidencia de infección por VIH-1 en la población.
At the time of writing, eight types of assays have been developed as tests for recent HIV infection. Although some of these have been developed specifically to identify recent infection, others are modifications of HIV diagnostic tests,1 with the first HIV incidence study using recent infection assays being published in 1998.2 As well as being used for the estimation of incidence,3 less frequently, these tests are used for individual diagnosis of recent HIV infection, with suitable caveats.3
Many of these early serological approaches to the detection of recent infection were based initially on changes in antibody titer, using dual sensitive/less sensitive detuned enzyme immunoassay (3A11, Abbott Laboratories, Abbott Park, IL) and (Vironostika, BioMerieux INC., Durham, NC). Later, a quantitative enzyme immunoassay for the determination of the proportion of HIV-1 specific IgG was commercialized.4 More recently, commercial antibody avidity assays have been developed and marketed.5–8 These avidity assays are based on the rationale that in the early phase of infection, antibodies show a low avidity (binding capacity) for HIV antigen and that over a period of 6–12 months the avidity matures and rises. Techniques based on limiting antigen avidity EIA (LAg-Avidity EIA) to identify recent HIV infections in cross-sectional studies using a multi-subtype gp41 recombinant protein have been described recently and although LAg assays are promising, they are also subject to misclassification.9
A significant problem of serological assays used to detect recent HIV infection is the misclassification of long standing infection (LSI) as recent infection (RI), so-called the false recent rate (FRR) which can lead to an over-estimate of HIV incidence.10,11 False-recency occurs more frequently in HIV positive patients with LSI, advanced infection (defined by a diagnosis of AIDS, CD4 count <200cell/μL) or among those on antiretroviral therapy (ART) and elite controllers (HIV positive patients with low or undetectable viral loads in the absence of ART).12,13
Recent studies have shown that the FRR can be minimized by using algorithms for detecting recent HIV infection which include more than one serological assay and complementing the results with additional clinical information or laboratory tests such as CD4+T cell count or antiretroviral drug testing.14,15
In Catalonia, recent infection algorithms have been part of the HIV surveillance system since 2003,16 initially using Vironostika and later BED-CEIA.17 BED has been found to have a high FRR18 and in addition its application in our setting incurs a relatively high cost. The Vitros system is already used at our laboratory for HIV diagnosis, has a rapid turnaround time and has been shown to accurately detect recent HIV infections.8 It has the additional advantage of not needing official approval by the regulatory authority since it is a modification of existing methods and requires only a preparation sample.5,8
Key aims of this study were (i) to evaluate the precision of an automated antibody avidity assay for qualitative detection of antibodies to HIV 1+2 virus; (ii) to validate the accuracy of an automated guanidine-based antibody avidity assay to discriminate between RI (≤6 months after seroconversion date) and LSI (>12 months after the diagnosis of HIV infection) using the VITROS® Anti-HIV 1+2 assay (Ortho Clinical Diagnostics Inc, Cardiff, Wales, UK) in a VITROS 3600 platform; (iii) to compare this method with BED-CEIA assay (Calypte Biomedical Inc, Portland, OR, USA), which is the only commercial assay developed specifically for HIV incidence surveillance; and (iv) to evaluate the occurrence of false recent misclassifications by the VITROS antibody avidity assay in patients with a CD4 count <200cells/μL and in patients on combination antiretroviral therapy (cART).
Materials and methodsPrecision studyThe precision of the VITROS HIV antibody avidity assay was assessed following the recommendations of Clinical and Laboratory Standards Institute (CLSI) document EP5-A2.19 We selected nine serum samples from nine different individual known to have tested positive for HIV 1+2 using the VITROS® Immunodiagnostics Products HIV 1+2 and confirmed as positive by Inno-LiA test (Innogenetics Inc, Alpharetta, GA, USA). Two of these nine specimens had a weak positive signal (a sample/cutoff [S/CO] ratio of less than eight). For each sample, we tested two aliquots of 150μL; one aliquot was diluted 1:10 with phosphate buffer saline (PBS) and the other one was diluted 1:10 with 1M guanidine (dissociation agent). All samples were vortexed and incubated at room temperature for 10min. Two replicates each of nine samples were tested by VITROS® Immunodiagnostics Products HIV 1+2 using the VITROS 3600 instrument on two separate runs every day for 10 days.
The Vitros Anti HIV1+2 assay is a qualitative chemiluminescence immunoassay using Intellicheck® Technology. In the first stage of this assay, HIV antibodies present in the sample bind with HIV recombinant antigen coated on the wells (four recombinant antigens derived from HIV-1 core (p24), HIV-1 envelope (p10 and p13) and HIV-2 envelope AL). In the second stage, the conjugate (horseradish peroxidase (HRP)-labeled) binds specifically to any human anti-HIV-1 or anti-HIV-2 (IgG and IgM) captured on the wells in the first stage. The bound conjugate is measured by a luminescent reaction. A reagent containing luminogenic substrates is added to the wells with an electron transfer agent. The conjugate catalyzes the oxidation of the luminol derivate, producing light, which is measured by a luminometer. Results are reported as the S/CO ratio with positive values being ≥1.00. The avidity index (AI) was calculated using the following formula: AI=(S/CO ratio of the guanidine aliquot)/(S/CO ratio of the PBS aliquot).
Statistical analysis of the precision studyWe calculated the mean and standard deviations of the AI obtained on each sample in each run and every day. The intra-day, inter-day and total imprecision of procedure were analyzed by analysis of variance and expressed as coefficients of variation.
Validation studyWe performed a validation study to evaluate the accuracy of the VITROS antibody avidity assay to detect RI and the ability to discriminate between RI and LSI. A total of 154 serum samples from 47 HIV-1 positive patients identified according to standard HIV algorithms for screening and confirmatory testing were included in the study. For these patients the seroconversion date could be estimated as the midpoint between the last HIV-negative test and the first HIV-positive test (interval between the two tests ≤24 months). For each patient, at least one serum sample was collected within the first year of the seroconversion date, plus one or more samples (two to seven specimens per patient) collected at different points after seroconversion. Nineteen of these patients were known to be infected with subtype B, one patient was infected with subtype CRF01_AE and in the remaining patients the subtype was unknown. The study received approval from the local Ethics Committee.
Statistical analysis of the validation studyThe distribution of the AI at different times after seroconversion date was graphically represented with box plots. ROC analysis was used to determine the accuracy of the VITROS antibody avidity assay to discriminate between recent and established HIV-1 infection. Sensitivity, specificity, positive and negative predictive values of this assay were calculated.
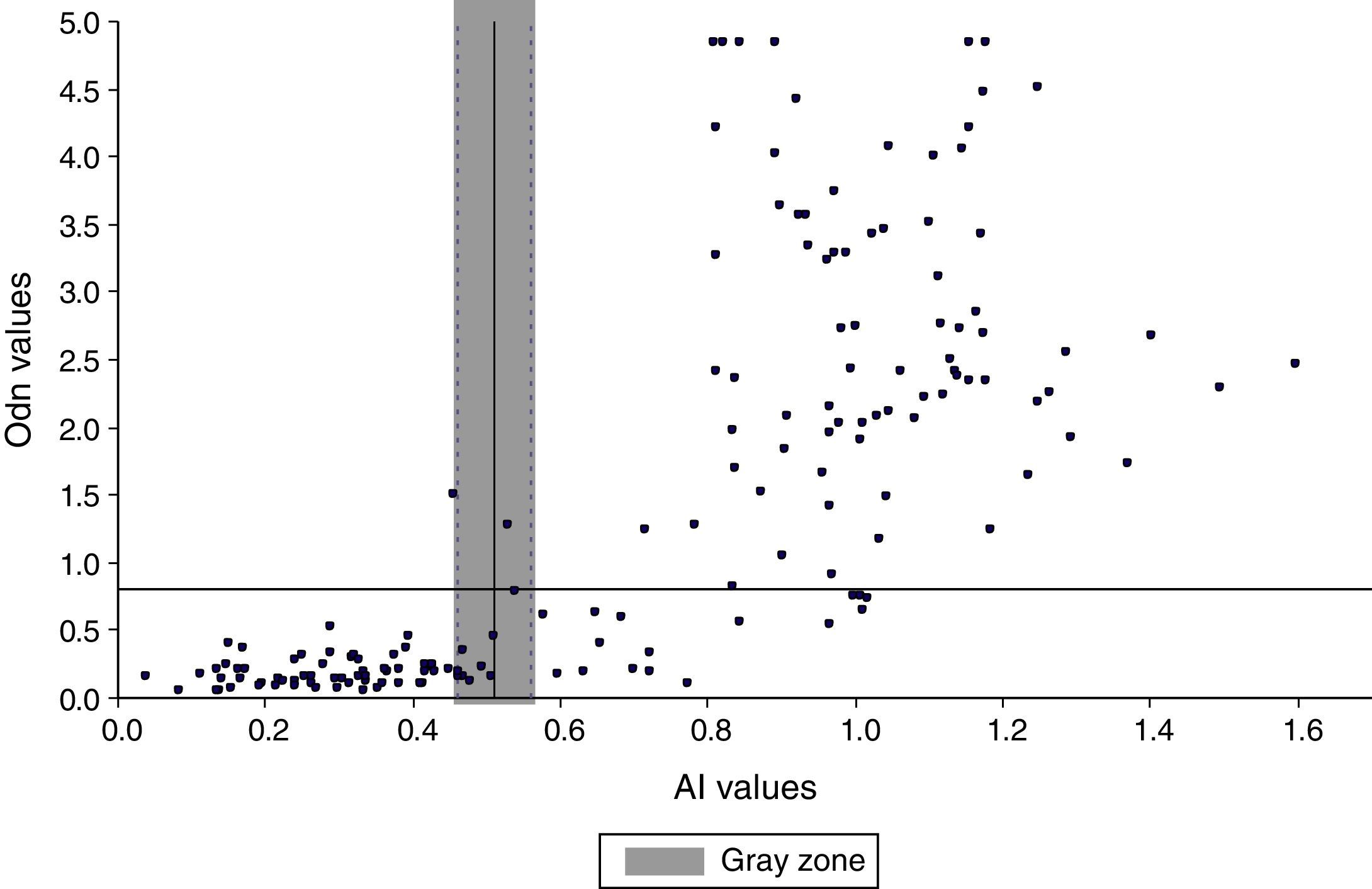
Comparison of the VITROS HIV antibody avidity and BED-CEIA assays for detecting recent HIV-1 infectionsA total of 164 serum samples from HIV-1 infected patients with known seroconversion date were collected to compare the ability of both assays to detect RIs. Kappa coefficient was used to assess agreement between results obtained by both, using a cut off of ≤0.51 for the VITROS antibody avidity assay and a normalized median optical density (ODn) of ≤0.8 for BED-CEIA assay as recommended by the manufacturer (Kappa values interpreted according to Viera and Garrett20). We defined a “gray zone” for VITROS antibody avidity assay based on the precision study, which showed a coefficient variation of the avidity index <5%. The BED-CEIA assay procedure has been described previously.21 Briefly, serum samples and controls were diluted 1:101. Specimens were tested singly and the controls (high and low positive and calibrator) were tested in triplicate and their median values were used to calculate the normalized ODn. Specimens with ODn ≤1.2 were tested in triplicate to confirm their ODn values. In confirmatory testing, those specimens with ODn <0.8 were considered as recent infections.
Evaluation of the frequency of false recent misclassification using the VITROS HIV antibody avidity assayWe selected 73 patients with CD4 count <200cell/μL and 138 patients on long cART to investigate the ability of the VITROS HIV antibody avidity assay to correctly discriminate RI from LSI in advanced and treated HIV-1 infection.
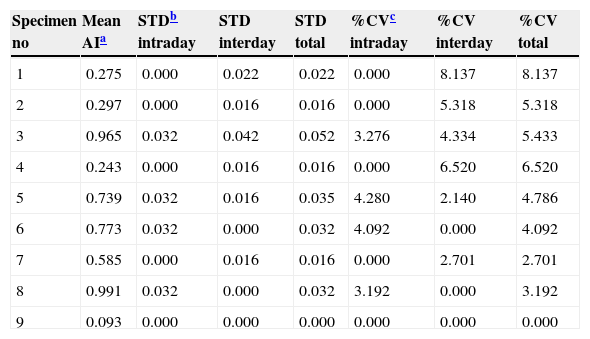
ResultsPrecision studyThe results obtained in the precision study are shown in Table 1. The intra-day, inter-day, coefficients of variation and the total variance of the VITROS antibody avidity assay were consistently lower than 5%.
Precision of the VITROS antibody avidity assay to detect recent HIV infection.
| Specimen no | Mean AIa | STDb intraday | STD interday | STD total | %CVc intraday | %CV interday | %CV total |
|---|---|---|---|---|---|---|---|
| 1 | 0.275 | 0.000 | 0.022 | 0.022 | 0.000 | 8.137 | 8.137 |
| 2 | 0.297 | 0.000 | 0.016 | 0.016 | 0.000 | 5.318 | 5.318 |
| 3 | 0.965 | 0.032 | 0.042 | 0.052 | 3.276 | 4.334 | 5.433 |
| 4 | 0.243 | 0.000 | 0.016 | 0.016 | 0.000 | 6.520 | 6.520 |
| 5 | 0.739 | 0.032 | 0.016 | 0.035 | 4.280 | 2.140 | 4.786 |
| 6 | 0.773 | 0.032 | 0.000 | 0.032 | 4.092 | 0.000 | 4.092 |
| 7 | 0.585 | 0.000 | 0.016 | 0.016 | 0.000 | 2.701 | 2.701 |
| 8 | 0.991 | 0.032 | 0.000 | 0.032 | 3.192 | 0.000 | 3.192 |
| 9 | 0.093 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
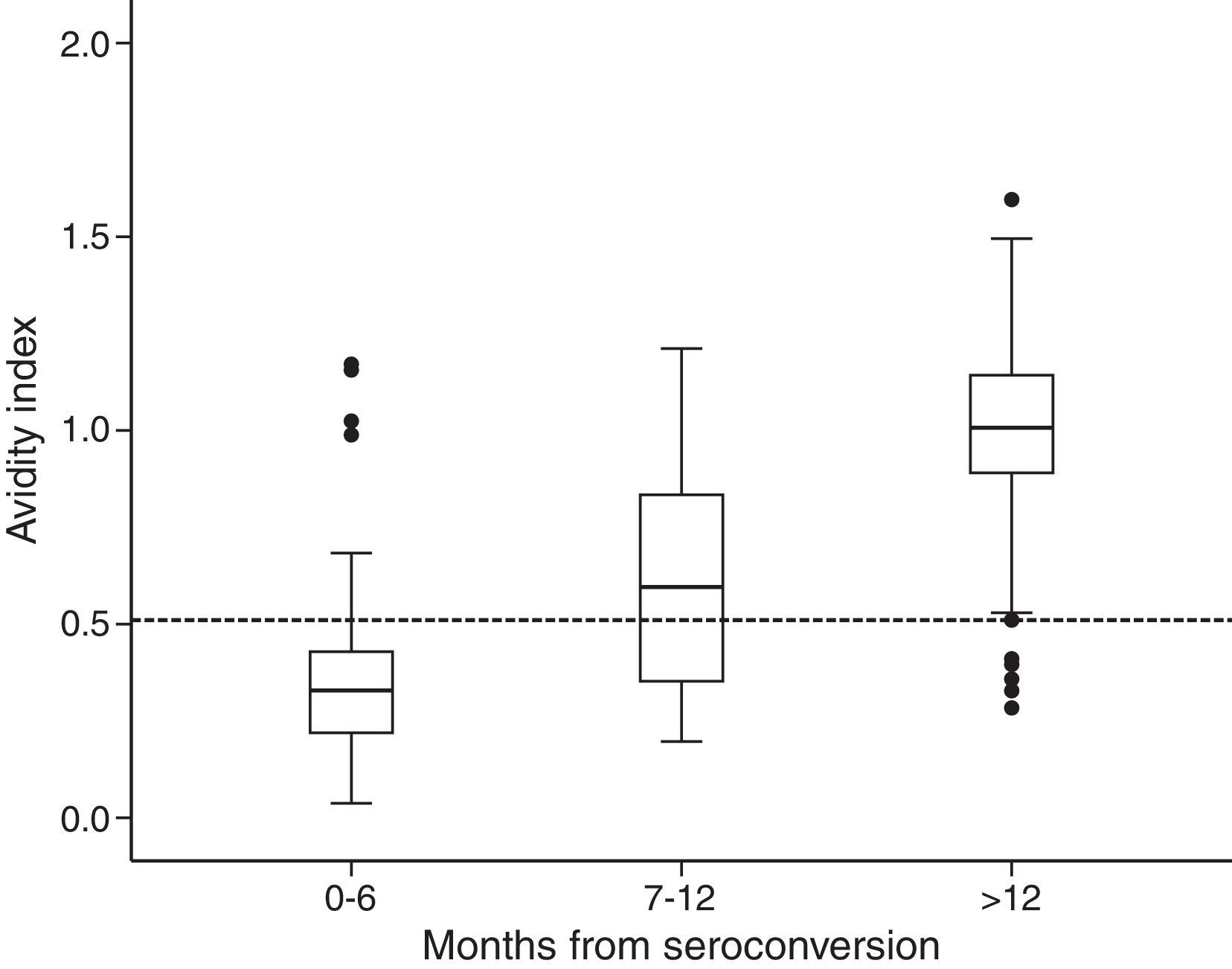
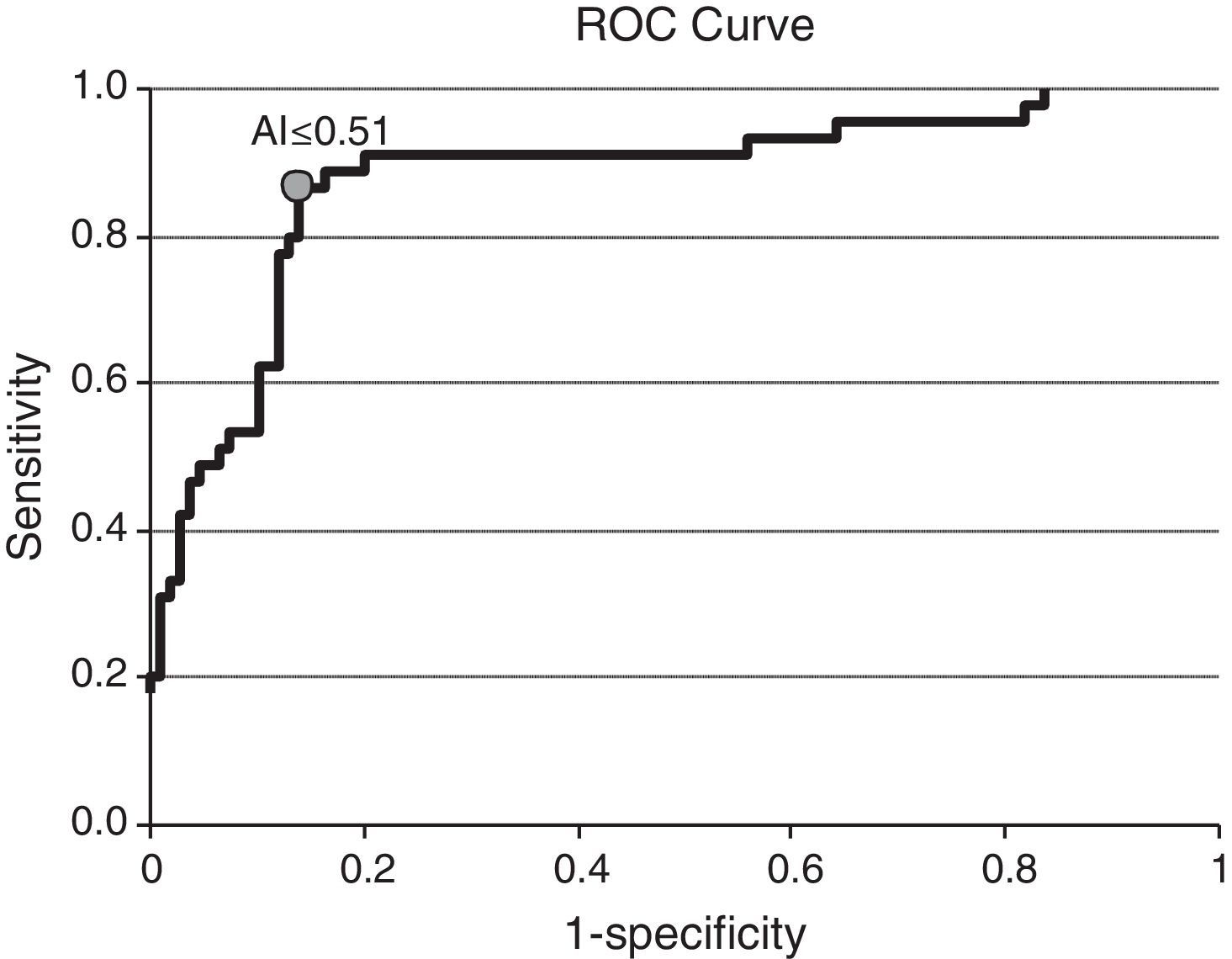
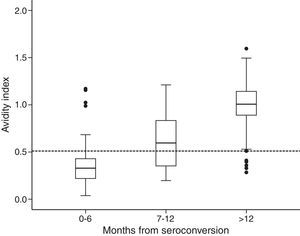
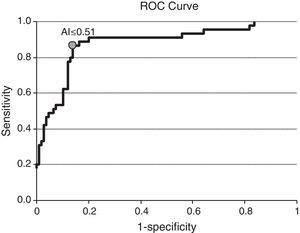
Among the 154 samples selected, 45 samples were collected 0–6 months after seroconversion, 25 samples were collected 7–12 months after seroconversion, and 84 were collected more than 12 months after seroconversion. The distribution of avidity index by the time elapsed since seroconversion is shown in Fig. 1. The AI reached approximately 1 at the end of the first year after seroconversion. For serum samples collected within 6 months from seroconversion, the 75th percentile was lower than 0.51. The median avidity indices increased with time since seroconversion, from 0.33 IQR (0.22–0.44) to 1.01 IQR (0.89–1.14). There were significant differences between groups (p<0.001 Kruskal–Wallis test). The ROC curve analysing the accuracy of the VITROS HIV antibody avidity assay to detect recent HIV-1 infection is shown in Fig. 2. We explored several cut offs for the VITROS antibody avidity assay and the optimal cut off point on the ROC curves for clinical use was an AI equal or lower than 0.51, with a sensitivity and specificity values of 86.67% (IC 95%: 72.51–94.46) and 86.24% (IC95%: 78.00–91.84), respectively.
Once the sensitivity and specificity were calculated, we estimated the positive and negative predictive values (PPV and NPV, respectively) of the VITROS antibody avidity assay if we were to use this test for identification recent HIV-1 infections among HIV-positive populations. Considering the prevalence of recent HIV infection obtained in our previous study, which was 19.5%14; we could estimate a PPV and NPV for this assay of 61.16% and 97.34%, respectively.
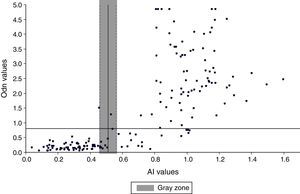
Comparative study between VITROS HIV antibody avidity and BED-CEIA assays for identification of recent HIV-1 infectionsThe overall agreement between the VITROS HIV antibody avidity and BED-CEIA assays was substantial (κ=0.77 IC95%: 0.67–0.86), with both assays concordant in classifying 66 specimens as RI and 79 specimens as LSI. The remaining 19/164 (11.58%) specimens were discrepant. Eighteen specimens were classified as RI by the BED assay and as LSI by the VITROS antibody avidity assay. Among these 18 discrepant results, 15 specimens were collected more than 12 months after seroconversion and 3 specimens were collected 9, 8 and 1 months respectively after seroconversion. One specimen was classified as an RI by the VITROS antibody avidity assay and as LSI by the BED assay; this specimen was collected 6 months after seroconversion. The AI was in the gray zone for 10 specimens with VITROS antibody avidity assay (Fig. 3). The agreement between BED-CEIA and VITROS antibody avidity assays remained similar when the 10 specimens in the gray zone were excluded (κ=0.78 IC95%: 0.68–0.87).
Misclassifications among patients with CD4 count <200cell/μL and patients on long cART therapyOverall the VITROS antibody avidity assay misclassified 6 (8.2%; 95% IC 3.4–17.6%) of 73 patients with CD4 count <200cell/μL and 12 (8.7%; 95% IC 4.8–15.0%) of 138 patients on cART as recently infected.
DiscussionThe purpose of our study was to evaluate the precision and accuracy of an automated guanidine-based HIV antibody avidity index method to detect recent HIV-1 infections using the VITROS 3600 instrument, which is available to clinical laboratories in Europe and other countries worldwide. The VITROS system has a rapid turnaround, allowing clinical laboratories to run the sensitive HIV diagnostic test followed by the antibody avidity modified version.
The results of the precision study showed that the VITROS HIV antibody avidity assay is highly reproducible with a variance of less than 5%. Accuracy of the assay was shown in the validation study by ROC curves results, which showed that an AI cut off of ≤0.51 optimized the values for sensitivity and specificity (86.67% and 86.24%, respectively). These data are consistent with a study published by Suligoi et al., in which the authors obtained a sensitivity and specificity of 87.9% and 86.3% using AXSYM antibody avidity assay and an AI lower than 0.9.22 The selection of an AI cut off depends on the study purpose. For epidemiological HIV surveillance, a higher AI cut off and thus a higher sensitivity may be desirable, whereas a lower cut off, which increases specificity could be used by clinicians to decide on early ART. Overall, the VITROS HIV antibody avidity assay performed well compared to results obtained by BED-CEIA assay, and the proportion of sera correctly classified as LSI was slightly higher with the VITROS antibody avidity assay. After a single 1:10 dilution, this assay can be fully automated using the VITROS 3600 instrument, which leads to better reproducibility. In this comparative study, 10 specimens (6%) had a result within the gray zone using VITROS antibody avidity assay. When these were excluded from analysis, no significant changes in the overall agreement between both assays were observed, a result that is consistent with other study.6
The VITROS antibody avidity assay accurately distinguished LSI from RI in patients on cART and patients with CD4 count <200cell/μL. Misclassification of these groups as RIs could be expected as a consequence of changes in qualitative and quantitative antiviral antibody responses in patients with advanced illness or those on ART.
The performance of recent infection testing algorithm (RITA) assays on as many as new diagnosis as possible has contributed to a better assessment of late diagnosis and its determinants. Nevertheless, incidence estimates rely on having the lowest FRR possible and at present, none of the available serological assays used for detection recent HIV infections are ideal due to the high false recent rates. In order to minimize false recent misclassification and thereby improve the accuracy of incidence estimations, the best approach is the use of more than one serological assay in a multistep parallel or serial algorithm as suggested by the WHO Technical Working Group on HIV Incidence assays.1 Clinical and laboratory information incorporated into the assay can be used to exclude from testing those with known late or advanced infection and the application of a screening sensitive assay (BED-CEIA) followed by a more specific assay (VITROS antibody avidity assay) may well provide the best balance between cost and minimized FRR in our setting. Formal comparison of a new RITA incorporating more than one serological assay would need to be undertaken to determine the best algorithm for detection of RI and estimation of HIV incidence and therefore consolidate its applicability in Public Health.
Conflict of interestVITROS® Anti-HIV1+2 reagents were provided by Ortho Clinical Diagnostics (a Johnson & Johnson Company, Madrid, Spain). However, this commercial sponsor had no involvement in study design; collection, analysis, or interpretation of data; writing the manuscript; and the decision to submit the manuscript for publication.
The authors would like to thank Núria Margall and Aurora Casanova their help in specimen collection. We also thank Ortho Clinical Diagnostics (Spain) for providing us the required reagent kits.