Influenza viruses and respiratory syncytial virus (RSV) can cause an acute respiratory disease that occurs seasonally in epidemic waves. This retrospective study was conducted to evaluate the Sofia® Influenza A+B and the Sofia® RSV fluorescence immunoassays (FIAs), two novel rapid detection tests (RDTs) for influenza A and B and RSV.
MethodsTwo hundred and nine breath samples were selected from patients with respiratory symptoms determined to be positive/negative for influenza A, influenza B or RSV using one of the reference diagnostic techniques, cell culture and/or RT-PCR (Simplexa™Flu A/B & RSV). The Sofia Influenza A+B FIA was tested on 123 samples (63 from children and 60 from adults) and the Sofia RSV FIA was tested on 86 pediatric samples. Sensitivity and specificity values of both assays were calculated assuming the reference techniques as the gold standard.
ResultsSensitivity and specificity values for the Sofia Influenza A+B FIA were 73.1% and 97.8%, respectively. Sensitivity and specificity values for the Sofia RSV FIA were 87.5% and 86.7%, respectively.
ConclusionThe sensitivity results obtained for the two assays were considerably higher than those reported for other RDTs. In conclusion, the Sofia Influenza A+B and the Sofia RSV FIAs are appropriate tools for the rapid diagnosis of these viruses.
Los virus influenza A y B y el virus respiratorio sincitial (VRS) causan infecciones respiratorias agudas de forma estacional en olas epidémicas. Este estudio restrospectivo se llevó a cabo para evaluar dos nuevos tests de diagnóstico rápido para detectar los virus influenza A y B y VRS: Sofia® Influenza A+B y Sofia® RSV Fluorescence Immunoassays (FIAs).
MétodosSe seleccionaron 209 muestras respiratorias procedentes de pacientes con síntomas respiratorios en las que se había establecido el diagnóstico de presencia o ausencia de influenza A, influenza B o VRS mediante las técnicas de referencia: cultivo celular o RT-PCR PCR (Simplexa™Flu A/B & RSV). Sofia Influenza A+B se realizó en 123 muestras (63 de niños y 60 de adultos) y Sofia RSV en 86 muestras pediátricas. Se calcularon los valores de sensibilidad y especificidad de ambos test tomando las técnicas de referencia como gold standard.
ResultadosLos valores de sensibilidad y especificidad de Sofia Influenza A+B fueron 73,1 y 97,8%. Los valores de sensibilidad y especificidad de Sofia RSV fueron 87,5 y 86,7%.
ConclusionesLos valores de sensibilidad obtenidos para ambos tests son mayores que los descritos para otras técnicas de diagnóstico rápido. Por lo tanto, Sofia Influenza A+B FIA y Sofia RSV FIA son test adecuados para el diagnóstico rápido de influenza y VRS.
Influenza viruses and respiratory syncytial virus (RSV) can cause an acute respiratory disease that occurs seasonally in epidemic waves. RSV is especially prevalent in young children and often involves the lower respiratory tract, primarily causing bronchiolitis. Influenza viruses and RSV are common nosocomial pathogens and a rapid diagnosis can help clinicians take control measures and, if necessary, administer appropriate and timely treatment.1–4
Cell culture and real-time reverse transcriptase polymerase chain reaction (RT-PCR) are considered to be the methods of reference to detect these viruses in respiratory specimens. There are also rapid diagnosis tests (RDTs) designed for a rapid detection of influenza and RSV antigens directly from respiratory specimens.5,6 RDTs are easy to perform, yield results within few minutes and, in the case of RDTs for influenza detection they can differentiate between influenza A and B viruses. However, their sensitivity values described ranges from 11% to 80%.2,4,7,8 Other disadvantages of RDTs are the subjective interpretation of the results and the fact that they are less sensitive in adult samples than in children.9
The Sofia Influenza A+B fluorescence immunoassay and Sofia RSV fluorescence immunoassay (FIAs) have recently become available. They employ a different technology than other RDTs: they are immunofluorescence assays using an europium dye that enhances the overall sensitivity. In addition, the assays are automated, reducing the subjective interpretation of results that can lead to errors with other RDTs.10–13
This study was conducted to evaluate the Sofia Influenza A+B and Sofia RSV FIAs for the rapid detection and differentiation of influenza A and B viruses and RSV in respiratory specimens compared with the reference diagnostic methods.
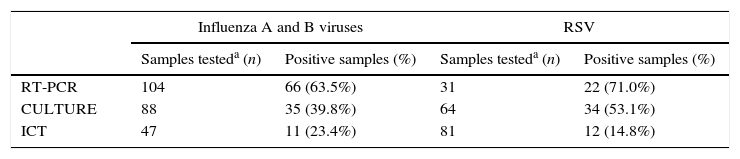
MethodsA comparative retrospective study was conducted using a selected group of 209 frozen at −80°C respiratory samples collected from November 2012 to March 2013: nasopharyngeal swabs, collected using flocked swabs (Copan, Brescia, Italy) or nasopharyngeal aspirates. A total of 123 samples (50 nasopharyngeal aspirates and 73 nasopharyngeal swabs) were selected to evaluate the Sofia Influenza A+B FIA from patients visiting the hospital with influenza-like symptoms (63 pediatric patients and 60 adults) and who were previously diagnosed for influenza A (n=49), influenza B (n=24) or negative for influenza (n=45) using cell culture (conventional cell culture and shell-vials) and/or RT-PCR (Simplexa™Flu A/B & RSV Kit, Focus Diagnostics, Cypress, CA, USA) as reference diagnostic methods. In 47 samples an ICT (SD BIOLINE Influenza Ag A/B/A (H1N1) Pandemic, SD Standard Diagnostics, Seoul, Korea) was also performed (Table 1).
Total number of samples tested by each of the diagnostic techniques used routinely for Influenza virus and RSV diagnosis.
Eighty-six nasopharyngeal aspirates were selected to test the Sofia RSV FIA from pediatric patients (under 16 years) with clinical suspicion of RSV infection and diagnosed as RSV-positive (n=56) or RSV-negative (n=30) using cell culture (conventional cell culture and shell-vials) and/or RT-PCR (Simplexa™Flu A/B & RSV Kit, Focus Diagnostics, Cypress, CA, USA) as a reference diagnostic methods. In 81 samples an ICT (Clearview®, Wampole Laboratories, Princeton, NJ, USA) was also performed (Table 1).
Cell cultures and shell-vial cultures were performed on the cell lines A-549, MRC-5 and MDCK. Conventional cell cultures were incubated for 7 days at 37°C and we investigated daily for the presence of characteristic cytopathic effects. The shell-vial cultures were incubated 48–72h at 37°C and were tested for the presence of RSV, influenza A and B, parainfluenza and adenovirus by direct immunofluorescence using monoclonal antibodies (Monofluo™Screen, BioRad, Marnes-la-Coquette, France).
For the molecular diagnosis, RNA was extracted from 200μl of the specimens using the NucliSENS® easyMAG instrument (bioMerieux Diagnostics, Marcy l’Etoile, France) eluting in 50μl. Five μl of the elution were employed to perform the nucleic acid extraction-dependent Simplexa™Flu A/B & RSV Kit real-time RT-PCR assay. The test is run in the 3M Integrated Cycler instrument and employs a bi-functional fluorescent probe–primer together with a reverse primer to amplify a specific target (conserved targets of influenza A, influenza B and RSV and the RNA internal control). Positive and negative controls were run for each reaction. According to the manufacturer instructions, Ct values of the samples should be ≤40 for result interpretation. All the samples positive for influenza A virus by RT-PCR or cell culture were subtyped using an in-house RT-PCR as previously described.14
The ICTs used for influenza and RSV were chromatographic immunoassays for the qualitative detection of antigens from influenza virus type A, type B, type A 2009 H1N1 and RSV directly from respiratory specimens.
A multiplex RT-PCR for RSV and influenza viruses detection was performed on samples from hospitalized patients, immunocompromised or patients with underlying pulmonary disease. Meanwhile, cell culture was performed for patients attending the Emergency Ward without risk factors who were discharged from the hospital.
The Sofia Influenza A+B and Sofia RSV FIAs (Quidel Corporation, San Diego, CA, USA) are lateral flow immunoassays which employ a fluorescence reaction (europium dye) to detect influenza A, influenza B or RSV nucleoprotein antigens. A total of 260μl of sample are inoculated into the Reagent Tube (lyophilized buffer with detergents and reducing agents that disrupt the virus particles present in the specimen, thus exposing internal viral nucleoproteins). Next, 120μl of the mixture are dispensed into the cassette sample well. The cassette is introduced into the Sofia Analyzer and after 15min of incubation, the analyzer detects the fluorescence signal and submits a qualitative result. Sofia may be set in two different modes: walk away mode and read now mode. In the first one the cassette is incubated inside the Sofia Analyzer and in the second one outside of the instrument. Both lecture methods were used in this study.
Sensitivity and specificity for the Sofia Influenza A+B and for the Sofia RSV FIAs were determined compared with the reference diagnostic methods (cell culture or RT-PCR). The same parameters were calculated for the Sofia Influenza A+B FIA based on patient age (pediatric patients under 16 years and adult patients over 16) as well as the type of sample (nasopharyngeal aspirate or nasopharyngeal swab). In case of discrepancies between the Sofia FIA kit and the reference methods, results were confirmed using a second RT-PCR (Influenza A/B r-gene™ and RSV/hMPV r-gene™, Biomerieux), performed on the same residual clinical samples stored at −80°C used for SOFIA tests. PCR cycle threshold (Ct) values were analyzed for all the samples positive by RT-PCR. The Sofia Influenza A+B and Sofia RSV FIAs performance was determined in a subgroup of discrepant samples negative by ICT but positive by one of the reference methods, in order to evaluate if the Sofia FIA assays improved the virus detection with respect to the ICT.
The performance parameters such as sensitivity and specificity were expressed as 95% confidence interval (95% CI) and the Mann–Whitney test was used for continuous variables (Epi Info™ 7).
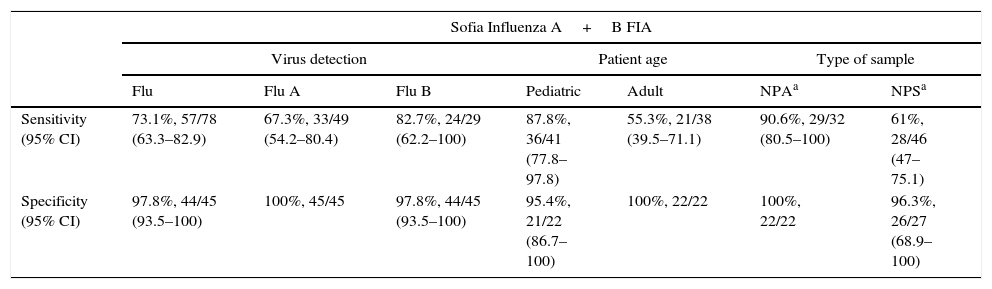
ResultsAn overall sensitivity of 73.1% and a specificity of 97.8% were obtained for the Sofia Influenza A+B FIA (Table 2). The Sofia Influenza A+B FIA showed higher sensitivity for influenza B detection than for influenza A: 82.7% vs. 67.3%, respectively. Specificity was 100% for influenza A and 97.8% for influenza B detection. There was one false positive result for the Sofia Influenza A+B, which affected both the overall and the influenza B specificities. When this sample was run in the RT-PCR assay it was positive for influenza B, confirming the result of the Sofia Influenza A+B. When the assays were compared according to age groups, the sensitivity value obtained for the pediatric population (87.8%) was higher than for the adult population (55.3%). The specificity value for children was 95.4% and for adults it was 100% (Table 2). Based on the sample type, the sensitivity values of the Sofia Influenza A+B for nasopharyngeal aspirates and nasopharyngeal swabs were, respectively, 90.6% and 61%. The specificity value was 100% for nasopharyngeal aspirates and 96.3% for nasopharyngeal swabs (Table 2).
Sofia Influenza A+B FIA performance vs. influenza reference diagnostic methods (RT-PCR and cell culture).
| Sofia Influenza A+B FIA | |||||||
|---|---|---|---|---|---|---|---|
| Virus detection | Patient age | Type of sample | |||||
| Flu | Flu A | Flu B | Pediatric | Adult | NPAa | NPSa | |
| Sensitivity (95% CI) | 73.1%, 57/78 (63.3–82.9) | 67.3%, 33/49 (54.2–80.4) | 82.7%, 24/29 (62.2–100) | 87.8%, 36/41 (77.8–97.8) | 55.3%, 21/38 (39.5–71.1) | 90.6%, 29/32 (80.5–100) | 61%, 28/46 (47–75.1) |
| Specificity (95% CI) | 97.8%, 44/45 (93.5–100) | 100%, 45/45 | 97.8%, 44/45 (93.5–100) | 95.4%, 21/22 (86.7–100) | 100%, 22/22 | 100%, 22/22 | 96.3%, 26/27 (68.9–100) |
A subgroup analysis was performed with the samples positive for influenza A by RT-PCR in order to determine if the results of the Sofia Influenza A+B varied according to the virus subtype. Of the 49 influenza A identifications, 41 were influenza A 2009 H1N1 and 8 were influenza A (H3N2). The Sofia Influenza A+B identified 29 of the 41 influenza A 2009 H1N1 (71%) and 4 of the 8 influenza A (H3N2) (50%).
A sensitivity of 87.5% (49/56; 95% CI 79–96.1) and a specificity of 86.7% (26/30; 95% CI 68.9–100) were obtained for the Sofia RSV FIA. The specificity value was low because four of the Sofia RSV FIA positives were negative by cell culture. These samples were determined to be positive by RT-PCR.
PCR cycle threshold (Ct) values were analyzed in the 65 RT-PCR influenza-positive samples and in the 22 RT-PCR RSV-positive samples. Samples were divided into two groups: Sofia FIA true positives and Sofia FIA false negatives with respect to the RT-PCR. The mean Ct value was calculated for each group: for the true positives group it was 26.67 (20–34.6) for the Sofia Influenza A+B and 22.21 (18.5–31.2) for the Sofia RSV; for the false negatives group it was 29.56 (22–35.2) for the Sofia Influenza A+B and 29.76 (24.1–34.1) for the Sofia RSV. The difference between the two groups was statistically significant (p=0.007 for Sofia Influenza A+B and p=0.005 for Sofia RSV).
Rapid testing with ICT for influenza was performed on 47 samples. Thirty-one of these samples were negative by ICT but positive with cell culture or RT-PCR, which demonstrated that the ICT negative results were false negatives. When the results of the Sofia Influenza A+B were compared in the same 31 samples, there were only six false negative results with the Sofia Influenza A+B assay, yielding a net gain of 25 positive results for the Sofia assay compared to ICT. With respect to the RSV analysis, there were 81 samples in which the ICT for RSV detection was performed; 51 of these were negative by ICT but positive by one of the reference methods. When the results of the Sofia RSV FIA for that group of 51 ICT false negative results were compared, there were only eight false negative results for the Sofia RSV FIA, yielding a net gain of 43 positive results for the Sofia assay compared to ICT.
DiscussionWhen the Sofia FIA assay was compared with the two reference diagnostic methods for viral detection (RT-PCR and cell culture), the Sofia Influenza A+B FIA sensitivity and specificity were, respectively, 73.1% and 97.8%. Sensitivity and specificity for the Sofia RSV FIA were 87.5% and 86.7%. It is noteworthy that specificity values of both Sofia Influenza A+B and Sofia RSV FIAs decreased because of five samples in which the Sofia assays detected the virus and cell culture did not. However, the Sofia assays discrepant positive results were confirmed as positive by the second RT-PCR. Hence, the Sofia FIA kits were able to detect the viruses in five samples in which those viruses had not been detected by cell culture. Although cell culture on shell-vial is a reference method, its sensitivity is highly dependant on personnel training.
The sensitivity values of the two assays are high compared to the ICT assays performed in this study and the sensitivity values for similar RDTs described in the literature.2,4,7,8 Usually, RDTs for influenza detection have higher sensitivities in pediatric specimens because children have higher titers of virus in the respiratory secretions and more virus antigen can be detected. This is also why RDTs yield better results when nasopharyngeal aspirates are used, as these are the most optimal respiratory specimens to detect viruses,10 but this sample type is only taken from children. The higher sensitivity of the Sofia Influenza A+B in pediatric samples and in nasopharyngeal aspirates corresponds with results previously described in the literature.9
It was also interesting that the Sofia Influenza A+B FIA showed increased sensitivity for influenza B detection compared to influenza A detection, in contrast to most previous Influenza RDT reports.10–13 The influenza A subtypes analysis suggested that the Sofia Influenza A+B assay showed higher sensitivity to viruses belonging to the subtype A 2009 H1N1 than to the subtype (H3N2), but the influenza A (H3N2) group had too few samples to establish a meaningful comparison. Of note is that the sensitivity of Sofia Influenza A+B was not reduced with the influenza A 2009 H1N1 as previously reported in the literature for other RDTs.15,16
RDTs are currently the most suitable diagnostic tool for influenza and RSV detection at point-of-care (POC) locations due to their ease of use as well as their rapid turnaround time that permits clinicians to make quick decisions. However, the poor sensitivity of RDTs is problematic and it is recommended that all RDT-negative results in patients with clinical suspicion of influenza or RSV infection be confirmed with viral culture or RT-PCR.17,18 This strategy increases the cost of influenza and RSV diagnosis and the time-to-result. This cost might be reduced using Sofia FIA kits because these tests have higher sensitivity, yielding a lower number of negative results to be confirmed.
As expected, the sensitivity of the Sofia Influenza A+B and the Sofia RSV was correlated with the viral load in the sample as assessed by the PCR cycle threshold value.15,19 The Ct analysis demonstrated that false negative results were more frequent in samples with low Ct values.
This study demonstrates some limitations. The techniques considered as gold standard (RT-PCR and culture) have differing sensitivities, although the majority of the samples were compared to the most sensitive method, real-time RT-PCR. Due to the retrospective nature of this study not all samples were tested using each one of the mentioned diagnostic method.
In summary, Sofia Influenza A+B and Sofia RSV FIAs demonstrated high sensitivity values compared with the two reference methods, cell culture and RT-PCR. These sensitivity and specificity values were higher than the values reported in the literature for other RDTs. Regarding the ability of the Sofia Influenza A+B FIA to differentiate between the influenza A and influenza B viruses, the Sofia assay showed higher sensitivity for the detection of influenza B virus. As expected, the Sofia Influenza A+B proved more sensitive in pediatric specimens than in adults, but the sensitivity in adult specimens is improved compared with other RDTs. The Sofia Analyzer is also highly automated and provides objective results, which potentially reduces errors that might occur with the subjective visualization of other RDT reactive strips. Besides, the walk away or the read now mode of lecture can be chosen depending on the work load of the laboratory. The walk away mode is more suitable to read cassettes individually and the read now mode can be use to analyze several cassettes each time, taking care in not exceeding the time of incubation. In conclusion, based on their high clinical sensitivity, novel technology and automated instrumentation, the Sofia Influenza A+B FIA and the Sofia RSV FIA are currently the most appropriate RDTs for influenza and RSV diagnosis.
Conflicts of interestThe authors have no conflict of interest to declare.
The authors thank Michele Hill for her thorough review of the manuscript.