Infections caused by multidrug resistant Gram-negative bacteria are becoming a worldwide problem due to their increasing incidence and associated high mortality. Carbapenem-resistant bacteria such as Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii are the most important in clinical practice. The objective of these guidelines is to update the recommendations for the diagnosis and treatment of infections caused by these multidrug resistant bacteria. Although ‘old’ antibiotics such as aminoglycosides, colistin, or tigecycline are frequently used for therapy of these bacteria, the ‘new’ beta-lactams such as ceftazidime–avibactam, ceftolozane–tazobactam, meropenem–vaborbactam, imipenem–cilastatin–relebactam or cefiderocol are progressively becoming the first-line therapy for most of these microorganisms. The Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica) designated a panel of experts in the field to provide evidence-based recommendations in response to common clinical questions. This document is primarily focused on microbiological diagnosis, clinical management, and targeted antimicrobial therapy of these infections, with special attention to defining the role of the new antimicrobials in the treatment of these bacteria.
Las infecciones causadas por bacterias gramnegativas multirresistentes se han convertido en un problema mundial debido a su creciente incidencia y alta mortalidad asociada. Las bacterias resistentes a carbapenémicos como Klebsiella pneumoniae, Pseudomonas aeruginosa y Acinetobacter baumannii son las más importantes en la práctica clínica. El objetivo de este documento de consenso es actualizar las recomendaciones sobre diagnóstico y tratamiento de las infecciones causadas por estas bacterias multirresistentes. Aunque los antibióticos ‘antiguos’ como aminoglucósidos, colistina o tigeciclina se utilizan con frecuencia en el tratamiento de estas bacterias, los ‘nuevos’ betalactámicos como ceftazidima-avibactam, ceftolozano-tazobactam, meropenem-vaborbactam, imipenem-cilastatina-relebactam o cefiderocol se están convirtiendo de forma progresiva en el tratamiento de primera elección para la mayoría de estos microorganismos. La Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica ha designado un grupo de expertos en la materia para elaborar una guía de recomendaciones basadas en la evidencia sobre las cuestiones clínicas más habituales. Este documento está principalmente centrado en el diagnóstico microbiológico, el manejo clínico y el tratamiento dirigido de estas infecciones, con especial referencia a definir el papel de los nuevos antimicrobianos en el tratamiento de estas bacterias.
Carbapenem resistance in Gram-negative bacteria (GNB) has become a worldwide problem. On the global priority list of antibiotic-resistant bacteria published by the World Health Organization in 2017, three of the four microorganisms designated as being of critical priority for research and development of new antibiotics are carbapenem-resistant pathogens, including carbapenem-resistant Enterobacterales (CRE) or carbapenemase-producing Enterobacterales (CPE), carbapenem-resistant Pseudomonas aeruginosa (CR-PA), and Acinetobacter baumannii (CR-AB). These microorganisms are the most important carbapenem-resistant GNB (CR-GNB) in clinical practice due to the increasing incidence of these bacteria worldwide in recent years, the lack of alternative antimicrobials for therapy, and the high mortality rates associated with these infections.
Justification and aimsIn 2015, the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [SEIMC]) published a Clinical Guideline on the management of invasive infections due to multidrug-resistant Enterobacterales, including CRE. In recent years, there has been an increasing spread of CRE, as well as other CR-GNB. The clinical importance of infections caused by these bacteria has led to the recent publication of clinical guidelines for the management of these multidrug-resistant bacteria. In addition, a number of new antimicrobials with activity against these organisms have been developed and introduced into clinical practice with promising results, modifying the current therapy of infections caused by CR-GNB.
In the present consensus document, we review the diagnosis and treatment of the most frequent infections caused by CR-GNB, especially isolates with a high level of resistance, such as multidrug-resistant, extensively drug-resistant, pandrug-resistant and those included in the new definition of ‘difficult-to-treat’ resistant isolates, focusing on the role of the new antimicrobials for the therapy of infections caused by these bacteria. The treatment recommendations assume that the causative organism has been identified and in vitro activity of antimicrobials has been demonstrated.
Recommendations will address the most severe infections caused by these bacteria, including invasive infections such as bloodstream infections, hospital-acquired pneumonia and ventilator-associated pneumonia (VAP), complicated intra-abdominal infections, pyelonephritis, and complicated urinary tract infections (UTI). Recommendations for less severe infections such as surgical site infections, skin and soft tissue infections, and non-complicated UTI will be briefly reviewed when considered appropriated. These guidelines have been developed for specialists in infectious diseases, critical care, internal medicine, clinical microbiologists, and primary care physicians, as well as all other health professionals responsible for the management of patients with resistant GNB infections. The complete document is available online.
MethodologySEIMC nominated two coordinators for this project (VP and PRG, an infectious diseases specialist and a clinical microbiology specialist, respectively). The coordinators selected the rest of the members of the panel of experts, which included infectious diseases specialists, clinical microbiologists, paediatricians, and a pharmacologist. The coordinators selected a set of questions designed to form the basis of the document. Answers include a brief summary of the evidence that supports the recommendations. The recommendations are based on a systematic critical review of the literature including, when necessary, the opinion of expert members of SEIMC. The criteria used to evaluate the strength of the recommendations and the quality of the evidence are summarized in Table 1. The scientific committees of the SEIMC approved the proposal.
Strength of recommendation and quality of evidence.
| Strength of recommendation | |
| A | Strongly supports a recommendation for use |
| B | Moderately supports a recommendation for use |
| C | Marginally supports a recommendation for use |
| Quality of evidence | |
| I | Evidence from at least one randomized controlled trial supporting the recommendation being made |
| II | Evidence from at least one well-designed clinical trial without randomization, cohort study or case-controlled study |
| III | Evidence from expert opinion based on clinical experience or descriptive cases |
The present document was written following the SEIMC guidelines for consensus documents (www.seimc.org), as well as the recommendations of the AGREE collaboration (www.agreecollaboration.org) for evaluating the methodological quality of clinical practice guidelines. The PubMed search engine (http://www.ncbi.nlm.nih.gov/pubmed) was used to perform a literature search of the MEDLINE database for relevant scientific publications. No specific period of inclusion was defined, although authors were instructed to inform mainly on the most recent evidence in the literature. The complete text has been discussed and approved by all authors. Before final publication, the manuscript was made available online for all SEIMC members to read and to make comments and suggestions. Possible conflicts of interest for all members of the panel of experts are listed at the end of the document.
DefinitionsThe definitions of multidrug-resistant (MDR), extensively drug-resistant (XDR) and pandrug-resistant (PDR) bacteria were established according to the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention consensus criteria. MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories, XDR was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remaining susceptible to only one or two categories) and PDR was defined as non-susceptibility to all agents in all antimicrobial categories.
In 2018, the concept of “difficult-to-treat” resistance (DTR) was proposed to define the resistance of GNB to first-line agents for therapy (all beta-lactam categories, including carbapenems, and quinolones). DTR is used in these guidelines to define P. aeruginosa isolates that are resistant to all of the following antimicrobials: piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem, ciprofloxacin, and levofloxacin.
RecommendationsMicrobiological diagnosis of carbapenem-resistant Gram-negative bacteriaHow do the new EUCAST definitions of S, I, R affect the susceptibility information in carbapenem-resistant GNB?
- 1.
The previous EUCAST intermediate (I) category is now interpreted as ‘susceptible, increased exposure’, which occurs when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or due to the increased concentration at the site of infection (A-III).
- 2.
Some breakpoints have been modified to adapt them to the new “I” definition. These new breakpoints have special relevance for the wild type Pseudomonas spp. population as they are now categorized as “I” for several relevant antimicrobials (piperacillin-tazobactam, ceftazidime, cefepime, aztreonam, imipenem, ciprofloxacin, and levofloxacin) (A-III).
- 1.
Microbiology laboratories must be able to distinguish carbapenemase-producing from carbapenem-resistant organisms with mechanisms of resistance other than carbapenemases due to their different epidemic potential. Carbapenemase detection is crucial from a clinical, public health and infection control point of view (A-III).
- 2.
The epidemiology of carbapenemase-producing organisms reflects important geographical variations in terms of prevalence and carbapenemase type distribution. Microbiology laboratories should also discriminate the type of carbapenemases. This is necessary to select the most appropriate antimicrobial treatment, especially considering the new antimicrobials targeting specific enzymes (A-I).
- 1.
Carbapenem MICs in CPE isolates may be below the clinical breakpoints. Screening cut-off values defined by EUCAST have been recommended for the accurate detection of CPE (A-III).
- 2.
The clinical breakpoint for meropenem susceptibility offers the best compromise between sensitivity and specificity. The breakpoint for ertapenem, on the other hand, is the most sensitive, but has low specificity since isolates with ESBL and/or AmpC beta-lactamases, in combination with other resistance mechanisms, may be resistant (A-III).
- 1.
The initial suspicion of a CPE must always be based on the antimicrobial susceptibility testing assay or when bacterial growth is detected on selective media containing a carbapenem (A-III).
- 2.
When reduced susceptibility to carbapenems is detected, phenotypic or genotypic methods for the screening of carbapenemases should be performed to confirm the production of a carbapenemase, or at least of the most prevalent enzymes (A-III).
- 3.
When choosing the detection/confirmation method to be used, cost, time to results, test performance (accuracy), and the information provided by the test should be considered (A-III).
Which methods should be used to detect carbapenemase enzymes?
- 1.
The identification of acquired carbapenemases in P. aeruginosa and A. baumannii is of great importance in order to select the best antimicrobial treatment, to gain knowledge of the local epidemiology, and to design appropriate infection control measures (A-III).
- 2.
For P. aruginosa and A. baumannii, specific recommendations for the phenotypic detection of carbapenemases have been made, however, no test seems to be specific enough to be used as a stand-alone method without genetic confirmation (B-III).
- 1.
Colistin testing for Enterobacterales, P. aeruginosa and Acinetobacter spp. must be done by the ISO-standard broth microdilution method (UNE-EN ISO 20776-1:2007) with cation-adjusted Mueller-Hinton broth without additives included in the testing process (no polysorbate-80 or other surfactants), in trays of plain polystyrene and using sulphate salts of colistin (B-III).
- 2.
Susceptibility testing by other methods, including agar dilution, disk diffusion and gradient diffusion, cannot be recommended (A-III).
- 1.
Agar dilution is the reference method for testing fosfomycin. MICs must be determined in the presence of glucose-6-phosphate (25mg/L) in the medium (A-III).
- 2.
No MIC and zone diameter breakpoints have been defined for fosfomycin and P. aeruginosa but infections by wild-type isolates can be treated with this drug in combination with other agents. Wild-type isolates are identified by ECOFF values (i.e. MIC 128mg/L or zone diameter 12mm using the 200μg disks supplemented with 50μg glucose-6-phosphate) (B-III).
What other antibiotics could be reported in the antibiogram?
- 1.
TMP-SMX EUCAST breakpoints are currently available. Susceptibility test results for agents other than TMP-SMX should be treated with caution, as there are no data to support an association between susceptibility testing results and clinical outcome in S. maltophilia infections (A-III).
- 2.
Susceptibility testing for TMP-SMX is more reproducible than testing for other agents and can be performed using diffusion or dilution methods (B-III).
- 3.
Despite limited data supporting an association between susceptibility testing results and clinical outcome for antimicrobial agents other than TMP-SMX, CLSI has set clinical breakpoints for ticarcillin-clavulanate, ceftazidime, cefiderocol, minocycline, levofloxacin and chloramphenicol (B-III).
- 4.
The Spanish Antibiogram Committee (COESANT) has recommended the inclusion of TMP-SMX, minocycline and levofloxacin for in vitro susceptibility testing when commercial panels of automated systems are used (A-III).
- 1.
For the detection of CR-GNB carriage in asymptomatic patients, specifically of CPE given the epidemiological implications, surveillance culture should be performed, guided by local epidemiology and patient risk assessment (A-III).
- 2.
Populations to be considered for such surveillance include patients with previous CR-GNB colonization, contacts of CR-GNB colonized or infected patients, and patients with a history of recent hospitalization in endemic CR-GNB settings (A-III).
- 3.
Rectal swabs are considered the most suitable specimens to detect asymptomatic colonization, especially if due to CPE (A-III).
- 4.
In selected cases, molecular tests performed directly from rectal swab samples may be used to detect carbapenemase enzymes (B-III).
- 5.
The procedure strategy for the screening of CR-GNB has to be selected based on local epidemiology, target population, laboratory expertise and equipment, as well as economic resources. In most settings, the combination of phenotypic and genotyping testing will result in the best performance (A-III).
- 1.
The presence of CR-GNB in blood, cerebrospinal fluid or other samples obtained from usually sterile sites (i.e. those obtained in the operating room) should be considered proof of infection unless contamination of the cultures is suspected (A-III).
- 2.
The presence of CR-GNB in clinical samples from non-sterile sites such as urine, respiratory tract or cutaneous ulcers can represent either infection or colonization (A-III).
- 3.
The differentiation between infection and colonization mainly depends on the absence or presence of clinical signs or symptoms of infection. When in doubt, laboratory and imaging testing can help to differentiate between colonization and infection (A-III).
- 1.
Empirical therapy for CR-GNB should be considered in patients with suspected, yet unconfirmed, severe CR-GNB infections (sepsis, septic shock) when the risk of CR-GNB is high enough, or in patients known to be colonized by CR-GNB who develop an infection potentially caused by a GNB. In severely immunocompromised patients (i.e. neutropenic patients and transplant recipients) with systemic, although less severe infections, the same principle applies. Validated CR-GNB infection prognostic scores can assist with therapeutic decision making (A-II).
- 2.
The overall rate of carbapemem-resistance among the most epidemiologically relevant GNB at the institution/ward can be used as a surrogate of the risk of CR-GNB. A 10–20% rate of carbapenem-resistance among GNB could be considered as a reasonable threshold to start antimicrobial therapy for CR-GNB in these circumstances (B-III).
- 1.
Source control (debridement of infected tissues, drainage of infected collections and removal of infected devices) as adjunctive therapy to antibiotic treatment should be attempted as soon as reasonably possible, as it is one of the strongest predictors of favorable outcomes among patients with CR-GNB infections (A-II).
- 2.
Salvage therapy of long-term CR-GNB infected intravascular catheters with antimicrobial lock solutions should be avoided (A-II).
- 1.
Several dosing strategies can contribute to pharmacokinetic/pharmacodynamic targets that may be associated with better clinical and microbiological outcomes:
- -
Extended and continuous infusion for beta-lactams (A-I).
- -
Loading dose for colistin, aminoglycosides, tigecycline and fosfomycin (A-II).
- -
Extended interval dosing for aminoglycosides (A-II).
- 1.
Antibiotic concentrations should be monitored to guide dosing in patients with CR-GNB infections treated with aminoglycosides in order to ensure safety and efficacy (A-II).
- 2.
Carbapenem drug monitoring may improve clinical outcomes in patients with CR-GNB infections and should be considered to guide therapeutic decisions, especially in critically ill patients, the morbidly obese and patients with renal impairment or difficult to treat infections (C-II).
- 3.
Antimicrobial drug monitoring in patients with CR-GNB infections may also be beneficial for the new beta-lactams but there is insufficient information to make a formal recommendation (C-III).
- 4.
Antimicrobial drug monitoring should be performed when possible for both colistin and polymyxin B in patients with CR-GNB infections (B-II).
- 5.
There is insufficient evidence to support the recommendation to monitor other drugs such as tigecycline, aztreonam or fosfomycin in patients with CR-GNB infections (C-III).
- 1.
Aerosolized colistin as adjunctive therapy to intravenous (IV) colistin may be clinically beneficial for some patients with VAP caused by MDR-GNB, including CR-GNB, as increased clinical response rates have been observed in several studies. Other aerosolized antibiotics, such as aminoglycosides, may have a comparable effect (B-I).
- 2.
Patients with VAP receiving aerosolized antibiotics may have an increased risk of respiratory complications, which could preclude their use in patients with unstable respiratory disease (A-III).
- 1.
Intestinal decolonization therapy with non-absorbable antibiotics should not be routinely used in patients colonized by CR-GNB, mainly CPE. Instead, it should be saved for those patients at the highest risk of CR-GNB infection (neutropenic patients or those patients undergoing gastrointestinal invasive procedures such as high-risk surgery, including transplantation) (C-II).
- 2.
Although fecal microbiota transplantation may eradicate CR-GNB (mainly CPE) from the gastrointestinal tract of some patients, it cannot be recommended currently as a routine procedure in patients colonized by CR-GNB (C-II).
Since 2019, EUCAST breakpoints are used to classify the results of antibiotic susceptibility testing into three new categories: “S” (Susceptible, standard dosing regimen) when there is a high likelihood of therapeutic success using a standard dosing regimen of the agent; “I” (Susceptible, increased exposure) when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or due to its concentration at the site of infection; and “R” (Resistant) when there is a high likelihood of therapeutic failure even when there is increased exposure.
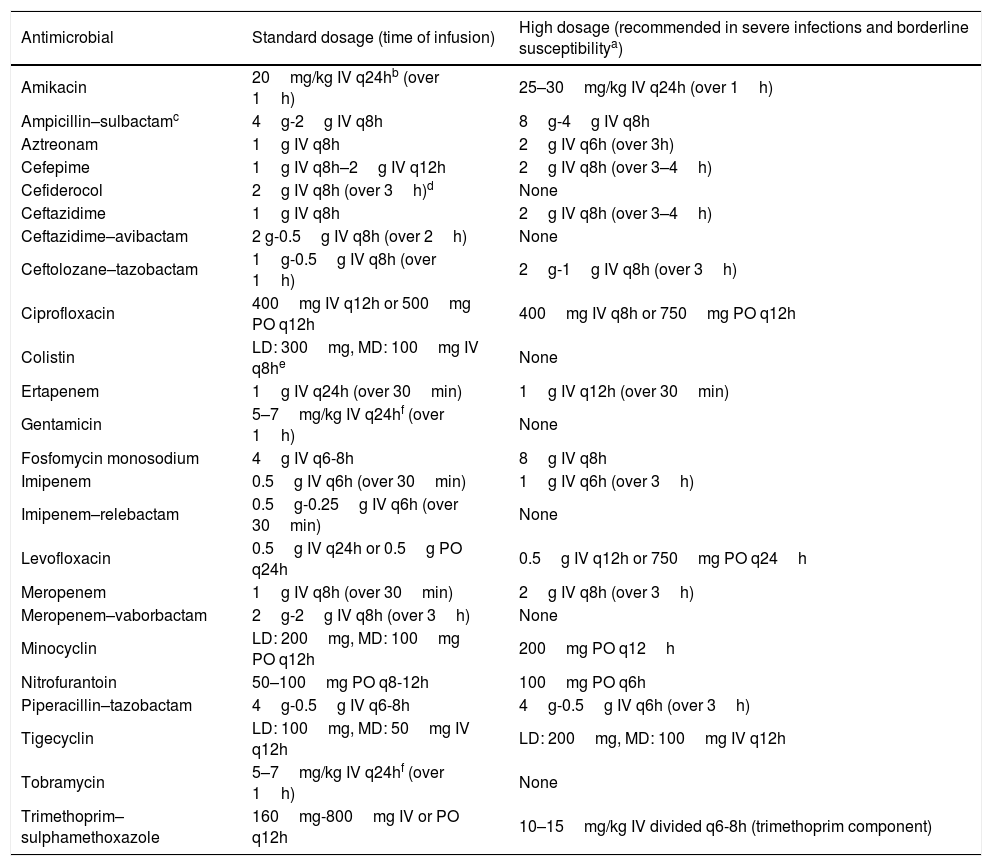
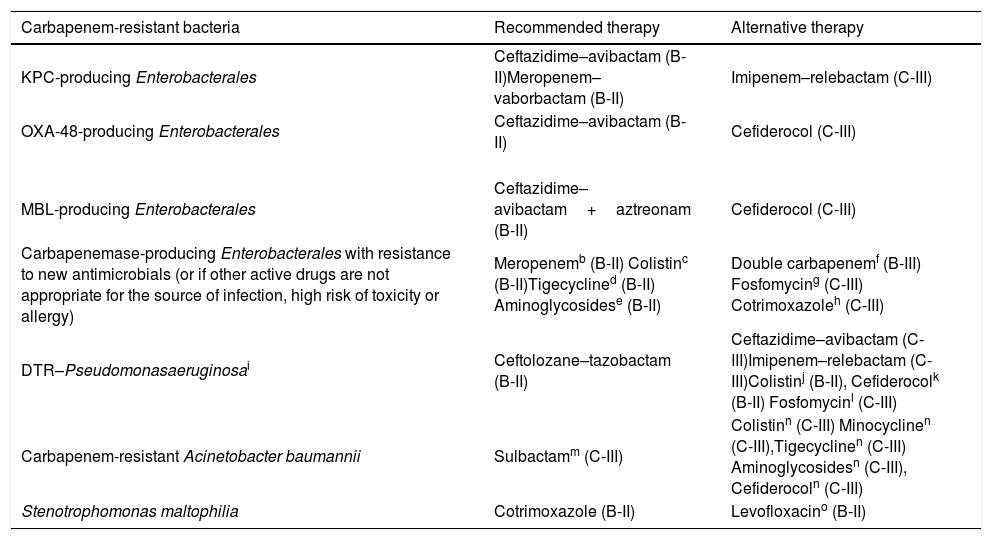
Table 2 shows the standard doses of the most frequently used antibiotics for therapy of GNB infections, and those recommended for severe infections or infections caused by strains with borderline susceptibility (such as ‘susceptible, increased exposure’), assuming normal renal function. The recommendations for the treatment of infections caused by CR-GNB are summarized in Table 3.
Recommended dosing for the most frequently used antimicrobials against Gram-negative bacteria in adult patients.
| Antimicrobial | Standard dosage (time of infusion) | High dosage (recommended in severe infections and borderline susceptibilitya) |
|---|---|---|
| Amikacin | 20mg/kg IV q24hb (over 1h) | 25–30mg/kg IV q24h (over 1h) |
| Ampicillin–sulbactamc | 4g-2g IV q8h | 8g-4g IV q8h |
| Aztreonam | 1g IV q8h | 2g IV q6h (over 3h) |
| Cefepime | 1g IV q8h–2g IV q12h | 2g IV q8h (over 3–4h) |
| Cefiderocol | 2g IV q8h (over 3h)d | None |
| Ceftazidime | 1g IV q8h | 2g IV q8h (over 3–4h) |
| Ceftazidime–avibactam | 2 g-0.5g IV q8h (over 2h) | None |
| Ceftolozane–tazobactam | 1g-0.5g IV q8h (over 1h) | 2g-1g IV q8h (over 3h) |
| Ciprofloxacin | 400mg IV q12h or 500mg PO q12h | 400mg IV q8h or 750mg PO q12h |
| Colistin | LD: 300mg, MD: 100mg IV q8he | None |
| Ertapenem | 1g IV q24h (over 30min) | 1g IV q12h (over 30min) |
| Gentamicin | 5–7mg/kg IV q24hf (over 1h) | None |
| Fosfomycin monosodium | 4g IV q6-8h | 8g IV q8h |
| Imipenem | 0.5g IV q6h (over 30min) | 1g IV q6h (over 3h) |
| Imipenem–relebactam | 0.5g-0.25g IV q6h (over 30min) | None |
| Levofloxacin | 0.5g IV q24h or 0.5g PO q24h | 0.5g IV q12h or 750mg PO q24h |
| Meropenem | 1g IV q8h (over 30min) | 2g IV q8h (over 3h) |
| Meropenem–vaborbactam | 2g-2g IV q8h (over 3h) | None |
| Minocyclin | LD: 200mg, MD: 100mg PO q12h | 200mg PO q12h |
| Nitrofurantoin | 50–100mg PO q8-12h | 100mg PO q6h |
| Piperacillin–tazobactam | 4g-0.5g IV q6-8h | 4g-0.5g IV q6h (over 3h) |
| Tigecyclin | LD: 100mg, MD: 50mg IV q12h | LD: 200mg, MD: 100mg IV q12h |
| Tobramycin | 5–7mg/kg IV q24hf (over 1h) | None |
| Trimethoprim–sulphamethoxazole | 160mg-800mg IV or PO q12h | 10–15mg/kg IV divided q6-8h (trimethoprim component) |
Abreviations. IV: intravenous, LD: loading dose, MD: maintenance dose, PO: oral.
Loading dose of colistin base activity: steady stage concentration target (mg/L)×2.0×ideal body weight. Maintenance dose of colistin base activity: same dose. First dose after the loading dose should be administered 12h later. The dose indicated in the table corresponds to an average steady stage concentration of 2mg/L and a patient with creatinine clearance of 70–80mL/min and ideal body weight of 75kg. Colistin base activity 300mg is equivalent to approximately 9 million units of colistin and 720mg of colistimethate sodium.
Summary of recommendations for infections caused by carbapenem-resistant Gram-negative bacteria.a
| Carbapenem-resistant bacteria | Recommended therapy | Alternative therapy |
|---|---|---|
| KPC-producing Enterobacterales | Ceftazidime–avibactam (B-II)Meropenem–vaborbactam (B-II) | Imipenem–relebactam (C-III) |
| OXA-48-producing Enterobacterales | Ceftazidime–avibactam (B-II) | Cefiderocol (C-III) |
| MBL-producing Enterobacterales | Ceftazidime–avibactam+aztreonam (B-II) | Cefiderocol (C-III) |
| Carbapenemase-producing Enterobacterales with resistance to new antimicrobials (or if other active drugs are not appropriate for the source of infection, high risk of toxicity or allergy) | Meropenemb (B-II) Colistinc (B-II)Tigecyclined (B-II) Aminoglycosidese (B-II) | Double carbapenemf (B-III) Fosfomycing (C-III) Cotrimoxazoleh (C-III) |
| DTR–Pseudomonasaeruginosai | Ceftolozane–tazobactam (B-II) | Ceftazidime–avibactam (C-III)Imipenem–relebactam (C-III)Colistinj (B-II), Cefiderocolk (B-II) Fosfomycinl (C-III) |
| Carbapenem-resistant Acinetobacter baumannii | Sulbactamm (C-III) | Colistinn (C-III) Minocyclinen (C-III),Tigecyclinen (C-III) Aminoglycosidesn (C-III), Cefiderocoln (C-III) |
| Stenotrophomonas maltophilia | Cotrimoxazole (B-II) | Levofloxacino (B-II) |
The treatment recommendations are mainly focused on severe infections assuming that the causative organism has been identified and in vitro activity of antimicrobials has been demonstrated. The standard and high doses of the antimicrobials are summarized in Table 2.
Combination therapy with a carbapenem could be used if the carbapenem MIC is ≤8mg/L. Extended-infusion, high dose meropenem is the preferred treatment in these cases.
In patients with a high mortality risk, as combination therapy with other drugs (preferably tigecycline). In patients with a low mortality risk, as targeted monotherapy for infections with a source other than the urinary tract.
In patients with a high mortality risk (high dose), as combination therapy with colistin or with aminoglycosides or fosfomycin (in case of resistance to colistin). In patients with a low mortality risk (standard dose), as targeted monotherapy for skin and soft tissue infections or intraabdominal infections.
In patients with a high mortality risk, as combination therapy with fosfomycin for UTIs. In case of resistance to all beta-lactams and colistin, as combination therapy with tigecycline. In patients with a low mortality risk, as targeted monotherapy for UTIs.
In patients with a high risk of mortality, consider fosfomycin as combination therapy for isolates with resistance to all beta-lactams and colistin.
Cotrimoxazole could be considered for mild infections such as UTI due to susceptible KPC-producing K. pneumoniae.
DTR, “difficult-to-treat” resistance. Ceftolozane–tazobactam, ceftazidime–avibactam and imipenem–relebactam are indicated for carbapenemase negative-P. aeruginosa because these drugs are inactive against MBL-producing isolates. Carbapenemase (VIM)-producing P. aeruginosa may be susceptible to aztreonam.
For invasive infections due to P. aeruginosa with resistance to new antimicrobials and other beta-lactams, colistin should be administered in combination with other antimicrobials to which the isolate was susceptible. Colistin monotherapy could be considered for urinary tract infections (C-III).
Cefiderocol should be considered an effective treatment option for UTIs (uncomplicated and complicated, including pyelonephritis) caused by DTR-P. aeruginosa.
Fosfomycin at high doses may be considered as part of a combination salvage treatment for patients with limited treatment options.
Combination therapy with at least two antimicrobials active in vitro should be considered for severe infections and in critically ill or immunosupressed patients. High-dose sulbactam in combination with additional antimicrobials (including colistin) should be considered for therapy. The current high rate of resistance limits the role of sulbactam as an alternative therapy for carbapenem-resistant A. baumannii.
Alternative combinations with other active antibiotics such as colistin, high-dose minocycline, high-dose tigecycline, aminoglycosides, or cefiderocol should be considered for sulbactam-resistant isolates. Combinations of colistin with aminoglycosides should be avoided due to increased nephrotoxicity.
- 1.
Therapy must be tailored according to the source of infection, underlying diseases and microorganism susceptibility (B-II).
- 2.
Combination antibiotic therapy is not routinely recommended for the treatment of infections caused by CPE. Combination therapy is only recommended for patients with severe infections caused by CPE when ceftazidime–avibactam, meropenem–vaborbactam or imipenem-relebactam cannot be used (B-II).
- 3.
In patients in whom these antimicrobials cannot be used, monotherapy may be sufficient for patients with a lower mortality risk (B-II).
- 1.
Combination therapy with a carbapenem could be used if the carbapenem MIC is ≤8mg/L (B-II). This combination therapy is recommended if other drugs active in vitro are not appropriate for the source of infection or if other combinations have a high risk of toxicity (B-III).
- 2.
Extended-infusion, high dose meropenem (2g every 8h) is the preferred treatment in these cases (B-II).
- 1.
Double carbapenem therapy should be considered only when there are no other reasonable options (B-III).
- 1.
Ceftazidime–avibactam monotherapy is an effective treatment option for KPC- and OXA-48 producing Enterobacterales (B-II), but is not recommended for MBL-producing isolates as these are resistant in vitro.
- 2.
KPC-producing K. pneumoniae resistant to ceftazidime–avibactam but susceptible to carbapenems can emerge during treatment of CRE infections with ceftazidime–avibactam. Use of carbapenems is not recommended in these patients until more results become available (C-III).
- 3.
Ceftazidime–avibactam should not be used indiscriminately due to the possibility of development of resistance (B-II).
- 1.
The enhanced in vitro potency of meropenem–vaborbactam against KPC producers makes this antimicrobial an excellent treatment option for KPC-producing Enterobacterales (B-II) but it is not recommended for MBL- and OXA-48-producing isolates as these are resistant in vitro.
- 2.
Although meropenem–vaborbactam seems to have a decreased potential for selection of resistance among KPC-producers compared to ceftazidime–avibactam, resistance to meropenem–vaborbactam has been described recently (B-II).
- 1.
Colistin should be used for CPE infections with resistance to new antimicrobials and other beta-lactams:
- -
As combination therapy with other drugs (preferably tigecycline) in patients with a high mortality risk. A loading dose should be used, 9 million units of colistimethate sodium, followed by 9 million units/day in 2–3 doses (B-II).
- -
As targeted monotherapy in patients with a low mortality risk and with infections with a source other than the urinary tract (if susceptibility to aminoglycosides is confirmed), in order to preserve the new antimicrobials for the most severe cases. The need to administer a loading dose is controversial (B-II).
- -
As empirical monotherapy in patients with a low risk of mortality in the setting of a nosocomial outbreak or if risk factors are present in areas with a high prevalence of CPE. The need to administer a loading dose is controversial (B-II).
- 1.
Tigecycline should be used to treat infections due to CPE in patients with a high mortality risk (200mg loading dose, then 100mg/12h):
- -
In case of resistance to new antimicrobials and other beta-lactams, in combination with colistin (not for UTIs) (B-II).
- -
In case of resistance to all beta-lactams and colistin, in combination with aminoglycosides or fosfomycin (B-III).
- 2.
Tigecycline should be used to treat infections due to CPE in patients with a low mortality risk (100mg loading dose, then 50mg/12h):
- -
As targeted monotherapy for skin and soft tissue infections or intraabdominal infections in order to preserve new antimicrobials for the most severe cases (B-II).
- 2.
Aminoglycosides should be used to treat infections due to CPE in patients with a high mortality risk:
- -
In case of resistance to new antimicrobials and other beta-lactams, in combination with fosfomycin to treat UTIs. For gentamicin and tobramycin consider dose: 5–7mg/kg/day; for amikacin consider 15–20mg/kg/day (B-II).
- -
In case of resistance to all beta-lactams and colistin, in combination with tigecycline. Consider high dose (risk of toxicity) if hospital-acquired pneumonia: for gentamicin and tobramycin consider 10–15mg/kg; for amikacin consider 25–30mg/kg (B-III).
- 3.
Aminoglycosides should be used to treat infections due to CPE in patients with a low mortality risk:
- -
As empirical monotherapy for UTIs, in the setting of a nosocomial outbreak or if risk factors are present in areas with a high prevalence of CPE and high local susceptibility rates to aminoglycosides in CPE. For gentamicin and tobramycin consider dose: 5–7mg/kg/day; for amikacin consider 15–20mg/kg/day (B-II).
- -
As targeted monotherapy for UTIs, in order to preserve new antimicrobials for the most severe cases. For gentamicin and tobramycin consider dose: 5–7mg/kg/day; for amikacin consider 15–20mg/kg/day (B-II).
- 1.
Consider fosfomycin in combination to treat infections due to CPE in patients with a high risk of mortality, in case of resistance to all beta-lactams and colistin. Dose: 4g/6h to 8g/8h (C-III).
- 1.
The combination of aztreonam (dose: 1–2g/8h) plus ceftazidime–avibactam should be considered for MBL-producing isolates (B-II).
- 2.
Imipenem-relebactam should be considered an alternative treatment option for KPC-producing Enterobacterales (C-III). It is inactive against CRE that produce OXA-48 and MBLs enzymes.
- 3.
Cefiderocol should be considered an effective treatment option for MBL-producing Enterobacterales (C-III).
- 4.
TMP-SMX should be considered for infections due to susceptible KPC-producing K. pneumoniae (C-III).
- 5.
There is no clinical experience with aztreonam or cephalosporins for the treatment of infections due to susceptible MBL- or OXA-48-producing Enterobacterales, respectively. In vitro and animal model data suggest that they may be useful; if considered, we recommend using these drugs in combination, except in complicated UTIs (C-III).
- 6.
There is no clinical experience with temocillin for the treatment of infections due to susceptible KPC-producing Enterobacterales. No recommendations can be made for these infections (unresolved issue).
Carbapenem-resistant P. aeruginosa isolates may be susceptible to alternative antimicrobials such as ceftazidime, cefepime, aztreonam, piperacillin-tazobactam or ciprofloxacin, and these drugs may be used as directed therapy if the strain is susceptible. For this reason, most recommendations of these guidelines are mainly focused on infections caused by DTR-P. aeruginosa (DTR-PA).
In what situations is combination therapy indicated for infections caused by DTR-P. aeruginosa?- 1.
Although, in general, there is no evidence for combination therapy in P. aeruginosa infections, in the case of DTR-PA the use of combination therapy in severe infections or those with a high inoculum should be considered (C-III).
- 2.
The combination of an active beta-lactam agent, such as ceftolozane–tazobactam or ceftazidime–avibactam, with an active aminoglycoside or colistin is the preferred treatment in these cases (C-III).
- 1.
As ceftolozane–tazobactam is one of the most active beta-lactams against CR-PA in vitro, it is indicated for DTR-PA infections, if the strain is susceptible (B-II).
- 2.
The standard dose of ceftolozane–tazobactam for patients with complicated UTIs and complicated intra-abdominal infections is 1g/0.5g every 8h. The recommendation for patients with pneumonia or other high-inoculum infections is a dose of 2g/1g every 8h (A-I).
- 3.
In severe infections, optimized infusions of ceftolozane–tazobactam and/or combinations with other antimicrobials should be considered (C-III).
- 1.
Ceftazidime–avibactam is a good option for the treatment of DTR-PA infections, if the strain is susceptible, although clinical experience is limited (C-III).
- 2.
Ceftazidime–avibactam could be the best option for some DTR-PA strains, such as those harboring class A carbapenemases (GES enzymes) or combinations of certain ESBLs with OprD deficiency (C-III). The recommended dose is 2g/0.5g every 8h infused intravenously over 2h (B-II).
- 1.
Imipenem-relebactam is a reasonable alternative option for the treatment of DTR-PA infections, if the strain is susceptible, although clinical experience is limited (C-III).
- 1.
For invasive infections caused by DTR-PA, colistin should be administered in combination with other antimicrobials to which the isolate was susceptible (B-II). Colistin monotherapy could be considered for UTIs (C-III).
- 2.
Inhaled colistin should be considered for therapy of VAP due to DTR-PA that is susceptible only to polymyxins (colistin or polymyxin B), in combination with IV colistin (B-II).
- 3.
For meningitis or ventriculitis caused by DTR-PA, intraventricular or intrathecal colistin could be used at a dosage of 125,000IU/day with concomitant IV colistin (C-III).
- 1.
In patients with limited treatment options, IV fosfomycin at high doses (4–6g every 6h to 8g every 8h) may be considered as part of a combination salvage treatment for susceptible isolates (fosfomycin ECOFF≤128mg/L), including at least one more active agent (C-III).
- 2.
Cefiderocol should be considered an effective treatment option for UTIs (uncomplicated and complicated, including pyelonephritis) caused by DTR-PA (B-II).
- 3.
There is insufficient evidence to recommend the use of rifampin for the treatment of DTR-PA infections (C-III).
- 1.
Combination therapy has not been found to be superior to monotherapy in improving clinical outcomes in CR-AB infections, despite it being better for microbiologic eradication in some cases (C-III). Despite this lack of evidence, combination therapy with at least two antimicrobials active in vitro should be considered for therapy of severe infections due to CR-AB, and in critically ill or immunosuppressed patients (C-III).
- 2.
High-dose sulbactam in combination with additional antimicrobials (including colistin) should be considered for therapy of CR-AB infections (C-III). Alternative combinations with other active antibiotics such as colistin, high-dose minocycline, high-dose tigecycline, or aminoglycosides should be considered for sulbactam-resistant isolates. Combinations of polymyxins with aminoglycosides should be avoided due to increased nephrotoxicity (C-III).
- 3.
Combined therapy with cefiderocol plus colistin, or triple-drug combination with colistin, high-dose meropenem and high-dose sulbactam should be considered for PDR-AB infections (C-III).
- 4.
Although monotherapy with sulbactam, polymyxins, minocycline or aminoglycosides may be adequate in patients with mild infections such as UTIs or skin and soft tissue infections, considering the adaptability of this bacterium to antimicrobials and the rapid evolution to resistance, it is highly recommended to avoid monotherapy of CR-AB infections, especially in severe cases (B-III).
- 1.
Colistin should be considered for therapy of CR-AB infections. Although the combination of colistin with other active antimicrobials is widely used in severe A. baumannii infections, there is insufficient evidence to recommend the combination of colistin with a carbapenem or rifampicin for the treatment of CR-AB infections (C-III).
- 2.
Low plasma levels and heteroresistance of colistin have raised serious concerns regarding colistin monotherapy and the rapid emergence of regrowth (B-III).
- 3.
Inhaled colistin should be considered for therapy of hospital-acquired pneumonia or VAP due to CR-AB that is susceptible only to polymyxins, in combination with IV colistin (C-III).
- 4.
For meningitis or ventriculitis caused by CR-AB, intraventricular or intrathecal colistin could be used with concomitant IV colistin (A-II).
- 1.
The available evidence suggests that sulbactam-based therapy may have a similar efficacy to alternative antimicrobial therapies for A. baumannii infections (C-III).
- 2.
High-dose sulbactam (≥6–9g per day) in combination with additional antibacterial agents (including colistin) should be considered for therapy of CR-AB infections (C-III).
- 3.
Although high-dose cefoperazone-sulbactam can improve the antimicrobial activity of tigecycline in VAP caused by XDR-AB, there is insufficient evidence to recommend this combination (C-III).
- 4.
The current high rate of resistance to sulbactam limits its role as an alternative therapy for CR-AB infections (B-III).
- 1.
Minocycline IV should be considered as an alternative for therapy of MDR-AB infections (C-III), although there is insufficient evidence to recommend its use as monotherapy (C-III).
- 2.
Although the available evidence suggests that tigecycline may have a similar efficacy to alternative antibiotics in CR-AB infections, tigecycline therapy is associated with a significantly lower microbial eradication rate (C-III).
- 3.
There is no favourable evidence to recommend the use of a tigecycline-based regimen for CR-AB infections, especially in patients with bacteremia, due to inadequate plasma concentrations (C-III).
- 4.
In comparison with monotherapy, tigecycline combination therapy is associated with a similar clinical and microbiological response, and a similar mortality rate (C-III).
- 5.
There is insufficient evidence to recommend the use of eravacycline for therapy of MDR-AB infections (C-III).
- 1.
Cefiderocol should be considered for therapy of severe infections such as pneumonia or bacteremia due to CR-AB in patients with limited therapeutic options, as part of a combination regimen (C-III).
- 1.
Although combination therapy has been associated with a higher microbiological eradication, rifampicin combined with colistin should not be routinely used for therapy of CR-AB infections (B-I).
- 1.
Combination therapy should be considered for severe infections caused by S. maltophilia in immunocompromised patients. (B-II).
- 2.
In patients with S. maltophilia resistant to TMP-SMX or in whom this drug cannot be used, combination therapy with alternative drugs should be based on the in vitro activity against the tested isolate (B-II).
- 1.
For S. maltophilia infections, IV TMP-SMX should be used at a dosage of 15mg/kg/day (TMP component), administered in 3–4 divided doses and adjusted for renal function (B-II).
- 1.
Levofloxacin monotherapy is not inferior to TMP-SMX monotherapy for treating infections caused by S. maltophilia (B-II).
- 2.
When levofloxacin or other fluoroquinolones are used for the treatment of S. maltophilia infections, the emergence of resistance during treatment should be monitored (C-II).
- 1.
Second-line agents against infections by S. maltophilia in patients with limited therapeutic options should be used considering their in vitro activity against the considered isolate (B-II).
- 2.
Susceptibility testing results of ticarcillin-clavulanate, aztreonam (alone or combined with clavulanic acid or avibactam), ceftazidime, minocycline, tigecycline, colistin, chloramphenicol and aminoglycosides against S. maltophilia should be interpreted with caution (C-III).
- 1.
Currently, CR-GNB prevalence among children in Spain is low. CR-PA and CRE produce the highest burden of disease (B-II).
- 2.
The diagnostic approach in children follows the same principles as in adults (B-II).
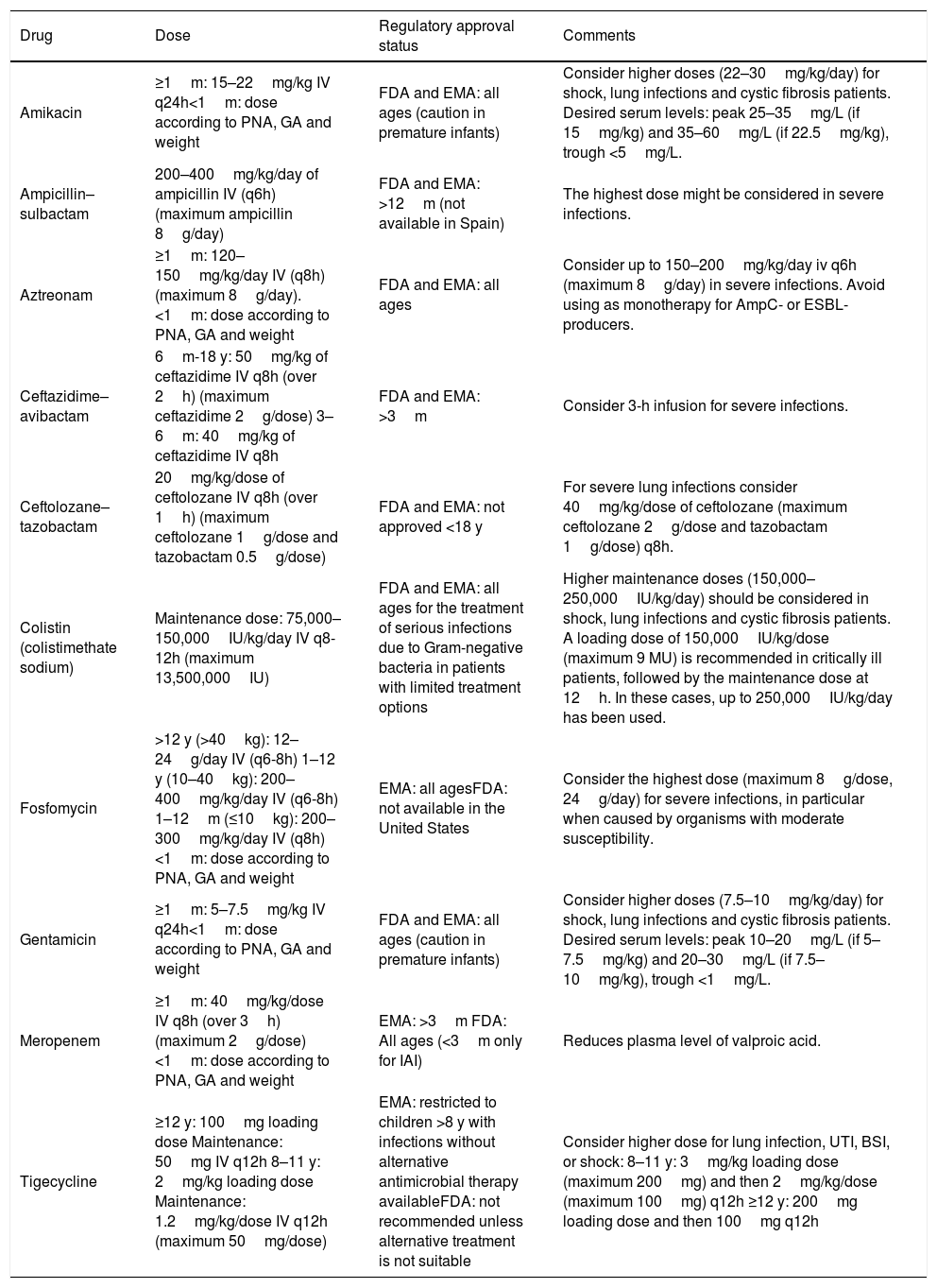
Pediatric drug dosing recommendations in children are summarized in Table 4.
- 1.
In children, high-dose extended infusion meropenem combined with a second active agent is recommended as first-line treatment of severe CR-GNB infections with a meropenem MIC ≤2mg/L (B-II). In the case of a meropenem MIC ≥4mg/L, a different non-carbapenem beta-lactam such as ceftazidime–avibactam (preferred), aztreonam, aztreonam plus ceftazidime–avibactam, ceftazidime or ceftolozane–tazobactam, should be considered initially, and high dose extended meropenem infusion combined with a second active agent as an alternative for isolates with an MIC ≤8mg/L (B-II)
- 2.
For cystitis and other mild infections, monotherapy with fosfomycin, fluoroquinolones or aminoglycosides may be considered (B-II).
Recommended dosing for the most frequently used antimicrobials against carbapenem-resistant Gram-negative bacteria in pediatric patients.
| Drug | Dose | Regulatory approval status | Comments |
|---|---|---|---|
| Amikacin | ≥1m: 15–22mg/kg IV q24h<1m: dose according to PNA, GA and weight | FDA and EMA: all ages (caution in premature infants) | Consider higher doses (22–30mg/kg/day) for shock, lung infections and cystic fibrosis patients. Desired serum levels: peak 25–35mg/L (if 15mg/kg) and 35–60mg/L (if 22.5mg/kg), trough <5mg/L. |
| Ampicillin–sulbactam | 200–400mg/kg/day of ampicillin IV (q6h) (maximum ampicillin 8g/day) | FDA and EMA: >12m (not available in Spain) | The highest dose might be considered in severe infections. |
| Aztreonam | ≥1m: 120–150mg/kg/day IV (q8h) (maximum 8g/day).<1m: dose according to PNA, GA and weight | FDA and EMA: all ages | Consider up to 150–200mg/kg/day iv q6h (maximum 8g/day) in severe infections. Avoid using as monotherapy for AmpC- or ESBL-producers. |
| Ceftazidime–avibactam | 6m-18 y: 50mg/kg of ceftazidime IV q8h (over 2h) (maximum ceftazidime 2g/dose) 3–6m: 40mg/kg of ceftazidime IV q8h | FDA and EMA: >3m | Consider 3-h infusion for severe infections. |
| Ceftolozane–tazobactam | 20mg/kg/dose of ceftolozane IV q8h (over 1h) (maximum ceftolozane 1g/dose and tazobactam 0.5g/dose) | FDA and EMA: not approved <18 y | For severe lung infections consider 40mg/kg/dose of ceftolozane (maximum ceftolozane 2g/dose and tazobactam 1g/dose) q8h. |
| Colistin (colistimethate sodium) | Maintenance dose: 75,000–150,000IU/kg/day IV q8-12h (maximum 13,500,000IU) | FDA and EMA: all ages for the treatment of serious infections due to Gram-negative bacteria in patients with limited treatment options | Higher maintenance doses (150,000–250,000IU/kg/day) should be considered in shock, lung infections and cystic fibrosis patients. A loading dose of 150,000IU/kg/dose (maximum 9 MU) is recommended in critically ill patients, followed by the maintenance dose at 12h. In these cases, up to 250,000IU/kg/day has been used. |
| Fosfomycin | >12 y (>40kg): 12–24g/day IV (q6-8h) 1–12 y (10–40kg): 200–400mg/kg/day IV (q6-8h) 1–12m (≤10kg): 200–300mg/kg/day IV (q8h) <1m: dose according to PNA, GA and weight | EMA: all agesFDA: not available in the United States | Consider the highest dose (maximum 8g/dose, 24g/day) for severe infections, in particular when caused by organisms with moderate susceptibility. |
| Gentamicin | ≥1m: 5–7.5mg/kg IV q24h<1m: dose according to PNA, GA and weight | FDA and EMA: all ages (caution in premature infants) | Consider higher doses (7.5–10mg/kg/day) for shock, lung infections and cystic fibrosis patients. Desired serum levels: peak 10–20mg/L (if 5–7.5mg/kg) and 20–30mg/L (if 7.5–10mg/kg), trough <1mg/L. |
| Meropenem | ≥1m: 40mg/kg/dose IV q8h (over 3h) (maximum 2g/dose)<1m: dose according to PNA, GA and weight | EMA: >3m FDA: All ages (<3m only for IAI) | Reduces plasma level of valproic acid. |
| Tigecycline | ≥12 y: 100mg loading dose Maintenance: 50mg IV q12h 8–11 y: 2mg/kg loading dose Maintenance: 1.2mg/kg/dose IV q12h (maximum 50mg/dose) | EMA: restricted to children >8 y with infections without alternative antimicrobial therapy availableFDA: not recommended unless alternative treatment is not suitable | Consider higher dose for lung infection, UTI, BSI, or shock: 8–11 y: 3mg/kg loading dose (maximum 200mg) and then 2mg/kg/dose (maximum 100mg) q12h ≥12 y: 200mg loading dose and then 100mg q12h |
Abbreviations. BSI: bloodstream infection, EMA: European Medicines Agency, ESBL: extended-spectrum beta-lactamase, FDA: Food and Drug Administration, GA: gestational age, IAI: intraabdominal infection, IV: intravenous, m: months, PNA: postnatal age, UTI: urinary tract infection, y: years.
- 1.
The management of carbapenem-resistant GNB infections in children with cystic fibrosis is the same as for other children, but higher doses of antimicrobials should be considered (C-II).
- 1.
Community-acquired carbapenem-resistant GNB infections are currently uncommon. While infections caused by CR-AB and CR-PA are extremely rare, CRE infections are currently uncommon but emerging in some geographic areas (A-III).
- 1.
Urinary tract infections are the most common community-acquired infections caused by CRE, while bacteremia, respiratory, and skin and soft tissue infections are reported less frequently (A-III).
- 2.
Pulmonary community-acquired infections caused by CR-PA remain very uncommon and usually appear in patients with cystic fibrosis and other chronic pulmonary diseases (A-II).
- 3.
Community-acquired pneumonia is the most frequent infection caused by A. baumannii, although the prevalence of resistance to carbapenems is very low in this setting (A-II).
- 1.
Oral ciprofloxacin, levofloxacin, TMP-SMX, fosfomycin and nitrofurantoin are adequate treatment options for uncomplicated cystitis caused by susceptible CRE (A-II). TMP-SMX could be considered for complicated UTIs due to susceptible KPC-producing K. pneumoniae (C-III).
- 2.
A single intramuscular dose of an aminoglycoside is an adequate treatment option for uncomplicated cystitis caused by susceptible CRE or DTR-PA (A-II).
- 3.
Oral TMP-SMX, ciprofloxacin or levofloxacin should be considered for therapy of other mild infections such as skin and soft tissue infections, or decubitus ulcer infections caused by susceptible MDR-GNB (C-III).
Since 2010, the Food and Drug Administration and the European Medicines Agency have approved 18 new antimicrobial agents, 6 of them with activity against CR-GNB. New antimicrobials in the pipeline and those most recently approved that are active against MDR-GNB are described in detail in the online version of the consensus statement.
Conflicts of interestVicente Pintado has participated in accredited educational activities sponsored by MSD, Pfizer and Shionogi and has been a consultant for Pfizer, Shionogi and Correvio. David Aguilera-Alonso has participated in accredited educational activities sponsored by MSD, Pfizer and Gilead and has been a consultant for Pfizer. Rafael Cantón has participated as speaker at scientific meetings sponsored by Chiesi, MSD, Pfizer and Shionogi and has received funding for research projects from MSD, Shionogi and Venatorx. Germán Bou has been a consultant for MSD, Pfizer, Menarini, Astellas and Shionogi, has served as speaker for MSD, Pfizer, Roche, Menarini, and Shionogi and has received research support from Pfizer and MSD. Nieves Larrosa has participated as speaker at scientific meetings sponsored by Menarini, MSD, Pfizer and Shionogi. Luis Martínez-Martínez has been a consultant for MSD and Shionogi, has served as speaker for Merck, Astra-Zeneca, Astellas, Becton Dickinson and Shionogi and has received research support from Shionogi, Janssen-Cilag, Pfizer and MSD. Antonio Oliver has participated as speaker at scientific meetings sponsored by MSD and Pfizer and has received funding for research projects from MSD and Shionogi. José Ramón Paño-Pardo has participated as speaker at scientific meetings and educational activities sponsored by MSD, Pfizer and Shionogi. The other authors declare no conflicts of interest.
The authors would like to thank Juan Pablo Horcajada for his excellent contribution and constant support in the elaboration of this document.