The main objective in the management of HIV-infected pregnant women is prevention of mother-to-child transmission; therefore, it is essential to provide universal antiretroviral treatment, regardless of CD4 count.
All pregnant women must receive adequate information and undergo HIV serology testing at the first visit. If the serological status is unknown at the time of delivery, or in the immediate postpartum, HIV serology testing has to be performed as soon as possible.
In this document, recommendations are made regarding the health of the mother and from the perspective of minimizing mother-to-child transmission.
El principal objetivo que debemos perseguir en una mujer gestante infectada por el VIH es la prevención de la transmisión vertical: Por ello, es fundamental realizar tratamiento antirretroviral en todas ellas, independientemente del número de linfocitos CD4 que tengan.
Es obligatorio ofrecer a toda embarazada la información adecuada y la realización de la serología frente al VIH. La serología frente al VIH se debe indicar en la primera visita y ha de realizarse lo antes posible. Si la situación serológica con respecto al VIH es desconocida en el momento del parto, o en el posparto inmediato, se debe indicar, con carácter urgente, la realización de pruebas serológicas rápidas.
En este documento se elaboran una serie de recomendaciones con respecto al tratamiento antirretroviral, tanto desde el punto de vista de la salud individual de la madre como con el objetivo de minimizar en lo posible el riesgo de transmisión vertical.
Current knowledge about the mechanisms of mother-to-child transmission (MTCT) is sufficient, as are data on the effectiveness of the different strategies to prevent it. Thus, if we identify infection early in pregnant women, we can almost guarantee prevention of MTCT, provided that we ensure adequate information and access to obstetric management and treatment.
The main objective of patient management is to prevent women from going into labor without knowing their HIV status. Therefore, all pregnant women should undergo serology testing in the first trimester (ideally before becoming pregnant) and again in the third trimester in order to identify seroconversion during pregnancy.1 If the woman goes into labor with an unknown HIV status, rapid testing must be performed to determine appropriate therapeutic interventions, including cesarean delivery, which can reduce MTCT by up to 50%.
Recommendations before pregnancy: preconception counseling, family planning, and assisted reproductionPreconception counselingThe objective of preconception counseling for HIV-infected women is to plan pregnancy under the best possible clinical conditions, by minimizing the risks for women, their partners, and the fetus. Preconception counseling should include effective contraception, advice on a healthy lifestyle, optimization of clinical monitoring, information on the risk of MTCT, prevention of sexual transmission, specialized information and advice, and basic evaluation of fertility.
Assisted reproduction techniques in HIV-infected individualsHIV-infected persons have access to assisted reproduction techniques for 2 main reasons: treatment of infertility and prevention of horizontal transmission in serodiscordant couples.
Reproductive choices will depend on whether the infected person is the man, the woman, or both.
HIV-infected man with a non-HIV-infected female partnerSperm washing with assisted reproductionThe rates for pregnancy in serodiscordant couples that undergo in vitro fertilization or intrauterine insemination with or without intracytoplasmic sperm injection (15–30% and 45–50% per cycle respectively) are comparable to those obtained in infertile couples who undergo the same techniques. Although the technique is probably not completely free of the risk of sexual transmission, assisted reproduction with sperm washing is considered the safest approach when the man is the infected partner. Therefore, it should be considered the method of choice in our setting.
Natural conceptionThe risk of heterosexual transmission is very low when the man is receiving combined antiretroviral therapy (cART) and has an undetectable plasma viral load. If the couple decide to continue with natural conception, in the knowledge that transmission cannot be prevented, the recommendations are as follows: adherence to cART and an undetectable viral load for more than 6 months (infected partner); urethral culture (man) and endocervical culture (woman) to rule out associated sexually transmitted infections (STIs); basic fertility study; unprotected sex restricted to the potentially most fertile periods (ovulation test); unprotected intercourse for no more than 12 months.
Periconceptional pre-exposure prophylaxisPericonceptional tenofovir (TDF) or TDF+emtricitabine (FTC) to prevent sexual transmission in uninfected individuals is currently under study. Doubts remain about its efficacy and safety and the need for adherence to treatment or risk of resistance in the case of seroconversion. This option has not been validated, since it is necessary to wait for the results of ongoing studies.
HIV-infected woman with a non-HIV-infected manHIV-infected women wishing to become pregnant should not systematically undergo assisted reproduction. Transmission to the uninfected man while attempting to become pregnant is easily prevented using self-insemination. Given the simplicity of self-insemination, natural conception is not justified in these cases.
The most suitable reproduction technique will be indicated for HIV-infected women, with stimulation patterns that are considered timely regardless of HIV infection (intrauterine insemination, in vitro fertilization, egg donation).
Both partners infected with HIVWhen both partners are HIV-infected, natural conception with unprotected intercourse can be considered. If the progress of the infection or the resistance pattern is very different between the partners, sperm washing or self-insemination could be considered with the aim of preventing superinfection by other strains. Fertility should also be assessed in these couples so as not to delay unnecessarily plans for becoming pregnant.
Family planningMost contraceptive methods can be used in case of HIV infection, after taking into account possible drug interactions. The family planning method used should ensure dual protection, that is, to prevent unwanted pregnancy and transmission of HIV or other STIs.
TerminationWith current MTCT prevention measures, the risk of transmission to the newborn is very low, and maternal HIV infection alone does not justify termination. Pregnancy does not worsen disease progression. However, termination could be considered in exceptional situations, e.g., for medical reasons related to HIV infection (advanced maternal disease, comorbid processes such as carcinoma of the cervix requiring surgery or exposure to teratogenic drugs or drugs with scant experience of use in pregnancy) or not.
Recommendations- 1.
Preconception counseling must form part of the management of HIV-infected women of childbearing age (A-II). In this context, easy access to assisted reproduction techniques should be provided for HIV-infected people (A-II).
- 2.
When only the man is HIV-infected, sperm washing is currently the safest option for preventing transmission of infection to the partner (A-II).
- 3.
When only the woman is infected, self-insemination is a simple and effective method for becoming pregnant (B-III).
- 4.
When assessing the desire to become pregnant, the decrease in fertility that may affect HIV-infected men and women should be taken into account (B-II).
- 5.
Most contraceptive methods can be used in HIV-infection, although family planning measures should ensure dual protection, to prevent both unwanted pregnancy and transmission of HIV or other STIs (A-II).
- 6.
All hormonal systems (pills, injections, implants, patches, or vaginal ring) can be used in HIV-infected women, although interactions with the antiretroviral drugs that can alter effectiveness should be taken into account (A-III).
In clinical practice, we can identify 3 different groups of HIV-infected pregnant women: women who were aware of their infection before pregnancy, women who were diagnosed during screening at the beginning of pregnancy, and women who were diagnosed at later stages of pregnancy and during childbirth.
A. Pregnant woman diagnosed with HIV infection before pregnancyInitiation of cART depends essentially on immunological and virological status and is governed by the general recommendations for treatment of adults. If treatment is not required initially, it will generally be recommended from the second trimester onward, with the exclusive purpose of preventing MTCT. cART should be started earlier in women with a high viral load. Pregnant women already receiving cART at the time of conception should not stop if it is not medically indicated.
B. Pregnant woman diagnosed with HIV infection during pregnancyOnly patients with test-confirmed HIV infection must be informed thereof. Information should be provided by the obstetrician and the infectious disease specialist they are referred to. Consultation with a pediatrician should also be considered to reduce the anxiety of parents.
C. Pregnant woman diagnosed with HIV infection in advanced stages of pregnancy or during childbirthAll nonmonitored pregnant women, those who have not undergone serology testing, and those whose serostatus is unknown should be adequately informed about the need for a rapid test. If the result is positive, she should be informed as soon as possible. When there is not enough time to perform a confirmation test, the patient will be informed of the positive result, as well as the possibility that it is a false positive. In the case of a positive result, clinicians will act as quickly as possible to reduce the risk of MTCT (e.g., cesarean delivery, administration of intrapartum intravenous zidovudine [ZDV]).
After delivery, the diagnostic evaluation of the patient will be completed, subsequent medical checks will be scheduled, and the woman will be provided with psychosocial support.
Recommendations- 1.
CD4 count should be determined at baseline and at least once per trimester (B-III).
- 2.
We recommend resistance testing in the following situations: pregnant women without current cART and a polymerase chain reaction (PCR) result above the usual limit of detection of resistance (500–1000copies/mL) (A-III); pregnant women who receive cART, but whose viral suppression is suboptimal or in whom a rebound of viral load is detected (A-II). In addition, when treatment is administered empirically without the results of the resistance test, it must be adapted to the result when this becomes available (B-III).
- 3.
Viral load should be determined at the first visit (A-I), 2–4 weeks after starting treatment or switching treatment (B-I), every month until it is undetectable, and at least every 3 months thereafter (B-III). Viral load should also be determined at 34–36 weeks to establish the most appropriate mode of delivery (A-III) for each patient.
Follow-up should be multidisciplinary (obstetric, clinical, immunological, virological, and psychosocial) and based on monitoring of analytical parameters related to HIV-infection, side effects of cART, and maternal and fetal well-being. The mother must be informed about behaviors that reduce MTCT (e.g., consumption of tobacco products and other toxic substances, avoiding unprotected sex with multiple partners, and artificial feeding).
Obstetric follow-up and complications associated with pregnancyHIV-infected pregnant women may have a series of health problems that can complicate pregnancy, and thus require more exhaustive monitoring, regardless of HIV infection. Therefore, HIV-infected pregnant women may have a greater risk of developing obstetric conditions such as premature rupture of membranes, preterm delivery, and intrauterine growth retardation.
Complications of cARTPossible side effects of cART should be monitored, as should poor adherence and resistance.
Recommendations- 1.
Prophylaxis against opportunistic infections should be considered in women with CD4 ≤200cells/mm3 after evaluating the potential adverse effects (A-II).
- 2.
Viral load should be determined at the first visit (A-I), as should the CD4 count (A-I). During follow-up, CD4 count should be determined every 3 months (B-III). Viral load should be determined during the second and third trimesters, the latter close to delivery (A-I).
- 3.
Screening for malformations should be performed, especially in women who had been receiving antiretroviral drugs with possible teratogenic effects, such as efavirenz (EFV) (B-III).
- 4.
Pregnancy should be closely monitored to identify potential complications secondary to antiretroviral therapy (A-III).
The results for the detection of chromosomal abnormalities are much better with combined tests, which are the approach of choice in HIV-infected pregnant women. These techniques should be applied to all HIV-infected men during the first trimester, or, if this is not possible, during the second trimester.
Chorionic villous sampling/amniocentesis biopsy is contraindicated in women a with viral load ≥1000copies/mL. In pregnant women with viral loads between 50 and 1000copies/mL, the possibility of delaying amniocentesis until the viral load is undetectable should be considered.
In certain circumstances it may be deemed necessary to perform amniocentesis or cordocentesis during the second or third trimester. No firm conclusions have been drawn on the specific risk in these situations; consequently, if the procedure is necessary in a woman receiving optimized cART, an in-depth evaluation of risk-benefit should be made.
Recommendations- 1.
Given potential alterations in serum markers for chromosomal abnormalities in HIV-infected pregnant women (caused viral load, the decrease in CD4 lymphocyte count, or antiretroviral medication) we recommend combined screening with biochemical techniques and ultrasound during the first trimester (B-II).
- 2.
If an invasive procedure is necessary during pregnancy (e.g., amniocentesis and fetal surgery), the risk-benefit ratio should be carefully assessed, and the procedure should be performed under optimized antiretroviral therapy with an undetectable viral load to prevent infection. Every effort should be made not to cross the placenta (A-III).
The main objective of cART in HIV-infected pregnant women is to prevent MTCT, preserve the health of the mother and child, and prevent the emergence of resistance, which could limit future therapeutic options. cART has been shown to be effective in preventing MTCT.
Recommendations- 1.
The main goal of ART during pregnancy is to maintain an undetectable viral load (A-II).
- 2.
cART is indicated for all pregnant women regardless of CD4 count and viral load (A-I).
- 3.
The treatment of choice in pregnancy is cART, irrespective of whether or not the women needs cART (A-II).
- 4.
Single-dose nevirapine (NVP) in monotherapy is not recommended at delivery in women who have been receiving cART at the usual doses during pregnancy (A-II).
- 5.
If cART is interrupted during pregnancy or after delivery in a woman receiving 2 nucleoside reverse transcriptase inhibitors and nevirapine (NVP), then NVP should be discontinued 7 days before (the ideal duration of the interval is unknown) (B-II).
- 6.
In triple therapy regimens with protease inhibitors, all drugs should be stopped simultaneously (A-II).
- 7.
The specific choice of drugs is based on the resistance study, drug safety, and ease of adherence (A-II).
- 8.
Adherence to cART should be a priority in pregnancy (A-II).
The criteria for the use of antiretroviral drugs in pregnant women differ from those applying to adults in general, since the safety of the mother and the child must be taken into consideration. The drugs used are those for which most experience is available, such as zidovudine (ZDV), which should be part of cART whenever possible (except in cases of documented resistance or intolerance). Potentially teratogenic drugs such as EFV should be avoided.
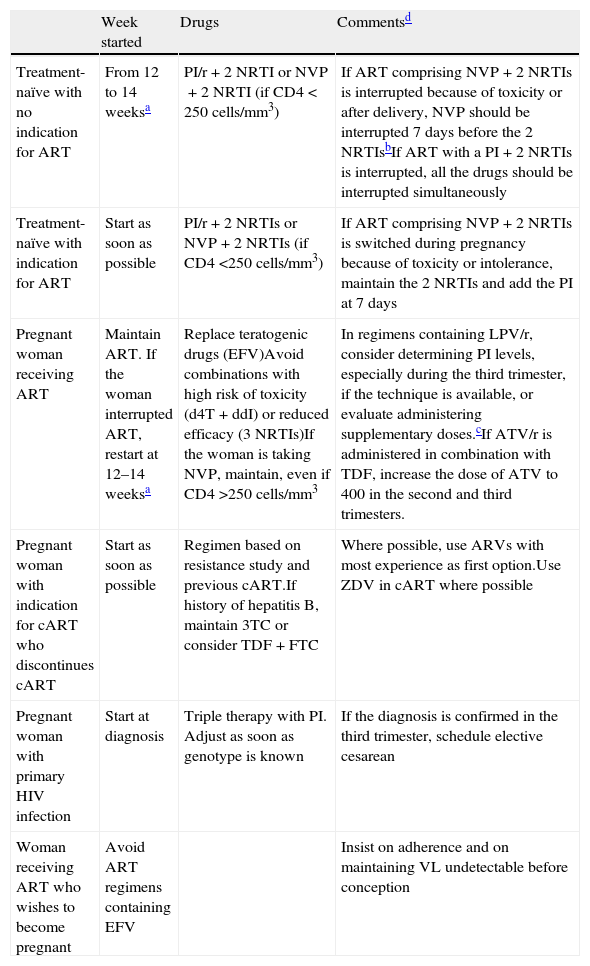
Specific recommendations for management of cART during pregnancy and postpartumMost cases of MTCT occur during the final weeks of pregnancy and/or during labor. Nevertheless, a small number of cases of transmission during the first weeks of gestation have been reported. Moreover, embryonic development ends at around 10–12 weeks, after which time the possibility of teratogenic disorders decreases. Table 1 shows the recommendations for management of cART in HIV-positive pregnant women in different situations. Specific recommendations for the use of drugs in pregnancy are set out below.
Management of antiretroviral therapy during pregnancy.
| Week started | Drugs | Commentsd | |
| Treatment-naïve with no indication for ART | From 12 to 14 weeksa | PI/r+2 NRTI or NVP+2 NRTI (if CD4<250cells/mm3) | If ART comprising NVP+2 NRTIs is interrupted because of toxicity or after delivery, NVP should be interrupted 7 days before the 2 NRTIsbIf ART with a PI+2 NRTIs is interrupted, all the drugs should be interrupted simultaneously |
| Treatment-naïve with indication for ART | Start as soon as possible | PI/r+2 NRTIs or NVP+2 NRTIs (if CD4 <250cells/mm3) | If ART comprising NVP+2 NRTIs is switched during pregnancy because of toxicity or intolerance, maintain the 2 NRTIs and add the PI at 7 days |
| Pregnant woman receiving ART | Maintain ART. If the woman interrupted ART, restart at 12–14 weeksa | Replace teratogenic drugs (EFV)Avoid combinations with high risk of toxicity (d4T+ddI) or reduced efficacy (3 NRTIs)If the woman is taking NVP, maintain, even if CD4 >250cells/mm3 | In regimens containing LPV/r, consider determining PI levels, especially during the third trimester, if the technique is available, or evaluate administering supplementary doses.cIf ATV/r is administered in combination with TDF, increase the dose of ATV to 400 in the second and third trimesters. |
| Pregnant woman with indication for cART who discontinues cART | Start as soon as possible | Regimen based on resistance study and previous cART.If history of hepatitis B, maintain 3TC or consider TDF+FTC | Where possible, use ARVs with most experience as first option.Use ZDV in cART where possible |
| Pregnant woman with primary HIV infection | Start at diagnosis | Triple therapy with PI. Adjust as soon as genotype is known | If the diagnosis is confirmed in the third trimester, schedule elective cesarean |
| Woman receiving ART who wishes to become pregnant | Avoid ART regimens containing EFV | Insist on adherence and on maintaining VL undetectable before conception |
In women with a high viral load or for whom cART is indicated (maternal health), treatment should be initiated as soon as possible, on the condition that it is with safe drugs and the woman does not have intractable hyperemesis gravidarum.
In order to minimize the appearance of resistance to NNRTIs when ART is suspended after delivery, some experts recommend continuing with 2 NRTIs and adding PI at 7 days and suspending ART at 4 weeks, given the long half-life of NVP.
Therapeutic levels can fall during the third trimester in women receiving LPV/r and ATZ/r. See text for more information.
ZDV is recommended as a component of ART during pregnancy, during delivery, and in the newborn according to protocol ACTG 076.
Abbreviations: ART: antiretroviral therapy; ARV: antiretroviral; ATZ: atazanavir; cART: combination antirretroviral therapy; EFV: efavirenz; FTC: emtricitabine; LPV/r: lopinavir/ritonavir; NVP: nevirapine; NRTI: nucleotide reverse transcriptase inhibitor; PI: protease inhibitor; TDF: tenofovir; VL: viral load; ZDV: zidovudine.
- 1.
The benefits of treating the mother and child with cART outweigh the potential risks of use in pregnancy (A-I).
- 2.
ZDV should be included in cART where possible (A-I).
- 3.
The treatment of choice comprises 2 nucleoside analogues plus a boosted protease inhibitor (A-I).
- 4.
The combination of choice in pregnancy is ZDV+lamivudine (3TC)+boosted lopinavir (LPV/r) (A-II). Other combinations with an acceptable level of scientific evidence are ZDV+3TC+boosted atazanavir (ATV/r) (B-II) and TDF+FTC+ATV/r at 400/100) (B-II). Abacavir (ABC)+3TC is also acceptable (C-II). The combination TDF+FTC is particularly indicated in patients coinfected with HBV. If resistance can compromise the efficacy of LPV/r and ATV/r, boosted darunavir (DRV/r) can be an alternative third drug if the virus is sensitive (B-II).
- 5.
NVP as a third drug in treatment-naïve patients can only be used in pregnant women with CD4 counts <250cells/μL and should be closely monitored for potential hepatotoxicity, especially in patients with chronic HCV and HBV infection (B-II).
- 6.
The combination of stavudine (d4T)+didanosine (ddI) should not be used, as it induces toxicity (A-II). EFV (A-II) or drugs with which clinicians have little experience (A-III) should not be used. EFV should be maintained only when pregnancy is confirmed more than 6 weeks previously in a woman receiving EFV (B-II).
- 7.
If the mother is already receiving cART, it should not be suspended during the first trimester; nevertheless, potentially teratogenic drugs (e.g., EFV) should be replaced (A-II), especially if pregnancy is confirmed before the sixth week of gestation and, whenever possible, by drugs with which clinicians have experience (A-III). In situations of resistance to first-line drugs and after an individualized assessment, raltegravir (RAL), DRV/r, and etravirine (ETR) could be used (C-III).
- 8.
TDF is not recommended as first choice because of its nephrotoxicity and effects on bone metabolism, except if it is required owing to failure or in patients coinfected with HBV (B-III).
- 9.
Changes in cART during pregnancy should be based on safety, side effects, and effectiveness (A-III).
Few conclusions have reached made about the management of obstetric disorders in patients receiving antiretroviral treatment. Nevertheless, we present specific recommendations that are complementary to the general recommendations made to non-HIV-infected women.
Obstetric disordersSuspected preterm laborPrevention of preterm labor should focus on trying to eliminate triggers, ensuring good monitoring during pregnancy, and reducing or eliminating consumption of toxins. The main approach to preterm delivery in HIV-infected patients, as in non-HIV-infected patients, is hospital-based.
Recommendations- 1.
If uterine contractions are regular during suspected preterm labor, even if cervical changes are minimal, intravenous ZDV should be administered (at the same doses as during labor) in combination with tocolytic therapy. In addition, patients on cART must continue therapy, and those who are not should initiate it (B-III).
- 2.
If tocolytic therapy fails, delivery will be completed vaginally or by cesarean section depending on viral load and obstetric conditions (B-III).
Treatment of premature rupture of membranes in HIV-positive pregnant women depends on gestational age, maternal viral load, and cART received, as well as on evidence of acute infection (chorioamnionitis). In the absence of chorioamnionitis or fetal distress, conservative treatment should be administered in cases of very early gestational age (<30 weeks) owing to the complications of severe prematurity. The protective role of cesarean section in preterm delivery in mothers with a low viral load receiving antiretroviral therapy is unclear. From week 34, the approach should be to complete the pregnancy using vaginal delivery if the appropriate conditions are met.
Metrorrhagia in the third trimesterVaginal bleeding in HIV-positive patients during the third trimester as a result of conditions such as placenta previa, abruptio placentae, and vasa previa may be accompanied by an increased risk of MTCT. No clear evidence exists of how to complete the pregnancy or of the method of delivery, since the risk of blood loss for the mother and fetus should be viewed against the risk of perinatal transmission and severe prematurity.
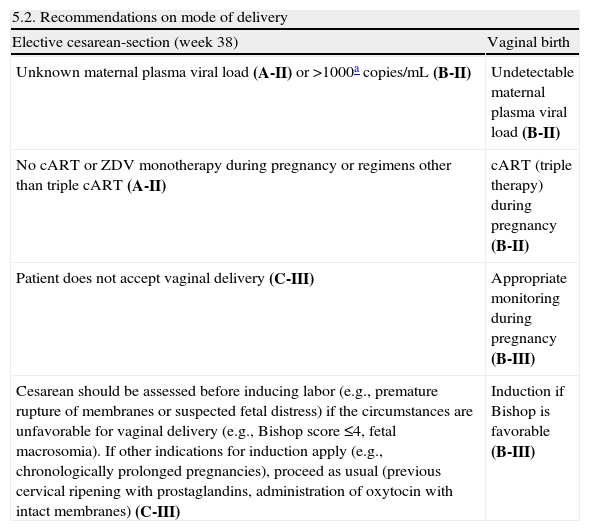
Intrapartum managementThe moment of greatest risk of transmission of HIV is at delivery. The risk factors with the greatest impact are viral load and cervicovaginal secretions, disease stage, duration of rupture of membranes and labor, cART received, and form of delivery. Therefore, the mode of delivery should be agreed with the mother and a multidisciplinary team (obstetrician, neonatologist, and infectious diseases specialist) after determining viral load at week 36 to discuss the risks and benefits of the different modalities.
Recommendations| 5.2. Recommendations on mode of delivery | |
| Elective cesarean-section (week 38) | Vaginal birth |
| Unknown maternal plasma viral load (A-II) or >1000acopies/mL (B-II) | Undetectable maternal plasma viral load (B-II) |
| No cART or ZDV monotherapy during pregnancy or regimens other than triple cART (A-II) | cART (triple therapy) during pregnancy (B-II) |
| Patient does not accept vaginal delivery (C-III) | Appropriate monitoring during pregnancy (B-III) |
| Cesarean should be assessed before inducing labor (e.g., premature rupture of membranes or suspected fetal distress) if the circumstances are unfavorable for vaginal delivery (e.g., Bishop score ≤4, fetal macrosomia). If other indications for induction apply (e.g., chronologically prolonged pregnancies), proceed as usual (previous cervical ripening with prostaglandins, administration of oxytocin with intact membranes) (C-III) | Induction if Bishop is favorable (B-III) |
In the group of patients who received cART during pregnancy and whose viral load remains detectable (between 50 and 1000copies near the time of delivery) but less than 1000copies/mL, the benefit of elective cesarean delivery with respect to the transmission of the virus is unclear. Therefore, each case should be dealt with on an individual basis and the mother should be informed about the risks and benefits associated with surgery so that agreement between the obstetrician and the mother can be reached (B-III).
- 1.
When the indication for elective cesarean section is HIV infection, the procedure should be scheduled for week 38 to prevent neonatal respiratory distress syndrome, and the patient should begin therapy immediately (B-II). If elective cesarean delivery is for an obstetric indication (e.g., breech presentation), it can be programmed for week 39.
- 2.
If labor has started or membranes ruptured before the date of the scheduled cesarean section, vaginal delivery should be allowed if progression is fast. Emergency cesarean section should be performed when labor is expected to be long (C-III).
- 3.
Intravenous antibiotic prophylaxis should be administered during cesarean section (A-II).
- 1.
In HIV-infected patients undergoing vaginal delivery, every effort must be made so as not to increase the risk of transmission of the virus (B-III).
- 2.
Oxytocin can be used to speed up labor (B-III).
- 3.
Artificial rupture of membranes should be avoided (B-II).
- 4.
Avoid invasive procedures to monitor fetal well-being, such as internal fetal scalp electrode and the determination of fetal scalp pH (B-III). Instrumental delivery (forceps or vacuum) and episiotomy should be performed only in selected circumstances (B-III).
- 1.
Intravenous ZDV is the recommended intrapartum treatment that has demonstrated the greatest efficacy in reducing the rate of MTCT, regardless of the woman's previous treatment (A-I). Dose: 2mg/kg for 1h followed by 1mg/kg/h until the end of labor. In the case of a cesarean section, treatment should be initiated ideally 2–3h before.
- 2.
Oral ART should not be discontinued during labor (B-III), except in patients treated with d4T during pregnancy. d4T should be interrupted during treatment with ZDV (A-I).
- 3.
In the case of a woman who has not received antiretroviral therapy during pregnancy or who has received it for a short time and who has a very high viral load close to the due date, intravenous ZDV can be combined with oral NVP at least 2h before delivery, especially in vaginal delivery (C-II). In this context, the addition of raltegravir (600mg/12h) can also be evaluated, given that it crosses the placenta quickly; however, experience with this drug is limited (C-III).
- 4.
This treatment is not recommended in patients who have received antiretrovirals during pregnancy, even if their viral load remains at >1000copies/mL (A-II).
- 5.
In women who receive NVP intrapartum, postpartum treatment should include ZDV+3TC for 7 days to prevent the emergence of resistance to NVP (A-II).
- 6.
Patients treated with protease inhibitors who present postpartum hemorrhage should be given prostaglandins, oxytocin, or misoprostol. Ergot-derived drugs should be avoided owing to the risk of excessive vasoconstriction; if necessary, use the lowest dose for the shortest time possible (C-III).
- •
Rapid HIV test.
- •
If the result is positive, initiate intravenous ZDV, administer a single dose of NVP 200mg at least 2h before delivery, and perform cesarean section.
- •
Confirm HIV serostatus as soon as possible.
- •
Antiretroviral therapy should be started when viral load and CD4 lymphocyte count are determined.
- •
Elective cesarean section at 38 weeks with intravenous ZDV should be started 2–3h before surgery and until the umbilical cord is cut. Evaluate addition of NVP in women with a high viral load.
- •
Continue treatment, since viral load is decreasing. However, since viral load has not yet reached undetectable levels, elective cesarean should be performed at 38 weeks under treatment with intravenous ZDV, as in the previous case.
- •
In these cases, there is no evidence that elective cesarean section reduces the risk of MTCT (≤1%) compared to vaginal delivery; however, the risk for the mother increases. Therefore, we recommend vaginal delivery, unless other obstetric indications contraindicate it.
- •
Intravenous ZDV should be initiated.
- •
The mode of delivery should be decided individually after evaluating the time of rupture of membranes, progression of labor, viral load, and whether the woman is receiving appropriate antiretroviral therapy.
Postpartum management involves the general care provided to all patients (monitoring of genital bleeding and uterine involution), regardless of their HIV status, and specific measures to control HIV infection. We can clearly distinguish 2 types of HIV-infected patient: women with well-controlled HIV infection during pregnancy and childbirth, in whom it is very important to ensure continuity of care for HIV infection (see below); and women diagnosed late in pregnancy, during labor, or in the immediate postpartum. These women must undergo a full assessment.
Gynecological controlThe gynecological examination should include a Papanicolaou smear if the patient did not have one in the previous year. Depending on the findings and the woman's previous history, cervicovaginal histopathology and genital cancer prevention should be considered.
Social services and social careSocial care systems have to adapt to the individual needs of the woman. Ideally, social support would begin before pregnancy and continue during pregnancy and in the postpartum.
Postpartum depressionGiven the potentially higher prevalence of depressive disorders in HIV-infected women in the postpartum, screening for depression should be conducted early in the postpartum, if not performed during pregnancy. The Edinburgh test should be used, given its greater reproducibility. Again, it is essential to ensure good coordination between all those responsible for the patient's care (obstetricians, pediatricians, infectious diseases specialists, social workers, and psychologists/psychiatrists [if necessary]).
Follow-up of HIV infectionIn previously diagnosed patients who received cART during pregnancy, it is very important to establish before delivery the form postpartum care will take. For patients with a late diagnosis (at the end of pregnancy, intrapartum, or immediately postpartum), a complete assessment must be performed before discharge. Treatment options should be discussed with an infectious diseases specialist, and follow-up should be planned. Breastfeeding cannot be allowed until the diagnosis of HIV infection is excluded completely. In the case of a positive rapid test, the child should be monitored by a pediatrician.
Recommendations- 1.
Control of HIV-positive pregnant women during the postpartum should follow the guidelines established in the general population, namely, blood test (complete blood count and blood biochemistry) and evaluation of the need for antithrombotic prophylaxis (B-III).
- 2.
HIV-infected women should have a Papanicolaou smear. Evaluate whether to follow up with cervicovaginal histopathology and genital cancer prevention. The postpartum is an important time to discuss safer sex and contraceptive options (A-III).
- 3.
We recommend screening for postpartum depression in women infected by HIV. Screening can be performed using the Edinburgh test. If depression is likely, the patient must be referred for follow-up, preferably with a mental health specialist (B-III).
- 4.
Before delivery, subsequent monitoring of HIV infection should be planned to prevent discontinuation of or poor adherence to treatment. Newly diagnosed patients must undergo a complete evaluation and follow-up before discharge from hospital (A-II).
Diagnosis of HIV infection in children aged >18 months is by serology testing, as is the case in adults: however, in children aged <18 months, virological testing should be performed, as follows: HIV DNA PCR, whose sensitivity increases with age (40% during the first week of life to 96% at 1 month, with a specificity of 99%) and/or HIV RNA PCR that detects free viral RNA in plasma. This technique is the most widely available in most centers. A child aged <18 months is considered to be infected after at least 2 PCR results are positive for HIV RNA and/or DNA in different blood tests. Children should be tested for HIV infection at baseline, at 4–6 weeks, and at 4 months, to determine their HIV status. In those aged <18 months, HIV DNA or RNA PCR should be used for diagnosis. HIV DNA PCR is preferable for children who are receiving combination antiretroviral prophylaxis. HIV antibody assays can be used in those aged >18 months.
Recommendations- 1.
HIV RNA and/or DNA should be determined in the first 48h of life (not using cord blood) (B-I).
- 2.
Determination of HIV RNA and/or viral DNA should be repeated between 15 and 21 days of age, at 4–6 weeks, and at ≥4 months (A-II).
The antiretroviral prophylaxis regimen administered to the neonate is determined by the theoretical risk of MTCT, which depends mainly on the mother's viral load, although other risk factors can be identified.
The different types of regimen are set out below.
Recommendations- 1.
The children of mothers who are receiving cART with a viral load <50copies/mL at delivery and no other risk factors must receive ZDV monotherapy for 4 weeks (A-I).
- 2.
In cases of a major risk of MTCT, triple therapy should be started, especially in mothers who did not receive cART during pregnancy and labor (A-I).
- 3.
In the case of preterm infants, especially those aged <32 weeks, ZDV should be administered in monotherapy for 4 weeks (A-II). A single dose of NVP administered to the mother or child 2h before delivery can be considered (B-III).
The most common neonatal condition in infants born to HIV-infected mothers has changed over the years. Currently, most pregnant women undergo adequate monitoring of their pregnancy and experience few comorbid conditions. However, some conditions are more common in infants born to HIV-infected women.
Recommendations- 1.
We recommend screening in children born to mothers with HIV infection and other infections that can be transmitted vertically, as well as other conditions affecting newborns (preterm birth, withdrawal syndrome), which pediatricians and neonatologists should be able to recognize and treat (A-II).
Hematological abnormalities are common in children exposed to cART during pregnancy. Exposure to NVP can result in nonsymptomatic elevation of transaminases. Clinical hepatitis and rash in the newborn are exceptional and have not been reported in children who received prophylaxis with 3 doses of NVP during the first week. In the neonate exposed in utero to ATV, bilirubin levels should be monitored during the first weeks of life.
Recommendations- 1.
We recommend a complete blood count/biochemistry workup in the samples taken to rule out MTCT so that hematological toxicity can be excluded (C-III).
- 2.
The determination of lactate for the study of mitochondrial toxicity is justified only in the symptomatic patient (C-III).
- 3.
In Spain, prophylaxis for Pneumocystis jiroveci pneumonia with trimethoprim-sulfamethoxazole from 6 weeks of age is recommended only in those cases where it is not reasonably possible to rule out MTCT (A-II).
At present, the only effective strategy is complete replacement of breastfeeding by formula feeding. If the child is breastfed before maternal infection is diagnosed, infection in the child should be ruled out immediately. Triple antiretroviral prophylaxis for 4–6 weeks should be evaluated in the child.
The mother should not chew the child's food, as cases of transmission by this route have been reported.
Recommendations- 1.
HIV-infected mothers must not breastfeed. The child should receive formula (A-I).
- 2.
The mother should not chew the child's food, as cases of transmission by this route have been reported (A-II).
No one knows the exact threshold at which the risk of transmission becomes significant. As for management, there are few data in the literature to clarify which is the best treatment for pregnant women coinfected with HIV and HCV. Children born to coinfected mothers cannot clear maternal HCV antibodies until after 18 months of age. Therefore, in order to rule out infection in the child, at least 2 negative PCR determinations are necessary between 2 and 6 months of age and/or HCV serology after 18 months.
Recommendations- 1.
All pregnant women should undergo determination of HCV antibodies (A-III).
- 2.
At least 1 quantitative HCV plasma viremia determination should be made, although no action can be taken as a result during pregnancy (C-III).
- 3.
Interferon is not recommended and ribavirin is contraindicated during pregnancy (A-II).
- 4.
The recommendations for use of antiretroviral drugs during pregnancy are the same for women coinfected with HCV and for those infected only with HIV (B-III).
- 5.
There are insufficient data to recommend universal elective cesarean based only on maternal HCV infection (B-III).
- 6.
Children born to mothers with HIV/HCV coinfection (A-III) should undergo HCV determination by HCV RNA at 2 and 6 months of age and/or determination of antibodies to HCV after 18 months age (C-III).
- 7.
Vaccination against hepatitis A and B is indicated in coinfected women who have not been exposed to these viruses (A-III).
Transplacental transmission of HBV is common if the mother has an acute infection very close to delivery or is a chronic carrier of HBsAg. Maternal HBV viral load is the most influential factor for MTCT. No definitive studies have been published on the safety of treatment of HBV infection during pregnancy and breastfeeding. All children born to mothers carrying HBsAg should receive HBV-specific immunoglobulin within the first 12h of life. Furthermore, the child should be vaccinated at a different anatomical site, according to habitual immunization practice. This regimen is >95% effective in the prevention of HBV infection.
Recommendations- 1.
All HIV-infected pregnant women should undergo HBV serology testing (A-II).
- 2.
Interferon-alpha and pegylated interferon-alfa are not recommended during pregnancy (A-III).
- 3.
HBV viral load should be determined in all HBsAg carriers or in cases where serology is doubtful. These data are useful when designing a cART regimen that is effective against HIV and HBV (C-III).
- 4.
HBV vaccine should be administered to women who are HBsAg-negative, anticore-negative, and negative for surface antigen antibodies (A-II).
- 5.
In women whose IgG is negative for hepatitis A, vaccination is recommended after delivery (A-II).
- 6.
Treating pregnant HIV/HBV-coinfected women involves drugs active for both viruses. The drugs of choice are TDF with 3TC or FTC according to the recommendations for use of antiretroviral therapy in pregnant women (A-III).
- 7.
Children born to HBsAg carriers should receive HBV-specific immunoglobulin within the first 12h of life. Furthermore, vaccination will be administered at a different anatomical site, with booster doses at 1 month and 6 months of life (A-I).
Diagnosis of tuberculosis (TB) in pregnant women is the same as in the general population. Chest radiography with abdominal shielding produces minimal fetal exposure to radiation and can be performed if deemed necessary.
Recommendations- 1.
HIV-infected pregnant women with active TB should start treatment for TB immediately (A-I).
- 2.
The treatment of choice for pregnant women with active TB is (isoniazid+rifampicin/rifabutin+ethambutol+pyrazinamide) (B-III). Experience with PZ is very limited, although it is recommended in HIV-infected pregnant women, especially those with disseminated or meningeal TB and when the susceptibility of the strain is unknown (C-III).
- 3.
Rifamycin should be part of TB treatment, with the dose adjusted if necessary based on cART (A-II).
- 4.
Streptomycin, kanamycin, amikacin, and capreomycin are contraindicated in pregnant women (A-II). There is little experience with other second-line drugs, such as para-aminosalicylic acid, quinolones, ethionamide, and cycloserine; however, if necessary, these can be used in multidrug-resistant TB (C-III).
- 5.
Pregnant women with active TB should be treated with antiretrovirals as soon as possible both for the health of mother and to avoid MTCT (A-II).
- 6.
Immune reconstitution inflammatory syndrome can occur after initiation of cART. Both treatments should be maintained and the syndrome managed (A-III).
- 7.
Monthly monitoring is recommended with liver tests during pregnancy and in the postpartum (C-III).
Genotypic resistance testing is recommended for all pregnant women before starting cART, as well as for those who have a detectable viral load while on treatment. It is sometimes necessary to initiate cART before the test result becomes available, although it can be modified depending on the outcome of the test.
The importance of adherence to prevent the emergence of resistance mutations should be underlined. For the reasons stated above, ZDV will be administered intrapartum.
Recommendations- 1.
Genotypic resistance testing is recommended for all pregnant women before starting cART and for those with a detectable viral load while receiving cART (A-III). It is sometimes necessary to initiate cART before the test result becomes available, although it can be amended depending on the outcome of the test (B-III).
- 2.
Women with documented resistance to ZDV during pregnancy will not include ZDV regimens to achieve virologic suppression, but may receive intravenous ZDV during labor (A-II).
- 3.
If the mother is infected by a resistant virus, treatment should be started in the newborn depending on the results of the maternal resistance test (A-II). If the result is not available at labor, therapy with ZDV+3TC and NVP should be started in the newborn. The regimen can be adjusted once the results of the resistance study are available.
- 4.
The optimal treatment for the newborn of a mother infected with a resistant virus is not known. It is wise to consult an expert in pediatric HIV infection before delivery (A-III).
- 5.
cART that suppresses viral replication should be used in HIV-infected pregnant women. This is the most effective strategy for preventing the development of resistance mutations and minimizing the risk of MTCT (A-II).
- 6.
In the case of maternal infection with multidrug-resistant virus, the cART prescribed should comprise those drugs that ensure the highest maternal viral suppression (A-III).
- 7.
Women who receive nonnucleoside-based ART only as prophylaxis against perinatal transmission and who discontinue ART after delivery should take nucleoside analogs for at least 7 days after withdrawal of the nonnucleoside in order to minimize the risk of developing resistance mutations (A-I). An alternative is to replace the nonnucleoside with a protease inhibitor before interruption and continue with the analogs for 30 days (B-III).
- 8.
The addition of a single dose of NVP at delivery or close to delivery in a woman receiving stable cART does not provide an extra benefit in reducing perinatal transmission and may cause the emergence of NVP resistance mutations in the mother or infant; therefore, it is not recommended (A-I).
The preparation of this document has been financed by the coordinating institutions own funds
Conflict of interestsIn order to avoid and/or minimize any conflicts of interests, the persons that made up the Expert Panel have made a formal declaration of interests. In this declaration, some of the authors have received funding to participate in conducting research, as well as having received payments as presenters on behalf of public institutions and pharmaceutical companies. These activities do not affect the clarity of the present document on not entering into that conflict of interest recommended with the fees and/or assistance received. It should be emphasized that, as regards the drugs in the document, it only mentions the active ingredient and not a commercial brand.
Writing Committee. Rosa Polo Rodríguez. Jefa del Área Asistencial y de Investigación. Secretaría del Plan Nacional sobre el Sida. MSSSI. Madrid. Eloy Muñoz Galligo. Especialista en Obstetricia y Ginecología. Hospital Universitario 12 de Octubre. Madrid. José Antonio Iribarren. Especialista en Medicina Interna. Unidad de VIH. Hospital Universitario Donostia. San Sebastián. Pere Domingo Pedrol. Especialista en Medicina Interna. Unidad de VIH. Hospital Universitario Sant Pau. Barcelona. María Leyes García. Especialista en Medicina Interna. Unidad de VIH. Hospital Universitario Son Dureta. Palma de Mallorca. Vicente Maiques Montesinos. Especialista en Obstetricia y Ginecología. Hospital Universitario La Fe. Valencia. Pilar Miralles Martín. Especialista en Medicina Interna. Unidad de VIH. Hospital Universitario Gregorio Marañón. Madrid. Antoni Noguera Julian. Especialista en Pediatría. Hospital San Joan de Deu. Barcelona. Antonio Ocampo Hernandez. Especialista en Medicina Interna. Unidad de VIH. Hospital Universitario Xeral de Vigo. María Lourdes Peres Bares. Especialista en Ginecología y Obstetricia. Hospital Universitario Xeral de Vigo Marta López Rojano. Especialista en Ginecología y Obstetricia. Hospital Universitario Clinic de Barcelona. Anna Suy Franch. Especialista en Obstetricia y Ginecología Hospital Universitario Vall d’Hebron. Barcelona. Mª Carmen Viñuela Beneitez. Especialista en Obstetricia y Ginecología. Hospital Universitario Gregorio Marañon. Madrid. María Isabel González Tomé. Especialista en Pediatría. Unidad de Inmunodeficiencias. Hospital Universitario 12 de Octubre. Madrid.
All members of the Expert Panel are authors of the article. Details of the Editorial Committee are given in Appendix 1.