A variable percentage of samples analysed using the Cobas 4800 assay can give an invalid result by PCR inhibition or erroneous due to incorrect DNA extraction with the Cobas 4800 CT/NG test.
MethodAn analysis was performed using the vortex agitation and dilution protocol on the original sample (swab or urine) for a total of 116 samples. In order to analyse the sensitivity of this method, 100 samples (swabs and urine) with known results were retested.
ResultsA total of 98.3% (114/116) of the samples analysed were resolved with this protocol with 100% agreement after reviewing clinical data, Gram stain, and other samples analysed in parallel from the same patient.
DiscussionThe data indicate no loss of sensitivity with this protocol; thus Cobas 4800 users could use this method without the need for alternative methods.
Un porcentaje variable de muestras analizadas por el equipo cobas 4800 pueden dar un resultado invalidado por inhibición de la PCR o erróneo al no extraerse el ADN correctamente con el test cobas 4800 CT/NG.
MétodoValoración de un protocolo de agitación y dilución de la muestra original (exudado u orina) en un total de 116 muestras. Para analizar la sensibilidad de este método, 100 muestras (exudados y orinas) con resultado conocido fueron retestadas.
ResultadosUn 98,3% (114/116) de las muestras se resolvieron con este protocolo con un 100% de concordancia al consultar con datos clínicos, tinción de Gram y otras muestras analizadas en paralelo del mismo paciente.
DiscusiónLos datos indican que no hay pérdida de sensibilidad con este protocolo, por lo que los usuarios de esta plataforma podrían usarlo sin necesidad de métodos alternativos.
Techniques based on nucleic acid amplification are among the techniques of choice for the detection of Chlamydia trachomatis (C. trachomatis) and are increasingly used for the detection of Neisseria gonorrhoeae (N. gonorrhoeae). The new generation of techniques based on nucleic acid amplification, such as the Cobas 4800 platform (Roche Diagnostics GmbH, Mannheim, Germany) installed in our laboratory in 2010,1 includes genetic sequences for detecting the new variant of C. trachomatis2,3 and, on the other hand, for reducing false negatives and cross-reactions with similar species of Neisseria spp.4,5 They have also improved the diagnostic sensitivity and have allowed the individual evaluation of cases with results that contradict the traditional culture.
Previous studies in this platform correlated patients with gonorrhoea and PCR invalidated by pipetting errors with the original sample due to mucopurulent secretion caused by the N. gonorrhoeae infection.6 Regarding the type of samples, rectal and pharyngeal exudates (samples not validated in Cobas 4800) may contain components that inhibit PCR,7 cervical exudates may interfere with results due to cervical mucus or, on the other hand, a high white blood cell count in samples (1×105) and red blood cells in both urine and exudates.8–10
The determination of these non-evaluable samples (defined as errors during extraction and sample processing or inhibition of PCR) is important in asymptomatic infections that often go unnoticed and untreated, posing a potential risk in both the patient and at the epidemiological level.11–13 In this study we evaluated a simple and reliable method to resolve this type of sample in this platform without the need for alternative techniques.
Material and methodsCollection of samplesThis analysis was carried out between 1 November 2013 and 30 April 2014. A total of 4224 samples were received from a sexually transmitted infection centre for the detection of C. trachomatis and N. gonorrhoeae in the Cobas 4800 platform. Most (64%) came from symptomatic patients, including both exudates (endocervical [31.4%], rectal [15.5%] and pharyngeal [5.3%]) and urine (47.8%). These samples were collected in Cobas PCR Female swab sample kit or Cobas PCR Urine Sample kit (Roche Diagnostics GmbH, Mannheim, Germany), respectively, following the manufacturer's recommendations and stored at room temperature (maximum 3 days) until processed and at −80°C up to 30 days if they are retested.
Resolution protocol of non-evaluable results of the original sampleThe resolution protocol used, depending on the type of sample, was as follows:
- -
In the case of exudates, intense mixing of the original sample with a vortex for 30s, an aliquot of 1ml was added to a new sterile tube of Cobas PCR Female swab sample kit containing 4.3ml of buffer (≤40% [v/v guanidine hydrochloride Tris-HCl]).
- -
For urine, intense mixing of the original sample with vortex for 30s, an aliquot of 3ml (minimum volume to process these samples) was added to a new sterile tube of Cobas PCR urine sample.
After this dilution, they were re-analysed by the Cobas 4800 device without further deviation from the protocol specified by the manufacturer. Samples were considered to have been resolved if a valid result was obtained with the Cobas 4800 device.
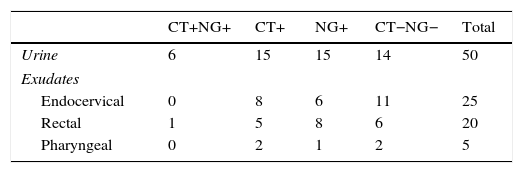
Validation of the resolution protocol for samples not evaluable from the original sampleThis protocol was validated using 100 samples with a valid and known result from the Cobas 4800 device with clinical data of each patient. A significant representation of each sample type included in this study was selected, as well as a range of amplification cycles (range of 25–39 cycles). These samples are described in Table 1.
These samples were analysed by the Cobas 4800 CT/NG test before and after dilution of the original sample.
Statistical analysisThe statistical analysis of this study was performed with SPSS Statistics v22 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as a median (Q1-Q3) and categorical variables as numbers (percentage).
EthicsThis study was designed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Hospital Universitario de Valme.
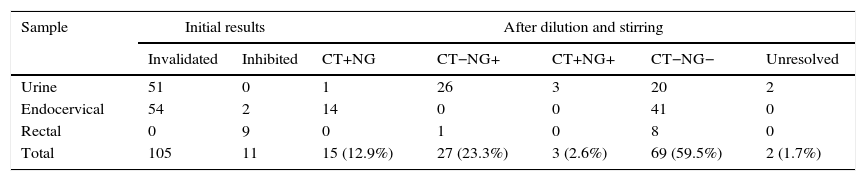
ResultsOf the 4224 samples analysed, 116 (2.7%) had an unevaluable result, which, after being re-analysed with the described method, obtained a valuable result of 114 (98.3%): 69 negative samples, 15 positive for C. trachomatis, 27 positive for N. gonorrhoeae and 3 positive for both micro-organisms. Two urine samples, from 2 symptomatic male patients with suspected gonorrhoea and Gram-positive staining, could not be resolved after dilution of the original and subsequent retested samples. These data are summarised in Table 2.
Results obtained with Cobas 4800: invalidated or inhibited results before and after the dilution protocol.
| Sample | Initial results | After dilution and stirring | |||||
|---|---|---|---|---|---|---|---|
| Invalidated | Inhibited | CT+NG | CT−NG+ | CT+NG+ | CT−NG− | Unresolved | |
| Urine | 51 | 0 | 1 | 26 | 3 | 20 | 2 |
| Endocervical | 54 | 2 | 14 | 0 | 0 | 41 | 0 |
| Rectal | 0 | 9 | 0 | 1 | 0 | 8 | 0 |
| Total | 105 | 11 | 15 (12.9%) | 27 (23.3%) | 3 (2.6%) | 69 (59.5%) | 2 (1.7%) |
In order to confirm the results obtained, the clinical data of these patients were reviewed: positive samples for C. trachomatis related to symptomatic patients (12/15, 80%) or contact follow-ups with suspected infection. Those who were positive for N. gonorrhoeae were patients symptomatic with a positive culture of N. gonorrhoeae and/or Gram-positive staining, whereas those positive for both micro-organisms came from symptomatic patients with suspected infection, with other samples (rectal exudates) positive for both organisms.
Negative samples (41 cervical exudates, 20 urine and 8 rectal exudates) corresponded to 22 male patients and 47 female patients. Most were control patients with no suspected infection with other determinations that were also negative. The remainder (symptomatic patients) presented non-gonococcal infection, infection by herpes simplex virus 2 and condyloma, respectively. The remainder were follow-up contact or post-treatment control patients who had other samples from different areas with negative results for both chlamydia and gonococcus.
The 100 samples analysed for the validation of this protocol obtained a concordance of 100% with regard to the previous results from the Cobas 4800 device. The mean value of the threshold cycle (Ct) at which positive samples were amplified before and after dilution were as follows: positive for C. trachomatis presented a median Ct of 32.2 cycles (range of 24.1–38.2 cycles), which after dilution totalled 33.5 cycles. For the positives for N. gonorrhoeae, the median Ct without dilution was 27.6 (range of 23.5–39.3 cycles), and after dilution was 30.4 cycles.
DiscussionOur laboratory, the reference centre for the sexually transmitted infections centre has a percentage of positive samples for C. trachomatis and N. gonorrhoeae of 10.3% and 5% (internal data from 2013), where, 2–5% of the samples analysed produced an invalid result.
In this platform there are few studies on resolution of this type of sample, notably Miller et al.6 which used Sputasol (1.4% dithiothreitol; Oxoid Ltd., Basingstoke, UK) to reanalyse this type of sample with good results, or other studies that raise the need for alternative methods for the resolution of this type of sample.14
In this study it is noteworthy that after the reanalysis of samples with non-evaluable results, 39.5% produced a positive result. Of these, 66.7% were positive for N. gonorrhoeae mostly in urine samples from male patients who produced a “failed” result. The median Ct value in these samples was 27.8 cycles; they indicate a high level of bacterial load. This result is probably due to an excess of sediments or leukocytes in the urine.
One of the limitations of this study is the limited number of samples with low bacterial DNA tested, especially those with a Ct value ≥38 cycles, where the dilution of these samples could lead to a false negative. However, our data indicate that only 7–10% of the analysed samples had a Ct higher than this value, the result of which could be evaluated together with the clinical condition, Gram culture or confirmation by another test. In the literature there are few studies that support a positive N. gonorrhoeae result using this platform.15 Another limitation of the study is the use of samples not validated on the Cobas 4800 platform (rectal and pharyngeal exudates), but analysed and included in previous studies.7 On the other hand, the number of inhibited samples is low, which would require a greater number of this type of sample to confirm the sensitivity of the dilution protocol.
Therefore, this easy, sensitive method, which does not require alternative methods or the use of other reagents, would allow those users of the Cobas 4800 device to confidently resolve those urine and mucopurulent exudates and, consequently, reduce the number of samples not amplifiable consequently saving time, resources by the laboratory and a new sampling.
Conflicts of interestNone declared.
Please cite this article as: Parra-Sánchez M, García-Rey S, Breval IZ-Y, Sierra-Atienza C, Bernal-Martínez S, Palomares-Folía JC. Valoración de un método de dilución para la resolución de resultados no valorables en la detección de Chlamydia trachomatis y Neisseria gonorrhoeae con la plataforma cobas 4800. Enferm Infecc Microbiol Clin. 2017;35:364–366.