Antibiotic prophylaxis in surgery is one of the most effective measures for preventing surgical site infection, although its use is frequently inadequate and may even increase the risk of infection, toxicities and bacterial resistance. As a result of advances in surgical techniques and the emergence of multidrug-resistant organisms, the current guidelines for prophylaxis need to be revised.
La Sociedad Española de Enfermedades Infecciosas (Spanish Society of Infectious Diseases and Clinical Microbiology) (SEIMC) together with the Asociación Española de Cirujanos (Spanish Association of Surgeons) (AEC) have revised and updated the recommendations for antibiotic prophylaxis to adapt them to any type of surgical intervention and to current epidemiology. This document gathers together the recommendations on antimicrobial prophylaxis in the various procedures, with doses, duration, prophylaxis in special patient groups, and in epidemiological settings of multidrug resistance to facilitate standardized management and the safe, effective and rational use of antibiotics in elective surgery.
La profilaxis antibiótica en cirugía es una de las medidas más eficaces para la prevención de la infección de localización quirúrgica, aunque su uso es con frecuencia inadecuado, pudiendo incrementar el riesgo de infección, toxicidades y resistencias bacterianas. Debido al avance en las técnicas quirúrgicas y la emergencia de microorganismos multirresistentes, las actuales pautas de profilaxis precisan ser revisadas.
La Sociedad Española de Enfermedades Infecciosas (SEIMC), conjuntamente con la Asociación Española de Cirujanos (AEC), ha revisado y actualizado las recomendaciones de profilaxis antimicrobiana para adaptarlas a cada tipo de intervención quirúrgica y a la epidemiología actual. En este documento se recogen las recomendaciones de los antimicrobianos utilizados en profilaxis en los diferentes procedimientos, las dosis, la duración, la profilaxis en huéspedes especiales, y en situación epidemiológica de multirresistencia, de tal forma que permitan un manejo estandarizado, un uso racional, seguro y efectivo de los mismos en la cirugía electiva.
Antibiotic prophylaxis (AP) in surgery is one of the most effective measures for the prevention of surgical site infection (SSI). Despite this, its use is frequently inadequate, in general because it is not administered according to the guidelines, not started at the right time or because it is prolonged. Inadequate administration can increase the risk of SSIs, toxicities and bacterial resistance, control of which is a priority objective of the health authorities.
Advances in surgical techniques and the appearance of new ones, the increased number of transplants, and the emergence and expansion of multidrug-resistant organisms make it necessary to revise the AP guidelines that have been used in earlier decades.
The last consensus document on AP for Spain was published in 2002. The Sociedad Española de Enfermedades Infecciosas (SEIMC) together with the Asociación Española de Cirujanos (AEC) have revised and updated the recommendations for AP in order to adapt them to any type of surgical intervention and to current epidemiology. The recommendations made in this document are based on scientific evidence. Where it has not been possible to find high-quality evidence, the coordinators and authors of the document have made their recommendations based on knowledge of etiopathogenesis and risk factors for SSI found in pharmacokinetic studies of the antibiotics used in prophylaxis, and on clinical experience.
The main objective of this Consensus Document is to provide guidelines that will enable standardized management of AP in elective surgery, with a safe, effective and rational use of antimicrobials for the prevention of SSIs.
Scope of the documentThis document is focused exclusively on AP in surgery and does not address other measures that have been shown to be effective for prevention of SSI, such as decolonization of Staphylococcus aureus or skin antisepsis. The document provides general recommendations for AP and specific indications by type of surgical procedure, with graded recommendations based on scientific evidence. Antimicrobial agents and doses are provided, although excluding some that the committee considers to be unsuitable for prophylaxis because of currently very high rates of resistance, too broad a spectrum, ecological impact or ability to induce resistance (e.g. quinolones or ertapenem). Recommendations have also been provided for duration and prophylaxis in special patients and in epidemiological settings of multidrug resistance.
One of the major limitations of this document is that recommendations cannot always be supported by the highest strength of evidence owing to the design of most of the studies, the scarcity of comparative studies with placebo or different antimicrobials to study the effectiveness of AP, or the low incidence of infections in most procedures. On occasions, recommendations are inferred from evidence in other types of surgery in the same anatomical area or with a similar microbial spectrum. Although certain antibiotic types are recommended, the selection should be adapted to local epidemiology and the antimicrobial stewardship programs designed to optimize antimicrobial use at each center. It is not possible to make general indications in complicated situations, such as patients undergoing multiple surgeries who have received several antimicrobials, where prophylaxis would need to be individualized depending on the risk of infection and the colonization status of the patient.
The document is directed at professionals in specialized care involved in surgical procedures, such as anesthetists and surgeons, and at those who participate in prevention of surgical infection, such as infectologists, microbiologists, preventivists and pharmacists.
MethodologyThe two societies nominated project coordinators: an infectologist (MDT) and a surgeon (JMB), who selected the rest of the panel of experts, which includes surgeons, infectologists, internists and microbiologists. The final manuscript was made available to members of both societies for their review and suggestions.
This document is an updated version of the one published in 2002 and is based on recently published guidelines answering questions of clinical interest.
In order to answer each question, a systematic literature search was conducted for relevant studies published between 1970 and October 2018 using the following databases (Biblioteca Cochrane Plus (Cochrane Library), Medline, EMBASE, Scopus, Tryp database, DARE). Some relevant studies published while the document was being revised have also been included. The search terms used are specified in each section of the original document.
In accordance with SEIMC regulations, the criteria established by the SEIMC were used to determine the strength and quality of evidence (Table 2 of the original document). The methodological quality of the studies was evaluated in accordance with the Agree Collaboration (www.agreecollaboration.org).
The complete text was revised and approved by all authors and was made available to all members for review on the web page of the SEIMC. The conflicts of interest of all members of the panel of experts are specified at the end of the document.
RecommendationsBasic principles of prophylaxis in surgeryWhen is prophylaxis indicated?AP in surgery is recommended:
- -
When there is a high probability of infection or when the consequences of postoperative infection are potentially serious for the patient (endocarditis, endophthalmitis, prosthetic joint infection) (A-III).
- -
In surgery classified as clean-contaminated and contaminated (A-II).
- -
In dirty surgery, when there is obvious suppuration or infection, antibiotics are administered as part of treatment (A-III).
- -
In clean surgery, the indication for AP depends on the type of intervention, the comorbidities of the patient and whether prosthetic material is involved, although in some cases, this has not been clarified completely.
The antibiotic must be active against the organisms most frequently isolated in each type of procedure, although the majority of experts advise the use of a first- or second-generation cephalosporin (A-III). Table 3 of the original document shows the organisms involved in SSI by type of surgery in thirteen European countries.
- -
The choice of antibiotics should take into account local epidemiology and antimicrobial susceptibility patterns of the organisms that cause surgical infections in the hospital (A-III).
- -
First- and second-generation cephalosporins, fundamentally cefazolin, are the antibiotics of choice for most indications (A-I).
- -
In cases of allergy to beta-lactams, a history of methicillin-resistant Staphylococcus aureus (MRSA) colonization or infection, or a very high prevalence of SSI caused by MRSA in the hospital, a glycopeptide may be used (A-I).
- -
In colorectal or gynecological surgery where anaerobic organisms and Enterobacteriaceae are highly likely to be involved in surgical wound colonization, it is recommended to choose an antibiotic or combination of antibiotics with activity against both groups of organisms (A-I).
- -
Antibiotic prophylaxis in surgery should be administered within 120min prior to incision (A-I).
- -
In the case of beta-lactams with short half-lives (e.g. penicillins and cephalosporins such as cefazolin, cefoxitin and cefuroxime), it is advisable to administer them within 60min prior to incision (B-II).
- -
In the case of vancomycin, aminoglycosides and fluoroquinolones, intravenous infusion should commence 90min prior to incision, since these antibiotics require long infusion times (B-II).
- -
In the case of surgery requiring limb ischemia, administer the prophylaxis before inflating the tourniquet (A-III).
It is generally accepted that the antibiotic dose used in prophylaxis is the same as the one used to treat the infection (A-III). Tables 4 and 5 in the original document show the initial dose of antibiotics for surgical prophylaxis, both oral and intravenous, in adults and children.
Should the dose be modified in obese patients?- -
In obese patients, the concentrations of some antibiotics may be modified due to pharmacokinetic alterations. Pharmacokinetic parameters such as volume of distribution and drug clearance may be greater in obese patients, but frequently not proportional to total body weight (A-II).
- -
Obese patients may require higher starting doses. The conventional dose for non-obese patients can lead to obese patients being underdosed for some drugs. By the same token, dosing based on total bodyweight may lead to overdose in the obese patient (A-II).
- -
The use of surrogate descriptors of total bodyweight, such as ideal weight or adjusted weight, may correct the problem of overdosing based on total weight in the obese (A-II).
- -
Calculating maintenance doses for prolonged prophylaxis in the obese (in lengthy surgeries, for example) is not well resolved, although the approach to dose selection based on estimates of renal function may be a reasonable, clinically useful alternative (A-II).
- -
An additional intraoperative dose is recommended when the procedure is more than two times the half-life of the antibiotic (B-II).
- -
With cefazolin or other antibiotics with a similar half-life, a second intraoperative dose should be administered at 3h (B-II).
- -
An additional dose is recommended when the half-life of the antibiotic is decreased (burns, very high glomerular filtration rates) or there is significant bleeding (>1500mL in adults or 25mL/kg in children) (B-II).
- -
For most surgical procedures, a single dose of antibiotic whose half-life ensures sufficient drug concentrations in serum and tissue for the duration of the surgical intervention will be appropriate (A-I).
- -
There is an increased risk of C. difficile infection with some antibiotics used in AP, such as the cephalosporins, carbapenems, fluoroquinolones and clindamycin (A-II).
- -
There is an increased risk of C. difficile infection if AP is prolonged (A-II).
- -
In the perioperative phase, the procedures in major and trauma surgery may expose the patient to non-specific acute kidney injury, even when there is no previous kidney disease. Furthermore, these patients may receive prophylaxis with antimicrobials such as aminoglycosides or glycopeptides, which are associated with nephrotoxicity (A-II).
- -
In major surgery patients, serial serum and urinary creatinine measurements should be requested in the preoperative assessment as well as ≥24h after surgery to check the degree of renal function, paying special attention to patients who have received prophylaxis with aminoglycosides or glycopeptides (A-II).
- -
Use of single doses in surgical prophylaxis, with special exceptions (such as prolonged surgery and significant loss of blood, among others) helps minimize acquired resistance to antimicrobials (A-II).
- -
Clarifying possible beta-lactam allergy should be a routine part of the pre-anesthesia evaluation and pre-operative care and attention (B-II).
- -
Patients with a history of severe allergic reaction, whether immediate (occurring within the first hour of beta-lactam administration) or non-immediate, should not receive beta-lactams as prophylaxis when there are other effective therapeutic alternatives (B-III).
- -
Other beta-lactams may be employed (with a different side chain from the beta-lactam implicated in the allergic reaction), provided that the allergy study has corroborated it through exposure tests (B-II).
- -
Local AP guidelines should consider alternatives to beta-lactams that are of proven efficacy for those patients who have allergies (B-III).
- -
In high-risk surgery (cardiac, orthopedic) in patients with MRSA colonization, a glycopeptide plus a beta-lactam can be given as prophylaxis, accompanied by other measures for decolonization (A-II).
- -
For patients with extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae colonization, prophylactic coverage should only be considered in high-risk patients (B-III).
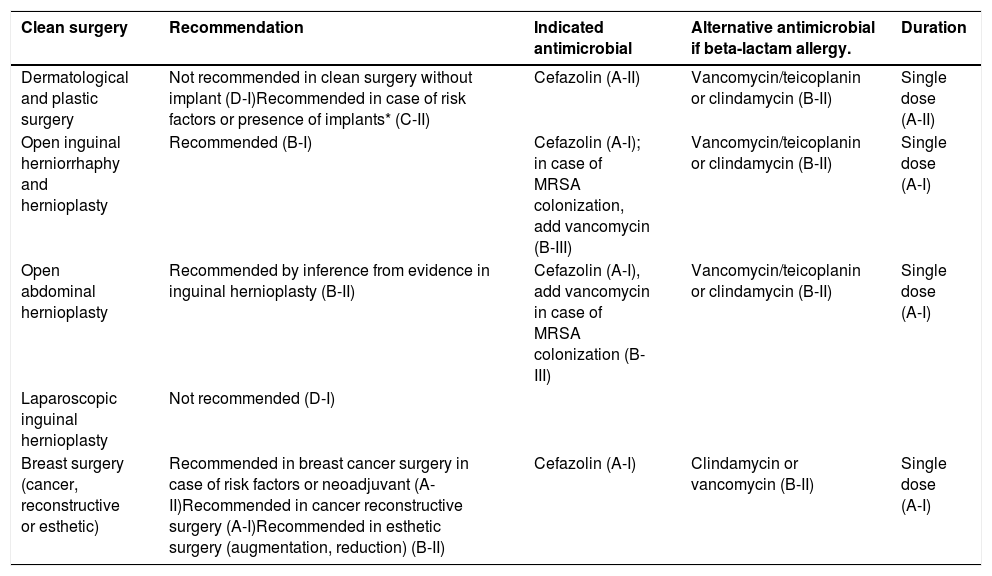
The antibiotics indicated for each type of surgery are shown in Table 1 of this document, and the dosages in Tables 4 and 5 of the original document.
Recommended indications for surgical antibiotic prophyl axis to prevent ssi, by type of surgery and surgical procedure.
| Clean surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy. | Duration |
|---|---|---|---|---|
| Dermatological and plastic surgery | Not recommended in clean surgery without implant (D-I)Recommended in case of risk factors or presence of implants* (C-II) | Cefazolin (A-II) | Vancomycin/teicoplanin or clindamycin (B-II) | Single dose (A-II) |
| Open inguinal herniorrhaphy and hernioplasty | Recommended (B-I) | Cefazolin (A-I); in case of MRSA colonization, add vancomycin (B-III) | Vancomycin/teicoplanin or clindamycin (B-II) | Single dose (A-I) |
| Open abdominal hernioplasty | Recommended by inference from evidence in inguinal hernioplasty (B-II) | Cefazolin (A-I), add vancomycin in case of MRSA colonization (B-III) | Vancomycin/teicoplanin or clindamycin (B-II) | Single dose (A-I) |
| Laparoscopic inguinal hernioplasty | Not recommended (D-I) | |||
| Breast surgery (cancer, reconstructive or esthetic) | Recommended in breast cancer surgery in case of risk factors or neoadjuvant (A-II)Recommended in cancer reconstructive surgery (A-I)Recommended in esthetic surgery (augmentation, reduction) (B-II) | Cefazolin (A-I) | Clindamycin or vancomycin (B-II) | Single dose (A-I) |
| Cardiovascular surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Aortocoronary bypass and valve replacement | Not recommended in percutaneous procedures (D-II).Recommended in aortocoronary bypass and valve replacement (A-I) and in percutaneous transcatheter aortic valve implantation (B-III) | Cefazolin or cefuroxime (A-I) | Vancomycin (A-II) | Single dose (A-I)If cephalosporins are used, redose 1g every 3h during the procedure, do not continue after closure (A-II) |
| Pacemaker and defibrillator insertion | Recommended (A-I) | Cefazolin or cefuroxime (A-I) | Vancomycin (A-II) | Single dose (A-I) |
| Implantation of central vascular access catheters | Not recommended in implantation of central vascular access catheters (D-I).An antibiotic lock is not routinely recommended before inserting or manipulating an intravascular catheter (D-I) | |||
| Peripheral vascular surgery (percutaneous and open) | Recommended in high-risk vascular procedures, including those in which some type of prosthetic material is to be implanted (A-I) | Cefazolin (A-I).Adding a second antibiotic with activity against GNB (gentamicin) is suggested if there is risk of exposure to intestinal microbiota. (B-III) | Vancomycin (B-II) or clindamycin (C-III) | Single dose (A-I) |
| Ophthalmic surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Cataract surgery | Intracameral administration is recommended (A-I) | Intracameral cefuroxime or cefazoline (A-I) | Intracameral vancomycin or moxifloxacin (A-III) | Single dose (A-I) |
| Glaucoma surgery, corneal graft | Intracameral administration is recommended by inference from cataract surgery (A-II) | Intracameral cefuroxime (A-I) | Intracameral vancomycin or moxifloxacin (B-III) | Single dose (A-I) |
| Lacrimal surgery | Recommended (A-III) | Cefazolin or cefuroxime (A-III) | Vancomycin (B-III) | Single dose (A-I) |
| Penetrating eye trauma | Intravitreal injection is recommended (A-I) | Gentamicin plus clindamycin (A-II) or gentamicin plus vancomycin (A-III) | Single dose (A-I) |
| Neurosurgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Craniotomy | Recommended (A-I) | Cefazolin (A-I) | Vancomycin or clindamycin (A-II) | Single dose (A-I) |
| Placement of ventriculo-peritoneal or ventriculo-auricular shunt and external ventricular drainage | Recommended in ventriculo-peritoneal or ventriculo-auricular shunt (A-I)Recommended in external ventricular drainage (B-I) | Cefazolin (A-I)In context of high prevalence of MRSA: Vancomycin (A-I) | Vancomycin (A-II) | Single dose (A-I) |
| Intracranial pressure sensor placement | Not recommended (D-II) | |||
| Transsphenoidal or pharyngeal surgery | Recommended (A-III) | Amoxicillin-clavulanic acid (A-III) | Clindamycin or vancomycin, associate with an aminoglycoside (B-III) | Single dose (A-I) |
| Head and neck surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Clean head and neck surgery | Not recommended in clean head and neck surgery (D-I).Recommended in extended lymphadenectomies or cervical surgery with multivisceral resection (B-II) | Cefazolin (B-II) | Clindamycin or vancomycin (B-III) | ≤24h (A-II)Possibly a single dose is enough (A-III) |
| Clean-contaminated head and neck surgery: tonsillectomy, adenoidectomy, laryngectomy, tracheotomy, and any other surgery involving incision of the pharyngeal-laryngeal mucosa | Recommended in clean-contaminated head and neck surgery (A-II), except tonsillectomy and adenoidectomy (D-I).Recommended in head and neck cancer surgery (A-II) | Amoxicillin-clavulanic acid (A-III) | Clindamycin plus gentamicin (B-III) | ≤24h (A-II), possibly a single dose is enough (A-III) |
| Sinus and middle ear surgery | Not recommended in endoscopic sinus surgery (D-I).Not recommended in clean otologic surgery (D-I).Recommended topical application of antibiotic after tympanoplasty (A-I).Recommended in clean contaminated and contaminated surgery (B-III).Recommended in cochlear implant surgery (B-III) | In clean contaminated and contaminated surgery: amoxicillin-clavulanic acid (B-III).In cochlear implant: cefazolin (A-II) | In clean contaminated and contaminated surgery: clindamycin plus gentamicin (C-III).In cochlear implant: clindamycin or vancomycin (C-III) | Single dose for clean contaminated and contaminated surgery (A-II) and cochlear implant (A-I) |
| Maxillofacial Surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Septoplasty | Not recommended (D-I) | |||
| Simple rhinoplasty | Not recommended (D-I) | |||
| Complex rhinoplasty (revision, prosthesis) | Recommended (B-II) | Cefazolin (B-II) or amoxicillin-clavulanic acid (B-III) | Vancomycin or clindamycin (C-III) | Single dose (A-I) |
| Maxillofacial fractures | Recommended in maxillofacial fractures, especially mandibular fractures requiring open reduction (A-II) | Cefazolin (A-II) or amoxicillin-clavulanic acid (B-III) | Vancomycin or clindamycin (B-III) | Single dose (A-I) |
| Dental procedures | Recommended in intraoral bone graft implantation (B-II).Not recommended for dental extraction in patients without risk factors (D-II)Not recommended in oral implants or endodontics (D-II) | Amoxicillin 1g, oral (A-II) | Clindamycin (B-II) | Single dose (A-I) |
| Trauma and Orthopedic Surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Closed fracture reduction without osteosynthesis material and other clean orthopedic surgery without instrumentation. | Not recommended in clean orthopedic surgery without instrumentation (D-II).Consider the use of prophylaxis in patients with risk factors; it may be considered in ligamentoplasty by referring to recommendations in arthroplasty (B-III) | |||
| Closed fracture reduction with osteosynthesis material | Recommended (A-I) | Cefazolin or cefuroxime (A-I).In case of risk of MRSA SSI: vancomycin or teicoplanin (B-II) plus cefazolin or cefuroxime if there is risk of GNB SSI.In case of risk of resistant GNB SSI, add gentamicin (B-III) | Vancomycin or teicoplanin (B-II) plus gentamicin in case of risk of GNB SSI (B-III) | ≤24h (A-I), a single dose may be enough (A-II) |
| Open fracture surgery | Recommended (A-I) | Cefazolin (A-I) or amoxicillin-clavulanic acid (B-III) (Gustilo grade I and II fractures)Add an aminoglycoside (gentamicin) in Gustilo grade III fractures (B-II) | Vancomycin or clindamycin±gentamicin (B-III) | Start as soon as possible. In Gustilo grade I-II fractures, maintain until 24h after debridement (A-II) and in grade III-A fractures, up to 72h maximum, or until soft tissue closure (whichever occurs first) (B-III) |
| Removal of orthopedic implants used in fracture stabilization | Not recommended (D-II) | |||
| Arthroplasties (THP, TKP, tumor megaprosthesis, primary and revision) | Recommended (A-I) | Cefazolin or cefuroxime (A-I) | Vancomycin or teicoplanin (B-II) (plus gentamicin in case of risk of GNB SSI) (B-III) | ≤24h (A-I)Possibly a single dose is enough (A-II)In megaprosthesis: ≤24h (A-II) |
| Spinal surgery (laminectomies and discectomies with/without instrumentation) | Recommended in vertebral orthopedic surgery with/without instrumentation, including vertebral fusion, laminectomy and minimally invasive discectomy (A-I) | Cefazolin or cefuroxime (A-I) | Vancomycin or teicoplanin (B-II) (plus gentamicin in case of risk of GNB SSI) (B-III) | ≤24h (A-I)A single dose may possibly be enough (A-II) |
| Limb amputation | Recommended in amputation of lower limbs (A-II) | Cefazolin or cefuroxime or amoxicillin-clavulanic acid (A-II) | Vancomycin or teicoplanin plus gentamicin (B-III) | At least 24h, provided there is no infected tissue remaining post-amputation (B-III) |
| Thoracic surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Major and minimally invasive thoracic surgery | Recommended in major thoracic surgery (A-I).Recommended in minimally invasive thoracic surgery (videothoracoscopy, mediastinoscopy) (B-III) | Cefazolin for major thoracic surgery (A-I) and minimally invasive thoracic surgery (A-II) | Vancomycin or teicoplanin (B-III) | Single dose (A-I) |
| Thoracic tube placement, penetrating trauma | Not recommended in elective thoracic tube placement (D-II)Recommended in penetrating chest trauma (A-I) | Cefazolin (A-I) | Vancomycin or teicoplanin (B-III) | Single dose (A-I) |
| Digestive and abdominal surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Esophageal gastric or duodenal surgery with mucosal rupture (esophagostomy, gastrectomy, cephalic duodenopancreatectomy) | Recommended in esophageal surgery (A-II), gastric (A-I) and gastroduodenal (A-I) surgery | Cefazolin (A-I) | Vancomycin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Esophageal gastric or duodenal surgery without mucosal rupture (gastroesophageal reflux surgery, achalasia, vagotomy) | Not recommended in esophageal-gastric-duodenal surgery without mucosal rupture (D-II).Recommended in esophageal-gastric-duodenal surgery without mucosal rupture in high-risk patients (C-III) | Cefazolin (A-I) | Vancomycin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| PEG implantation (percutaneous endoscopic gastrotomy) | Recommended (A-I)Recommended: good antisepsis at implantation site (A-III) | Cefazolin or cefuroxime or amoxicillin-clavulanic acid (A-II) | Vancomycin plus gentamicin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Bariatric surgery | Recommended (A-II) | Cefazolin or cefuroxime or amoxicillin-clavulanic acid (A-II) | Vancomycin plus gentamicin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Small bowel surgery | Recommended in small bowel surgery with and without obstruction (A-III) | In surgery without obstruction: cefazolin (A-I).In surgery with obstruction: cefazolin plus metronidazole (B-II) or amoxicillin-clavulanic acid (B-III) | In surgery without obstruction: clindamycin plus gentamicin (B-III).In surgery with obstruction: metronidazole plus gentamicin (B-II) | Single dose (A-I) |
| Splenectomy | Not recommended in splenectomy without risk factors (D-II).Recommended in traumatic or elective splenectomy with risk factors (B-III) | Cefazolin (A-I) | Vancomycin plus gentamicin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Penetrating abdominal trauma | Recommended (A-I) | Cefuroxime plus metronidazole (A-I) or amoxicillin-clavulanic acid (A-II) | Metronidazole plus gentamicin (A-III) | Single dose (A-I)If hollow viscus injury ≤24h (A-II) |
| Appendicectomy | Recommended in uncomplicated appendectomy (A-I) | Cefuroxime plus metronidazole (A-I) or amoxicillin-clavulanic acid (A-II) | Metronidazole plus gentamicin (B-III) | Single dose (A-I) |
| Colorectal surgery | Recommended (A-I) | Cefuroxime plus metronidazole or amoxicillin-clavulanic acid (A-II), add gentamicin in case of high prevalence of resistant GNB (B-III) | Metronidazole plus gentamicin (B-III) | Single dose (A-I) |
| Oral antibiotic prophylaxis and mechanical bowel preparation (MBP) | Recommended in mechanical bowel preparation (A-II) associated with oral antibiotics (A-I) in elective colorectal surgery | Neomycin 1000mg plus metronidazole 500mg (A-II) | 3 preoperative doses 19, 18 and 9h before the start of the procedure |
| Hepatobiliary and pancreatic surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Cholecystectomy and biliary surgery | Not recommended in low-moderate elective laparoscopic cholecystectomy (D-I).Recommended in open cholecystectomy (A-I) or high-risk laparoscopic cholecystectomy (A-II) | Cefazolin (A-I) | Vancomycin plus gentamicin or clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Liver surgery | Not recommended in simple hepatectomy (D-I)Recommended in major hepatectomy (includes extrahepatic biliary resection) (A-II) | Cefazolin (A-I) or amoxicillin-clavulanic acid (A-III)In case of previous bile cultures: adjust according to sensitivity (A-II) | Vancomycin plus gentamicin (A-III) | ≤24h (A-I). Possibly a single dose is enough (A-II) |
| Pancreatic surgery | Recommended (A-II) | Low-risk surgery (no bile duct manipulation): cefazolin (A-I) or amoxicillin-clavulanic acid (A-III). Add gentamicin in case of high prevalence of resistant GNB (B-III).High-risk surgery with bile microbiological information: adjust to previous microbiology (A-II).High-risk surgery without bile microbiological information: amoxicillin-clavulanic acid plus gentamicin (B-III) | Vancomycin plus gentamicin (B-III) | ≤24h (A-I).Possibly a single dose is enough (A-II) |
| Advanced peritoneal surgery-peritonectomy | Recommended in advanced peritoneal cancer surgery (A-II) | Cefazolin plus metronidazole or amoxicillin-clavulanic acid (A-III).Add gentamicin in case of risk of resistant GNB SSI (B-III) | Metronidazole plus gentamicin (B-III) | Single dose (A-II) |
| Urological surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Simple cystoscopy (without manipulation) | Not recommended in simple cystoscopy or urodynamics without risk factors (D-I).Recommended in case of risk factors** (B-III) | Fosfomycin trometamol or cefuroxime or amoxicillin-clavulanic acid (A-II) | Fosfomycin trometamol (A-II) | Single dose (A-I) |
| Transurethral prostate resection | Recommended (A-I) | Fosfomycin trometamol (A-II) or cefuroxime or amoxicillin-clavulanic acid (A-III) | Fosfomycin trometamol (A-II) or gentamicin (A-III) | Single dose (A-I) |
| Transurethral resection of bladder tumor | Not recommended (D-III)Recommended in case of risk factors** or large tumors (B-III) | Fosfomycin trometamol (A-II) or cefuroxime or amoxicillin-clavulanic acid (A-III) | Fosfomycin trometamol (A-II) or gentamicin (A-III) | Single dose (A-I) |
| Ureteral stent insertion or removal. Ambulatory endourological surgery. | Recommended in case of patients with risk factors** (B-III) | Fosfomycin trometamol (A-II) or cefuroxime or amoxicillin-clavulanic acid (A-III).In patients with previous infection or catheter colonization, antibiotic prophylaxis should be adapted, based on previous urine cultures (B-II) | Fosfomycin trometamol (A-II) or gentamicin (A-III) | Single dose (A-I) |
| Ureteroscopic stone removal | Recommended (C-III), mainly in patients with risk factors** (B-III) | Fosfomycin trometamol (A-II) or cefuroxime or amoxicillin-clavulanic acid (A-III)In patients with previous infection or catheter colonization, antibiotic prophylaxis should be adapted, based on previous urine cultures (B-II) | Fosfomycin trometamol (A-II) or gentamicin (A-III) | Single dose (A-I) |
| Extracorporeal lithotripsy | Not recommended if there are no risk factors (D-I)Recommended in patients with risk factors** (B-III) | Fosfomycin trometamol (A-II) or cefuroxime or amoxicillin-clavulanic acid (A-III).In patients with previous infection or catheter colonization, antibiotic prophylaxis should be adapted, based on previous urine cultures (B-II) | Fosfomycin trometamol (A-II) or gentamicin (A-III) | Single dose (A-I) |
| Open or laparoscopic nephrectomy | Not recommended (D-II), except if it is considered a clean-contaminated surgery or in high-risk patients** (B-II) | Cefuroxime or amoxicillin-clavulanic acid (A-III).In patients with previous infection or catheter colonization, antibiotic prophylaxis must be adapted, based on previous urine cultures (B-II) | Gentamicin (A-III) | Single dose (A-I) |
| Percutaneous nephrolithotomy | Recommended (A-II) | Cefuroxime or amoxicillin-clavulanic acid (A-II).In patients with a history of UTI by ESBL-producing bacteria, antibiotic prophylaxis must be adapted (A-III) | Gentamicin (A-III) | Single dose (A-I) |
| Simple prostatectomy (abdominal and laparoscopic) | Recommended (A-II) | Cefuroxime or amoxicillin-clavulanic acid (A-III) | Gentamicin plus vancomycin (B-III) | Single dose (A-I) |
| Radical cystectomy with entry into the intestinal tract. Urinary diversions | Recommended (A-II) | Cefuroxime plus metronidazole or amoxicillin-clavulanic acid (A-II) (add gentamicin in case of high prevalence of resistant GNB) (B-III) | Gentamicin plus metronidazole (B-III) | Single dose (A-II) |
| Transrectal prostate biopsy | Recommended (A-I) | Fosfomycin trometamol (A-I) or cefuroxime or amoxicillin-clavulanic acid, orally before the procedure (A-II) (add gentamicin in case of high prevalence of resistant GNB) (B-III).If there is a history of UTI by multiresistant microorganisms, targeted prophylaxis is recommended (A-II) | Fosfomycin trometamol (A-I) or gentamicin (A-III) | Single dose 1–3h before the procedure (A-I) |
| Clean surgery: testicular, phimosis and other penile surgeries without prosthetic implantation, open renal biopsy | Not recommended (D-II) | |||
| Penile prosthesis | Recommended (A-III) | First- or second-generation cephalosporin (A-III) | Gentamicin plus vancomycin (B-III) | Single dose (A-I) |
| Gynecological surgery | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Cesarean section | Recommended in elective and urgent cesarean sections (A-I) | For elective cesarean section: cefazolin (A-I).For urgent cesarean section: cefazolin plus azithromycin (A-I) | Clindamycin plus gentamicin (B-III) | Single dose before the incision (A-I) |
| Hysterectomy | Recommended in abdominal and vaginal hysterectomy (A-I) | Cefazolin or cefoxitin or amoxicillin-clavulanic acid (A-I) | Clindamycin plus gentamicin (B-III) or vancomycin plus gentamicin (B-III) | Single dose (A-I) |
| Adnexectomy and tubal ligation | Not recommended (D-III) | |||
| Induced abortion or puerperal curettage | Recommended for induced surgical abortion in the first trimester (A-I), the second trimester or puerperal curettage (A-III).Not recommended in medical abortion (D-III) | Doxycycline 100mg orally 2h before or i.v. before the procedure (A-I) or azithromycin 1g orally or i.v. plus metronidazole 500mg orally (B-III) | Clindamycin plus gentamicin (B-III) | Single dose (A-II) |
| Postpartum vaginal tear repair | Recommended in postpartum vaginal tear repair (III/IV) (A-I) | Cefoxitin or amoxicillin-clavulanic acid (A-I) | Clindamycin plus gentamicin (B-III) | Single dose (A-I) |
| Transplants | Recommendation | Indicated antimicrobial | Alternative antimicrobial if beta-lactam allergy | Duration |
|---|---|---|---|---|
| Kidney transplant | Recommended (A-II) | Cefazolin (A-II), consider adding gentamicin if high prevalence of resistant GNB (B-III) | Vancomycin plus gentamicin according to the previous consideration (B-III) | Single dose (A-II) |
| Pancreas transplant -simultaneous pancreas/kidney (SPK) transplant | Recommended (A-II) | Cefazolin (A-II), or amoxicillin-clavulanic acid (B-II) (due to the frequent implication of enterococci).Consider adding an aminoglycoside according to local epidemiology or if prior colonization with multidrug-resistant organisms (B-III).Consider adding fluconazole if there is a high risk of infection with Candida spp (enteric drainage, vascular thrombosis, pancreatitis after reperfusion) (C-III) | Vancomycin plus gentamicin (B-III) | Single dose (A-II)Additional intraoperative doses if surgery is prolonged or there is major blood loss (B-II) |
| Liver transplant | Recommended (A-II) | Amoxicillin-clavulanic acid (A-III).Consider adding an aminoglycoside according to local epidemiology or prior colonization with multidrug-resistant microorganisms (B-III).Individualize prophylaxis according to pre-transplant colonization or infection (B-III) | Vancomycin plus gentamicin (B-III) | ≤24h (A-II)Additional intraoperative doses if surgery is prolonged or there is significant blood loss (B-II) |
| Small bowel transplant | Recommended (A-II) | If there are no risk factors for infection with multidrug-resistant organisms: amoxicillin-clavulanic acid plus gentamicin (A-III).Consider adjusting prophylaxis to previous microbiology, add fluconazole if there are risk factors for Candida spp (B-III) | Vancomycin plus gentamicin plus metronidazole (B-III) | ≤24h (A-II).Consider additional intraoperative doses if surgery is prolonged or there is major blood loss (A-III) |
| Heart transplant | Recommended (A-II) | Cefazolin (A-II)Patients diagnosed with an infection associated with a ventricular assist device about to have a heart transplant should receive prophylaxis that includes the organism involved (B-III) | Vancomycin. Consider adding gentamicin according to local epidemiology and risk of GNB infection (B-III) | Single dose (A-II) |
| Combined heart-lung transplant | Recommended (A-II) | Cefazolin (A-II). This regimen should be modified in patients with previous cultures or positive donor cultures (B-III).Patients diagnosed with an infection associated with a ventricular assist device who are going to have a heart-lung transplant should receive prophylaxis that includes coverage against the organism involved (B-III).Prophylaxis may sometimes include antifungals with activity against Candida spp. or Aspergillus spp. in certain patients (cystic fibrosis, donors or recipients colonized pre-transplant) (B-III) | Vancomycin; consider adding gentamicin according to local epidemiology and risk of GNB infection (B-III) | ≤24h (A-II)Do not maintain prophylaxis until drainage is removed.If cystic fibrosis, treatment should be for at least 7 days, with coverage against pre-transplant organisms isolated (B-II) |
Clean surgery, duration <2h, no prosthetic material, age <65 years, no comorbidities or obesity, no transfusion, no active infection at a remote site, a possible SSI does not imply severity.
Advanced age, anatomic abnormalities of the urinary tract, poor nutritional status, smoking, chronic corticosteroid use, immunodeficiency, external catheters, colonized endogenous or exogenous material, distant infection, prolonged hospitalization. MRSA: methicillin-resistant Staphylococcus aureus. GNB: gram-negative bacilli. SSI: surgical site infection. THP: total hip prosthesis. TKP: total knee prosthesis. UTI: urinary tract infection. ESBL: extended-spectrum beta-lactamases.
There are various general criteria when routine use of AP may be dispensed with (Table 6 of the original document). It is not necessary to give AP before clean, non-implant surgery, with little tissue attrition, where duration of surgery is under 2h, since the rate of infection should be below 3%. Prophylaxis is indicated for placement of a prosthesis or intravascular implant and when the infectious complications would be very serious for the patient (endophthalmitis, infected hernia mesh or vascular access device (VAD)).
Plastic and dermatological surgeryAP is not recommended for clean surgery not involving implants. It is recommended if the operation includes an implant or there are risk factors, see Table 6 of the original document.
Hernia surgery and repairThere is controversy about prophylaxis in hernia. Given the difficulty of predicting some of the risk factors for SSI in the preoperative period, prophylaxis is recommended in herniorrhaphy, open inguinal hernioplasty and other abdominal hernioplasty (by inferring from the evidence in inguinal hernia).
Breast surgery and breast cancer surgeryProphylaxis is effective in breast cancer surgery without reconstruction, in immediate reconstruction, breast reduction surgery and in implants for cosmetic purposes.
Cardiac and vascular surgeryAortocoronary bypass and valve replacement. In procedures of this kind, AP is effective for reducing the associated rate of infection. First- and second-generation cephalosporins are the antimicrobials mainly used. There is no clear evidence to support the use of a glycopeptide as prophylaxis in institutions with a high prevalence of MRSA. A single dose of antibiotic appears to be sufficient if serum levels are maintained throughout the procedure.
There is no evidence on AP before transcatheter aortic valve implantation (TAVI), although administration of a single dose of antibiotic before the procedure seems reasonable.
Insertion of pacemakers and defibrillators. AP has been shown to be effective in various studies and a meta-analysis.
Implantation of central vascular access catheter. Prophylaxis is not recommended before insertion of a central vascular catheter; antimicrobial lock solutions are not routinely recommended before inserting or manipulating an intravascular catheter.
Peripheral vascular surgery (percutaneous and open). AP is recommended for procedures involving placement of prosthetic material and high-risk procedures such as aneurysm repair, thromboendarterectomy or venous bypasses. AP does not appear to be of benefit in brachiocephalic procedures (carotid endarterectomy, brachial artery repair) not involving implantation of prosthetic material. The risk factors associated with stent placement include prolonged duration (>2h), reoperations, stents in the lower limbs, hematoma, patients with other intravascular devices and immunosuppressed patients.
Ophthalmic surgeryIntracameral antibiotic administration is recommended at the conclusion of cataract surgery, and glaucoma/corneal graft surgery. Intravitreal AP is recommended for penetrating eye injuries. AP is also indicated in lacrimal surgery.
Neurosurgical proceduresCraniotomy. AP reduces the incidence of meningitis and SSI after craniotomy.
Placement of ventriculo-peritoneal shunt (VPS) or ventriculo-auricular shunt (VAS) and external ventricular drainage (EVD). AP is recommended for the placement of VPS, VAS and EVD.
Transsphenoidal or pharyngeal surgery. Ultra-short AP seems to be safe, effective and economical for the prevention of meningitis after transsphenoidal surgery.
Head and neck surgeryClean surgery: salivary gland surgery, thyroidectomy, parathyroidectomy, lymphadenectomy not requiring incision of the pharyngeal/laryngeal or digestive mucosa. Administration of AP does not reduce SSI in patients undergoing clean head and neck surgery. When it is accompanied by cervical lymph node dissection, the extensive exposure of tissue may increase the risk of infection, so that prophylaxis may be considered in patients with extensive lymphadenectomy or cervical surgery with multivisceral resection.
Clean contaminated surgery: amygdalectomy, adenoidectomy, laryngectomy, tracheotomy and any other surgery involving incision of the pharyngeal/laryngeal mucosa. AP is recommended in clean head and neck surgery, except in the case of adenoidectomy and amygdalectomy. It is also advisable for head and neck cancer surgery in patients with neoplasms, where very high rates of wound infection have been observed.
Sinus and middle ear surgery. AP is not recommended for endoscopic sinus surgery or clean otologic surgery. Topical application of antibiotics is advisable after tympanoplasty, and AP in clean-contaminated and contaminated surgery. AP in cochlear implant surgery is controversial and is indicated by inferring from clean surgery with implant placement
Maxillo-facial surgery. The benefit of AP has not been shown in septoplasty or simple rhinoplasty despite the fact that these are clean-contaminated procedures. It is recommended in complex rhinoplasty (revision, prosthesis).
Maxillofacial fractures. There is limited Information about the impact of prophylaxis in surgery for maxillofacial fractures, although AP is generally recommended, especially for mandibular fractures requiring open reduction.
Dental procedures. AP is not recommended for tooth extractions, oral implants and endodontic procedures except when there are risk factors (age, surgery or previous infection, tobacco use, systemic disease and traumatic tooth extraction). AP is recommended for intraoral bone grafting procedures.
Orthopedic and trauma surgeryOrthopedic and trauma procedures are mostly classified as clean surgery, often accompanied by implants. Even though the incidence of SSI is low, complications in this area can be catastrophic and AP is recommended in most procedures. AP is not recommended either in patients undergoing clean orthopedic surgery without instrumentation, including arthroscopically assisted ligamentoplasty, or for the removal of orthopedic implants.
Closed fracture reduction with osteosynthesis material. Patients with hip fractures are normally elderly, with comorbidities, and often institutionalized. MRSA or polymicrobial infections with the involvement of gram-negative bacilli are often detected in these patients. Antibiotic prophylaxis is recommended for closed fracture reduction with osteosynthesis material, with coverage against MRSA if there are risk factors.
Open fracture surgery. AP should be administered as soon as possible after the time of fracture. For Grade I and Grade II fractures in the Gustilo classification, it is recommended to maintain it for 24h following debridement, and for Grade IIIA fractures, for a maximum of 72h or until soft tissue closure.
Arthroplasty (THR, TKR, tumor megaprosthesis, primary and revision). Single-dose AP has been shown to be beneficial. There is an increased risk of infection, and of infections caused by resistant organisms, in revision arthroplasty performed because of an infected prosthesis, so that broad-spectrum AP coverage of organism that caused the initial infection is recommended.
Laminectomy and discectomy with/without instrumentation. AP is recommended.
Limb amputations. AP is recommended for at least 24h after lower limb amputation.
Thoracic surgeryMajor thoracic and minimally invasive surgery. AP is recommended for both major thoracic surgery and minimally invasive surgery (video-assisted thoracoscopy, mediastinoscopy).
Thoracic tube placement, penetrating chest trauma. AP is recommended for penetrating chest trauma but not for elective thoracic tube placement.
Esophageal, gastric or duodenal surgeryWith rupture of the mucosa (esophagectomy, gastrectomy, cephalic duodenopancreatectomy). AP is recommended.
Without rupture of the mucosa (gastroesophageal reflux surgery, achalasia, vagotomy). AP is only recommended in high-risk patients (morbid obesity, intestinal obstruction, hypochlorhydria, gastrointestinal bleeding, tumor, perforation, immunosuppression or ASA class 3 or higher).
Implantation of PEG (percutaneous endoscopic gastrostomy). AP is recommended.
Bariatric surgery. By extrapolation from similar procedures, AP is recommended, adjusted for weight.
Small bowel surgeryAs it is clean-contaminated surgery, AP is recommended by inferring from evidence about other sites, particularly colorectal.
SplenectomyAP is not indicated for non-traumatic splenectomy. For traumatic or elective splenectomy, AP is indicated in high-risk patients (immunosuppressed or receiving immunosuppressive treatment, the elderly with debilitating diseases, surgery >120min or elevated blood loss).
Penetrating abdominal traumaPro-operative AP is indicated with coverage against aerobes and anaerobes, maintaining it for no more than 24h if there is hollow viscus injury. In cases of hemorrhagic shock, the dose should be 2–3 times greater and repeated after transfusion of every 10 units of blood.
AppendicectomyAP is recommended in uncomplicated appendicectomy. Complicated appendicectomy requires antibiotic treatment (abscess, plastron appendicitis, diffuse peritonitis or perforation).
Colorectal surgeryElective colorectal surgery is clean-contaminated and has the highest rates of SSI in gastrointestinal surgery. Elective AP reduces the rate of SSI. Mechanical bowel preparation associated with oral antibiotic prophylaxis is recommended in elective colorectal surgery.
Hepatobiliary and pancreatic surgeryCholecystectomy and biliary surgery. AP is not recommended in low-risk patients undergoing laparoscopic cholecystectomy, but should be considered in open cholecystectomy and all high-risk situations: emergency intervention, immunocompromised patients, diabetes, pregnancy, age >70 years, ASA score ≥3, episode of biliary colic within the 30 days prior to the procedure, jaundice, choledocholithiasis, cholangitis, previous biliary surgery, acute cholecystitis in the preceding 6 months, lithiasic pancreatitis, and bile duct prosthesis and antibiotic therapy in the preceding month.
Hepatic surgery. AP is not recommended in simple hepatectomy but is recommended in major hepatectomy (including extrahepatic bile duct resection). When there is a previous bile culture, it is advisable to adjust AP to the results.
Pancreatic surgery. AP is indicated by inferring from biliary tract surgery. When there are previous bile cultures, adjust antibiotic therapy to the drug susceptibility patterns of the pathogens isolated.
Advanced peritoneal surgery-peritonectomyThere are no prospective studies. Nevertheless, given the radical approach to peritoneal carcinomatosis, administration of AP seems to be recommended adapted to the procedure being performed (splenectomy, pancreatectomy, cholecystectomy, appendicectomy, lymphadenectomy and hollow viscus, gastrointestinal or colorectal resections).
Urological surgeryUrological surgery, whether open or laparoscopic, is classified as clean-contaminated and calls for AP. Simple cystoscopy, ambulatory endourological surgery and extracorporeal shock wave lithotripsy are invasive procedures which do not require prophylaxis, except where there are risk factors that affect the response to infection: advanced age, anatomic anomalies of the urinary tract, nutritional deficiencies, smoking, chronic use of corticosteroids, immunodeficiencies, external catheters, colonized endogenous or exogenous material, infection at a distant site or prolonged hospitalization. In open or laparoscopic urological procedures such as nephrectomy, the basis of the recommendation for prophylaxis is the general recommendation according to degree of contamination. When AP is indicated, a preoperative dose prevents infection and the type of antibiotic should be adjusted to local susceptibility patterns and programs for optimization of antimicrobial use in the center.
Transurethral prostate resection. Randomized studies advise administration of AP.
Transurethral resection of bladder tumor. AP is not recommended unless there are risk factors or very large tumors.
Ureteroscopic stone removal and extracorporeal shock-wave lithotripsy. It is suggested that AP be restricted to high-risk patients in uncomplicated ureteral lithiasis and to complicated or impacted ureteral lithiasis.
Open or laparoscopic nephrectomy. This is clean surgery and AP is not indicated except in high-risk patients.
Percutaneous nephrolithotomy. Percutaneous nephrolithotomy with sterile urine is clean-contaminated surgery and requires AP.
Simple prostatectomy (abdominal or laparoscopic) This is clean-contaminated surgery without intestinal resection and AP is indicated.
Radical cystectomy with entry into the intestinal tract. Urinary diversions. This is clean-contaminated surgery with intestinal resection and a high rate of postsurgical wound infection. AP is therefore recommended.
Transrectal prostate biopsy. Two meta-analyses have shown that single-dose AP is beneficial.
Gynecological surgeryCesarean. AP is recommended for urgent and elective cesareans. Various studies, meta-analyses and the most recent guidelines all support administration before incision.
Hysterectomy. Vaginal and abdominal hysterectomies are considered clean-contaminated and require AP.
Adnexectomy and tubal ligation. These are considered clean surgeries with a low risk of infection that do not require AP.
Induced abortion or puerperal curettage. AP is recommended in surgical abortion during the first and second trimesters or for puerperal curettage. It is not recommended in medical abortion.
Postpartum vaginal tear repair. Administration of AP is recommended for third- or fourth-degree postpartum vaginal tears (affecting the anus and rectum, respectively).
TransplantsKidney, pancreas, liver, small bowel, heart and lung transplants are associated with high rates of SSI, sometimes with graft loss. For this reason, AP is recommended. No high-quality comparative studies have evaluated the efficacy or best course of antibiotics. Recommendations are based on observational studies and on inference from other surgeries, adapted to the previous microbiology.
ConclusionsAntibiotic prophylaxis is one of the principal measures for prevention of SSI. Its objective is to achieve high concentrations of antibiotic in the relevant tissue before incision and maintain them throughout the procedure. In general, it is recommended when there is a very high likelihood of post-operative infection or when the consequences are potentially serious. It includes, at the very least, surgeries classed as clean-contaminated, contaminated, and clean surgery with prosthetic material implantation.
The antibiotics selected must be active against the organisms most frequently isolated in each type of surgical procedure and will usually be first- and second- generation cephalosporins. The drugs should be administered intravenously within the 120-minute interval prior to surgical incision, at maximum therapeutic doses, and modified in obese patients.
For most procedures, a single preoperative dose is recommended and should never be continued beyond the first 24h after surgery. Intraoperative redosing is more important than administration of a post-operative dose when the wound is already closed and is indicated when the surgical procedure is more than twice the half-life of the antibiotic.
Any protocol for antibiotic prophylaxis should include recording compliance with its guidelines, an analysis of its results, and feedback of those results to members of the surgical teams.
FinancingThe present study was sponsored by the SEIMC and has not received specific funding from agencies in the public or private sectors or from other non-profit organizations.
Conflict of interestsThe authors declare that they have no conflicts of interest related to this manuscript.
The authors would like to thank Antonio Gutierrez-Lizarraga for his assistance with the bibliographic support of this document, and to the SEIMC and AEC for entrusting us with the preparation of the manuscript.
The complete consensus statement is available as Appendix in Supplementary Material.
This document is published simultaneously in the journal: Cirugía Española. 2020: https://doi.org/XXXXXX with the consent of the authors and editors.