In this update, antiretroviral therapy (ART) is recommended for all patients infected by type 1 human immunodeficiency virus (HIV-1). The strength and grade of the recommendation varies with clinical circumstances, number of CD4 cells, comorbid conditions and prevention of transmission of HIV. The objective of ART is to achieve an undetectable plasma viral load. Initial ART should always comprise a combination of 3 drugs, including 2 nucleoside reverse transcriptase inhibitors and a third drug from a different family (non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase inhibitor). This update presents the causes and criteria for switching ART in patients with undetectable plasma viral load and in cases of virological failure. An update is also provided for the specific criteria for ART in special situations (acute infection, HIV-2 infection, and pregnancy) and with comorbid conditions (tuberculosis or other opportunistic infections, kidney disease, liver disease, and cancer).
Se recomienda el TAR en todos los pacientes infectados por el VIH-1. La fuerza y gradación de la recomendación varía según la circunstancia clínica, número de CD4+, presencia de comorbilidades y prevención de la transmisión del VIH. El objetivo del TAR es lograr una CVP indetectable. El TAR de inicio debe ser siempre una combinación de tres fármacos que incluya una asociación de 2 análogos de nucleósido y otro fármaco de distinta familia (inhibidor de la transcriptasa inversa no nucleósido, inhibidor de la proteasa o inhibidor de la integrasa). Se exponen las causas y criterios para cambiar un TAR estando con CVP indetectable, así como en el fracaso virológico. Se actualizan igualmente los criterios específicos del TAR en situaciones especiales (infección aguda, infección por VIH-2, embarazo) o comorbilidades (tuberculosis u otra enfermedad oportunista, afectación renal, hepatopatías y neoplasias).
Since 1996, when the arrival of antiretroviral drugs made it possible to build potent combinations, antiretroviral therapy (ART) has led to huge health care benefits (reduced morbidity and mortality and reduced transmission of the human immunodeficiency virus [HIV]). In parallel with these advances, ART has become complicated owing to the high number of drugs and families, as well as the many aspects affecting administration (efficacy, toxicity, resistance, tropism, pharmacologic interactions, use in special situations, and cost-effectiveness).
The complexity and speed with which changes occur necessitate frequent preparation and updating of guidelines on ART. For the last 15 years, GESIDA and the National AIDS Plan have jointly edited a consensus document on ART in adults.1 The present document updates previous recommendations in this population.
The objective of this consensus document is to provide health professionals who treat HIV-infected adults with up-to-date knowledge on ART and a series of recommendations based on scientific evidence that can act as guidelines in therapeutic decision making.
Clinical and laboratory evaluation as a guide for ARTClinical evaluationIt is important to take an exhaustive clinical history, including physical and psychological data, treatment, habits, and risk practices. Specific aspects applying to women (e.g., desire to become pregnant and contraception) should be analyzed and a complete physical examination performed.
Recommendation
- •
Every year, HIV-infected patients should undergo a physical examination. Pharmacological treatment should also be evaluated and a clinical history taken (A-II).
In addition to specific determinations associated with HIV infection and its consequences, other tests should be ordered to take account of previous infections or cardiovascular risk factors.
Recommendation
- •
The initial laboratory workup should include a complete blood count, general biochemistry, and serology testing (Toxoplasma, cytomegalovirus, syphilis, HAV, HBV, and HCV). Viral load, CD4+ T-lymphocyte count, and primary resistance to HIV and HLA-B*5701 should also be determined (A-II).
The number of CD4+ T lymphocytes is the main marker of the risk of progression and appearance of non-AIDS events.
Recommendation
- •
The absolute number and percentage of CD4+ T lymphocytes should be determined before initiating ART. Once therapy has started, these determinations should be made periodically to monitor the immune response (A-I).
Plasma viral load (PVL) is a marker of the risk of progression and transmission of HIV.
Recommendation
- •
PVL should be determined before initiating ART (A-II).
- •
PVL is the main parameter for evaluating the virological efficacy of ART and for defining virological failure (A-I).
- •
The objectives of virological suppression should be met both in ART-naïve patients and in those who have experienced previous therapeutic failure (A-II).
- •
PVL should be determined using a technique with a quantification limit of at least 50copies/mL. The same technique should always be used (A-II).
- •
If decisions on therapy are to be taken based on PVL, they should be confirmed with a second determination (A-II).
Plasma concentration of antiretroviral drugs is correlated with efficacy and toxicity; therefore, determination of their levels could prove useful in certain situations.
Recommendation
- •
Determination of the plasma concentration of antiretroviral drugs is not recommended for habitual monitoring of HIV-infected patients (A-II).
- •
Determination of the plasma concentration of antiretroviral drugs may be indicated in specific clinical situations (e.g., risk of pharmacological interactions, organ transplantation, extreme underweight or overweight [morbid obesity], pregnancy, and renal or hepatic insufficiency) and to confirm suspected poor adherence to therapy (B-III).
Viral genome mutations are the consequence of rapid HIV-1 turnover and error-prone reverse transcriptase. The emergence of resistant mutations is associated with virologic failure. Resistance mutations can be either primary or secondary to virologic failure.
Recommendation
- •
Genotyping should be performed for detection of HIV resistance mutations in all patients, both at diagnosis of HIV infection and before initiating ART (if ART is deferred) (A-II).
- •
Genotyping should be performed for detection of HIV resistance mutations in all patients whose therapy has failed (A-I).
The presence of the HLA-B*5701 allele is associated with hypersensitivity reaction to abacavir (ABC), a life-threatening multi-organ clinical syndrome observed during the first 6 weeks of treatment.
Recommendation
- •
HLA-B*5701 should be determined in all patients before initiating an ART regimen containing ABC (A-I).
- •
ABC should not be prescribed if the result of the HLA-B*5701 determination is positive (A-I).
A tropism assay is useful when prescribing maraviroc.
Recommendation
- •
Viral tropism should be determined before starting therapy with a CCR5 inhibitor (A-I)
The main objectives of ART are to reduce HIV-associated morbidity and mortality, restore and preserve immune function, prevent the harmful effect of viral replication on possible existing comorbid conditions, and impede transmission of HIV.
When should ART be initiated?Recommendation
- •
ART should be initiated in all HIV-infected patients to prevent disease progression, reduce viral transmission, and limit any harmful effects on possible co-existing comorbid conditions. The strength of the recommendation varies depending on the circumstances (see Table 1).
Table 1.Indications for ART in patients with chronic HIV infection.a
General recommendation ART should be administered to all HIV-infected patients.b The strength and grade of the recommendation varies depending on the circumstances, as follows: Condition/circumstance Strength and grade Diseases classed as B or C by the CDC A-I CD4+ T lymphocytes <350/μL A-I 350–500/μL A-II >500/μL B-III Comorbid conditions HIV-associated nephropathy A-II Chronic HCV infection A-II Chronic HBV infection A-II Age ≥55 years A-II High cardiovascular risk A-II Neurocognitive disorders A-II Cancer A-II Risk of transmission Pregnant women A-I Heterosexual transmission A-I Transmission between MSM A-III Abbreviations: HIV, human immunodeficiency virus; CDC, Centers for Disease Control and Prevention; HCV, hepatitis C virus; HBV, hepatitis B virus; MSM, men who have sex with men.
aIt is important to evaluate the antiretroviral drugs that comprise the initial regimen on an individual basis by weighing up the advantages and disadvantages of each of the options.
The patient's disposition and motivation is a critical factor that should be taken into account when deciding when to start therapy.
- •
Initiation of ART should always be evaluated on an individual basis. Both CD4+ T-lymphocyte count and PVL should be evaluated before taking the decision to initiate ART. Furthermore, the patient should be offered a series of options, and the therapeutic regimen should be adapted to lifestyle, comorbid conditions, and possible drug interactions. The risk of poor adherence should also be assessed (A-III).
Recommendation
- •
Initial ART can be a combination of 2 nucleoside reverse transcriptase inhibitors (NRTI) and 1 non-nucleoside reverse transcriptase inhibitor (NNRTI), 2 NRTI and 1 ritonavir-boosted protease inhibitor (PI/r), or 2 NRTI and 1 integrase inhibitor. Antiretroviral drugs are set out in Table 2 (A-I).
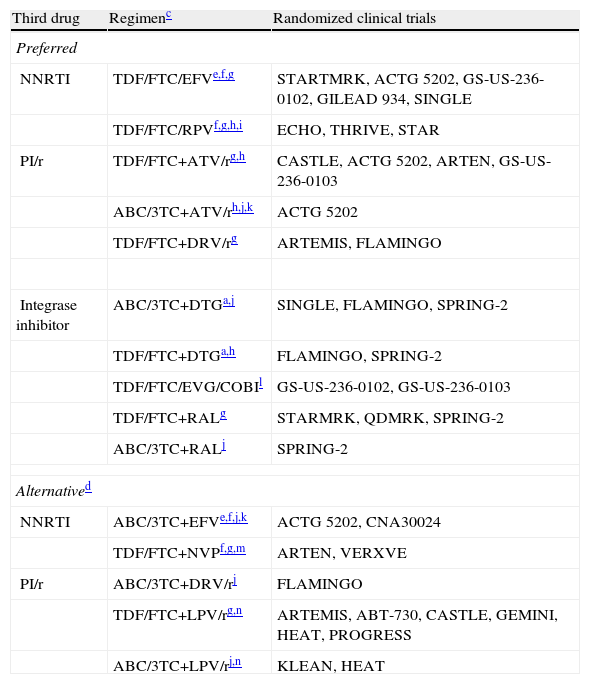
Table 2.Recommended initial ART regimens.b
Third drug Regimenc Randomized clinical trials Preferred NNRTI TDF/FTC/EFVe,f,g STARTMRK, ACTG 5202, GS-US-236-0102, GILEAD 934, SINGLE TDF/FTC/RPVf,g,h,i ECHO, THRIVE, STAR PI/r TDF/FTC+ATV/rg,h CASTLE, ACTG 5202, ARTEN, GS-US-236-0103 ABC/3TC+ATV/rh,j,k ACTG 5202 TDF/FTC+DRV/rg ARTEMIS, FLAMINGO Integrase inhibitor ABC/3TC+DTGa,j SINGLE, FLAMINGO, SPRING-2 TDF/FTC+DTGa,h FLAMINGO, SPRING-2 TDF/FTC/EVG/COBIl GS-US-236-0102, GS-US-236-0103 TDF/FTC+RALg STARMRK, QDMRK, SPRING-2 ABC/3TC+RALj SPRING-2 Alternatived NNRTI ABC/3TC+EFVe,f,j,k ACTG 5202, CNA30024 TDF/FTC+NVPf,g,m ARTEN, VERXVE PI/r ABC/3TC+DRV/rj FLAMINGO TDF/FTC+LPV/rg,n ARTEMIS, ABT-730, CASTLE, GEMINI, HEAT, PROGRESS ABC/3TC+LPV/rj,n KLEAN, HEAT NNRTI, non-nucleoside reverse transcriptase inhibitor; PI/r, protease inhibitor boosted with ritonavir.
aDTG has been recommended by the CHMP (Committee for Medicinal Products for Human Use) of the EMA, although it has not yet been marketed; therefore, combinations including this agent cannot be used at present.
bOrdered alphabetically according to third drug. Preparations combining fixed-dose drugs should be used. Evidence to support considering FTC and 3TC as therapeutic equivalents is insufficient; therefore, the use of one or the other drug in the regimens chosen depends mainly on experience with their use alongside the other drugs in the combination. See text for recommendations on dual therapy as initial treatment.
cThe comments reflect aspects to be taken into account when selecting a regimen; however they are not an exhaustive guide on the precautions to be taken when using these drugs. More detailed information can be obtained by reviewing the text and consulting the relevant summary of product characteristics.
dAlternative regimens are also efficacious and well tolerated, although they have lower-grade scientific evidence or are affected by potential disadvantages compared with the preferred regimens. They may be preferred for some patients.
eAvoid in women who are planning on becoming pregnant and in patients with non-stabilized neuropsychiatric disorders. Use with caution in patients who perform dangerous tasks if they present symptoms of somnolence, dizziness, and/or concentration disorders.
gUse TDF with caution in patients with risk factors for renal insufficiency. TDF is contraindicated in patients with GFR <30mL/min; the dose should be adjusted if GFR is 30–49mL/min. The combination of PI/r and TDF increases somewhat the risk of nephrotoxicity.
iNot authorized in patients with PVL >100,000copies/mL; however, in patients with PVL <100,000copies/mL, it has shown greater efficacy than treatment with TDF/FTC/EFV. Always take with meals.
- 1.
NRTI
The NRTI combinations of choice are considered to be those comprising tenofovir/emtricitabine (TDF/FTC) and those comprising abacavir/lamivudine (ABC/3TC). Co-formulated preparations are recommended.
Recommendation
- •
The NRTI combinations of choice for initial regimens are TDF/FTC and ABC/3TC (A-I). Co-formulated preparations are recommended (A-II).
- •
TDF/FTC should be used with caution in patients with renal insufficiency (A-II).
- •
ABC/3TC should be used with caution in patients with a high PVL (>100,000copies/mL) when combined with an NNRTI or a PI/r other than LPV/r (A-II).
- 2.
NNRTI
Recommendations
- •
The combination efavirenz (EFV)/TDF/FTC is considered a preferential option (A-I). The combination EFV+ABC/3TC should be avoided in patients with a PVL >100,000copies/mL (B-I).
- •
EFV is contraindicated during the first trimester of pregnancy. Other options are recommended in women who do not use effective contraception. Similarly, EFV should be avoided in patients who perform dangerous tasks if they present symptoms of somnolence, dizziness, and/or difficulty concentrating (A-III).
- •
Nevirapine (NVP) is contraindicated in women with CD4+ T-lymphocyte counts >250cells/μL and in men with >400cells/μL (A-II).
- •
Rilpivirine (RPV) should not be administered in patients with a PVL >100,000copies/mL (A-II). The combination RPV/TDF/FTC is preferred (A-I).
- 3.
PI/r
Recommendations
- •
The preferred PI-based regimens are ATV/r QD (once-daily boosted atazanavir)+TDF/FTC, and DRV/r QD (once-daily boosted darunavir)+TDF/FTC (A-I). The combination ATV/r+ABC/3TC is also a preferred option, although it should be administered with caution in patients with a PVL >100,000copies/mL (A-I).
- •
Alternative regimens include boosted lopinavir (LPV/r) BID or QD+TDF/FTC or ABC/3TC (B-I). DRV/r+ABC/3TC can also be used, although it has not been formally analyzed in clinical trials (B-III).
- •
ATV and DRV can be boosted indiscriminately with ritonavir (RTV) 100mg or cobicistat (COBI) 150mg (B-II).
- •
LPV/r+3TC and LPV/r+RAL (raltegravir) can be an alternative to conventional triple therapy when neither TDF nor ABC can be used (B-I).
- 4.
Integrase inhibitors
Recommendations
- •
RAL can be used as initial treatment in combination with TDF/FTC (A-I) or ABC/3TC (A-I).
- •
The combinations elvitegravir (EVG)/COBI/TDF/FTC (which should not be used in patients with an estimated glomerular filtration rate <70mL/min) (A-I) and dolutegravir (DTG) with ABC/3TC (A-I) or TDF/FTC (A-I) can be used as initial ART regimens (those including DTG can be used when it comes onto the market).
PVL is considered undetectable at <50copies/mL. Most clinical trials on switching ART include patients who have maintained virological suppression at this level for at least 6 months.
There are several reasons for changing an efficacious ART regimen (e.g., toxicity, comorbid conditions, drug interactions, and reducing the pill burden or number of daily doses). However, all switches in efficacious ART have the common and priority objective of maintaining an undetectable PVL.
Efficacious ART can be switched in 2 ways: proactively, which is recommended when attempting to prevent a severe or incurable adverse event, and reactively, which is mandatory in the case of an adverse effect.
After switching ART in this context, maintenance of virological suppression and performance of relevant laboratory tests should be evaluated within 3–6 weeks.
Virological considerations when switching efficacious ARTRecommendation
- •
Switching from a regimen comprising 2 NRTI+PI/r to one comprising 2 NRTI+1 NNRTI, RAL, or unboosted ATV should only be allowed if the antiviral activity of the 2 NRTI and the third drug can be guaranteed (A-I).
- 1.
Switching between drugs from the same family
- (a)
NRTI
- (a)
Recommendation
- •
Proactive switch from d4T or ZDV to TDF or ABC in order to prevent or try to reverse lipoatrophy associated with thymidine analogs (A-I).
Recommendation
- •
The association between ABC and increased incidence of cardiovascular events is open to debate. This committee cannot make a recommendation on the strength of evidence for switching from ABC/3TC to TDF/FTC.
Recommendation
- •
The switch from TDF to ABC is a valid option in patients with osteopenia or osteoporosis associated with TDF, as long as HLA-B*5701 is negative (A-II).
- (b)
NNRTI
Recommendation
- •
In patients with adverse central nervous system (CNS) effect caused by EFV/TDF/FTC, the switch to RPV/TDF/FTC is one of the options that can improve the symptoms associated with EFV (A-II). There are no data in favor of recommending a proactive switch in patients who do not have CNS symptoms or comparative data on switching other antiretroviral drugs that do not cause CNS effects.
Recommendation
- •
In patients with CNS adverse effects caused by EFV, the switch to ETR can lead to an improvement in EFV-associated neuropsychological symptoms (A-II). There are no data to recommend a proactive switch in patients who do not have CNS symptoms or data on switching to other antiretroviral drugs that do not cause CNS effects.
Recommendation
- •
In patients with CNS adverse effects caused by EFV, switching to NVP could improve EFV-associated neuropsychological symptoms (A-II). There are no data to recommend a proactive switch in patients who do not have CNS symptoms or data on switching to other antiretroviral drugs that do not cause CNS effects. Switching is also an option in patients with elevated LDL cholesterol caused by EFV (A-II).
Recommendation
- •
Switching to EFV/TDF/FTC is an option in patients taking ART with EFV and NVP whose daily pill burden should be reduced (A-I).
- (c)
Protease inhibitors
Recommendation
- •
In patients taking ATV/r+ABC/3TC, switching to ATV+ABC/3TC is a simplification option when attempting to avoid RTV, owing to the likelihood of increasing the toxicity of ATV (hyperbilirubinemia), toxicity (dyslipidemia, diarrhea), or the risk of interactions with RTV (A-I).
Recommendation
- •
In patients taking ATV/r+TDF/FTC, switching to ATV+ABC/3TC is an option in those cases where both TDF and RTV should be avoided (A-II).
- 2.
Switching to antiretroviral drugs from a different family
- (a)
Switching from TDF to RAL
- (a)
Recommendation
- •
Switching from TDF to RAL in patients who are also taking a PI/r is also an option in patients with reduced bone mineral density (A-II).
- (b)
Switching from EFV to RAL
Recommendation
- •
Switching from EFV to RAL is an option in patients with CNS adverse events caused by EFV (A-II). There are no data to recommend a proactive change in patients with no CNS symptoms or data on switching to other antiretroviral drugs that do not cause CNS effects.
- •
Switching from EFV to RAL is a valid option in patients with dyslipidemia caused by EFV (A-I).
- (c)
Switching from enfuvirtide (ENF) to RAL
Recommendation
- •
Switching from ENF to RAL is a safe option that obviates parenteral administration of enfuvirtide (A-I).
- (d)
Switching from a PI to an NNRTI
Recommendation
- •
Switching to EFV/TDF/FTC is an option in patients who are taking ART with PI. This approach makes it possible to reduce the daily pill burden, although patients may experience EFV-induced CNS adverse effects (B-I).
Recommendation
- •
Switching from a PI to NVP could be an option in patients taking a PI/r in order to avoid the adverse effects of NVP (B-III).
Recommendation
- •
Switching to an ART regimen comprising 2 NRTI and 1 PI/r to the co-formulation RPV/TDF/FTC is a valid option in patients with gastrointestinal disorders or dyslipidemia. It also enables the daily pill burden to be reduced (A-I).
Recommendation
- •
Switching to RAL+2 active NRTI is a valid option for patients with dyslipidemia taking ART with NRTI+1 PI/r (B-I).
- 3.
Monotherapy with PI/r
Recommendation
- •
Monotherapy with DRV/r once daily or LPV/r twice daily is a valid option for preventing adverse effects caused by NRTI if the patient fulfills the following criteria: (1) no chronic hepatitis B; (2) PVL <50copies/mL for at least 6 months; (3) no mutations in the protease gene and no previous virological failure with PI; and (4) good adherence to ART (B-I).
- 1.
Definitions
Virological failure. Two confirmed determinations of PVL >50copies/mL 24 weeks after initiating ART.
Transient rebound of low-level viremia (“blips”). Isolated and transient increase in PVL to between 50 and 200copies/mL.
Immunological failure. Inability to reach an adequate CD4+ T-lymphocyte count despite maintaining a PVL <50copies/mL.
- 2.
Incidence and determinants of virological failure
The determinants of virological failure can be patient-dependent (adherence), drug-dependent (dosing errors, potency, inadequate plasma concentrations, drug or food interactions), and HIV-dependent (pre-existing resistance mutations to any of the drugs making up ART).
- 3.
Objective of ART after virological failure
The objective of ART is to achieve maintained viral suppression. Therefore, a new regimen should be started with 3 or at least 2 active antiretroviral drugs. Rescue ART should not be delayed in order to prevent the accumulation of resistance mutations and increased PVL.
- 4.
Strategies for improving the success of rescue ART regimens
The measures to be taken when prescribing rescue ART are as follows: facilitating adherence, determining resistance mutations and viral tropism, reviewing previous therapy, and occasional monitoring of plasma concentrations of antiretroviral drugs.
- 5.
Clinical scenarios in virological failure
- 5.1.
Virological failure with low viral loads
- (a)
PVL between 50 and 200copies/mL. It is generally not recommended to modify ART, although some studies have demonstrated selection of new resistance mutations and an association between bacterial translocation and systemic inflammation.
- (b)
PVL between 200 and 1000copies/mL. This level is associated with selection of resistance mutations.
Intensification of ART by adding a single active drug is contraindicated in these situations.
- (a)
- 5.2.
Early virological failure
Early virological failure occurs after the first line of ART. Selection of resistance mutations and second-line regimens differ according to the initial ART regimen applied. It is generally recommended to use a PI/r with 2 antiretroviral drugs, preferably NRTI, that conserve their antiviral activity. DRV/r is the most efficacious PI/r of all the rescue lines analyzed. In patients whose first NNRTI-based ART regimen fails (i.e., one based on NVP or EFV), a dual therapy regimen with LPV/r+RAL is not inferior to LPV/r+2 or 3 NRTI (SECOND LINE study).
- 5.3.
Advanced virological failure
Advanced rescue therapy is a rescue regimen that is administered when virological failure has occurred with at least 2 ART lines. A regimen comprising 3 or at least 2 active antiretroviral drugs can be designed by combining drugs from different families.
- 5.4.
Virological failure in patients with no therapeutic options
In this setting, it is impossible to design an ART regimen with a minimum of 2 fully active antiretroviral drugs. Most patients continue to have relatively stable CD4+ T-lymphocyte counts. ART should not be suspended. The ART regimen should be non-suppressing and easy to take, with minimum toxicity. It should also be able to reduce viral replicative capacity and not generate resistance mutations. Furthermore, the patient should be referred to a specialized center with experience in treating this population and where access to new antiretroviral drugs is provided through clinical trials or expanded-access studies.
- 5.1.
Recommendations (switching ART because of virological failure)
- •
The objective of rescue ART is to achieve a PVL <50copies/mL (A-II).
- •
Switching ART because of virological failure should be performed early to avoid accumulation of mutations and to facilitate the response to the new treatment (A-III).
- •
The new ART regimen should contain 3 totally active antiretroviral drugs. If this is not possible, 2 fully active drugs should be combined with other drugs that maintain partial virological activity, especially in the case of advanced rescue in patients with limited therapeutic options (A-I).
- •
Resistance and viral tropisms should be assessed in order to design the best alternative regimen. The test should be performed while the patient is receiving the failed treatment or as soon as possible after suspension of the failed treatment. If the results of previous genotyping tests are available, all the resistance mutations detected should be evaluated (A-I).
- •
The causes of virological failure—adherence, drug or food interactions, previous therapy, and previous toxicity—should be analyzed. The new regimen should be comfortable and well tolerated (A-III).
- •
In patients with virological failure, DRV/r is the PI/r that has proven most efficacious in all rescue lines. In the case of patients with previous virological failure and accumulated resistance mutations, depending on the genotyping test used and viral tropism, the most efficacious combination is DRV/r±ETR+1–2 antiretroviral drugs from a family that has not previously been used (integrase inhibitors, MVC, or ENF) (A-I).
- •
In patients in whom therapy with RAL has failed, DTG (50mg, BID) combined with optimized therapy is the regimen of choice (A-II).
- •
The use of TPV (tipranavir)/r, ENF, or thymidine analogs is restricted to patients with no other therapeutic options (A-III).
- •
ART should not be suspended in patients with advanced virological failure and no therapeutic options (A-II). In this situation, the approach should involve antiretroviral drugs that reduce viral replicative capacity and do not lead to resistance mutations that might compromise future treatments (e.g., 3TC/FTC, TDF, and AZT). The evolution of the CD4+ T-lymphocyte count and PVL should be closely monitored (A-III).
- •
In advanced virological failure, it is recommended to consult with clinicians and virologists who are specialized in resistance and rescue ART and who have access to restricted use of antiretroviral drugs through expanded-access programs (A-III).
- 1.
Adherence
Adherence to ART is the patient's ability to become suitably involved in the choice, initiation, and completion of his/her treatment in order to achieve an undetectable PVL.
Recommendations
- •
Before initiating ART, the patient should be prepared and factors likely to limit adherence should be identified and corrected (A-III).
- •
Once ART has been initiated, a first check-up should be made after 2–4 weeks to verify adherence and correct adherence problems if necessary (A-III).
- •
Adherence should be monitored and reinforced at visits to the doctor (A-III).
- •
Adherence should be monitored by a multidisciplinary team including a doctor, nursing staff, specialists in psychological support, and a hospital pharmacist (A-III).
- •
In the case of patients whose adherence is irregular, it is preferable to use regimens based on PI/r in order to prevent the development of resistance (A-I).
- •
Using fixed dose combinations of antiretroviral drugs simplifies ART and thus facilitates continued adherence. The use of whole regimens in a single tablet is the most efficient strategy for preventing selective poor adherence (A-II).
- 2.
Tolerability and adverse effects
Tolerability depends on drug-related factors (number and size of tablets, administration requirements, and number and intensity of immediate side effects) and patient-related factors (age, sex, weight, clinical situation, and expectations from treatment).
- (a)
Immediate adverse effects
The immediate adverse effects are well defined. In some cases, these can be anticipated and are usually easy to control. Adverse effects are usually gastrointestinal, cutaneous, or neuropsychological.
Recommendations
- •
Avoid the use of antiretroviral drugs whose immediate adverse effects are similar to clinical manifestations or laboratory abnormalities that are already present in a specific patient (A-II). HLA-B*5701 typing is mandatory before prescribing ABC, since it has a positive predictive value of almost 100% for the risk of hypersensitivity reaction to this drug (A-I).
- •
The patient should be informed about the correct way to take an ART regimen and the possibility of immediate adverse events. In any case, the patient should be told how to deal with specific adverse events and always be able to contact the doctor directly. Mild immediate adverse events can be treated symptomatically by evaluating the patient's progress and tolerability. If the adverse effect is very intense or long-lasting or cannot be tolerated by the patient, the potential culprit antiretroviral drug(s) should be switched (A-I).
- (b)
Late adverse effects
Late adverse effects are worse and more difficult to prevent and control. They exacerbate the symptoms of chronic diseases associated with aging and affect the functioning of organs and systems. In general, the absolute risk of late adverse effects of currently recommended antiretroviral drugs is very small.
Recommendations
- •
ART should be tailored by evaluating the risk or presence of chronic diseases in such a way that the regimen selected does not contain antiretroviral drugs that can favor the onset or progression of these diseases (A-II).
- •
Withdrawal of some of the antiretroviral drugs involved in late adverse effects can improve—albeit partially—the underling clinical abnormality, although it is not known whether such a modification can alter the natural history of the specific chronic disease or survival. Antiretroviral drugs contribute collaterally to the risk or progression of specific chronic diseases, although other factors are generally considered to be more important. Priority should be given to interventions to address these factors (A-II).
- 3.
Drug interactions
Interactions between antiretroviral drugs or between antiretroviral drugs and other agents, food, or herbal products could have significant clinical consequences.
Recommendations
- •
All medications, natural products, and alternative medicines taken by the patient should be recorded in the clinical history in order to evaluate potential interactions (A-III).
- •
Contraindications should be taken into account and the corresponding dose adjustments made where necessary (A-I).
- •
Plasma levels should be monitored when prescribing 2 or more drugs with potential pharmacokinetic interactions in order to avoid toxicity or lack of efficacy (A-II).
- 1.
Acute HIV infection
In more than 50% of cases, acute HIV infection is characterized by self-limiting acute febrile syndrome similar to influenza or infectious mononucleosis. Acute infection (first 30 days) should not be confused with recent infection (patients diagnosed during the previous 6 months).
Recommendations
- •
Patients with acute symptomatic infection should initiate ART immediately in all severe cases (with involvement of the CNS or organ involvement, long duration of symptoms [more than 7 days], B or C events according to the 2003 CDC classification, or a CD4+ T-lymphocyte count <350cells/μL) (A-II). ART should be considered in all other patients (B-III).
- •
In asymptomatic patients with acute infection, ART should be initiated during the first 4 months if the patient has a CD4+ T-lymphocyte count <500cells/μL or a PVL >100,000copies/mL (B-II).
- •
ART should be initiated in all cases where there is a high risk of transmission of HIV (A-II).
- •
ART should be initiated in those indications where initiation is independent of the CD4+ T-lymphocyte count and which are set out in the section on chronic HIV infection (A-II) (Table 1) and when acute HIV infection occurs during pregnancy (A-I).
- •
If ART is to be initiated, it should be done so with the same preferential regimens used to treat chronic HIV infection (A-I) (Table 2). A regimen comprising 2 NRTI and an integrase inhibitor could reduce PVL more rapidly during the first 4–8 weeks than PI or NNRTI and, thus, make it easier to reduce transmission of HIV. The combination of RAL+2 NRTI (preferably TDF/FTC) would also have the advantage of reaching higher concentrations in genital tract secretions (B-III).
- •
Testing for resistance and viral tropism should always be performed at diagnosis of acute or recent infection, irrespective of whether ART is to be initiated (A-II).
- •
If the results of resistance testing are not available, it is preferable to begin with a regimen based on a PI/r until the results become available (A-II).
- •
If ART is initiated, it should be administered indefinitely (A-I). In patients with no criteria for ART, the indication should be re-evaluated at 6 months, when the infection becomes chronic.
- 2.
Infection by HIV-2
The genomic organization of HIV-2 is similar to that of HIV-1, except for certain structural differences that can significantly affect its pathogenicity and its sensitivity to antiretroviral drugs.
Recommendations
- •
The general principles of ART in patients infected by HIV-2 should be the same as those of HIV-1 infection (A-III).
- •
The preferred regimen for initial ART in these patients is the combination of 2 NRTI and 1 PI/r (A-III).
- •
The use of NNRTI, MVC, or ENF is contraindicated for the treatment of HIV-2 infection (A-I).
- 3.
Pregnancy
A specific GESIDA and PNS document on women and pregnancy is available. The most important recommendations are summarized below.
Recommendations
- •
All pregnant women must undergo HIV serology testing. If the result is negative, testing must be repeated during the third trimester (A-II).
- •
Pre-pregnancy counseling must form part of health care for HIV-infected women of childbearing age (A-II).
- •
ART is indicated in all pregnant women, irrespective of CD4+ T-lymphocyte count and PVL, in order to ensure that PVL remains undetectable (A-I).
- •
The choice of specific antiretroviral drugs should be based on resistance studies, drug safety, and ease of adherence. If there are no resistance mutations, the regimen of choice is ZDV+3TC+LPV/r (A-I); if resistance mutations are detected, patients can receive any of the “recommended” and “alternative” antiretroviral drugs after a personalized evaluation (A-III).
- •
Intrapartum treatment should be with intravenous ZDV, especially in women with PVL >400–1000copies/mL, irrespective of the ART previously taken by the patient (A-I).
- •
Elective cesarean delivery is indicated at week 38 in women with a pre-labor PVL of >1000copies/mL (A-II).
- •
Mothers cannot breastfeed. Adapted formula food must be used (A-I).
- 4.
Comorbid conditions
- (a)
Initial ART in patients with opportunistic infections other than tuberculosis
Recommendations
- •
ART should be started within the first 15–30 days of treatment of the opportunistic infection (A-II).
- •
In the case of cryptococcal meningitis, it seems prudent to wait 15 days until CSF has cleared, or at least until the antigen load has fallen, before initiating ART (A-II). In addition, intracranial hypertension should be closely monitored (A-I).
- (b)
ART and tuberculosis
Treatment of tuberculosis in HIV-infected adults was the subject of a recent consensus document from GESIDA/Secretariat of the National AIDS Plan, which is available for consultation.
Optimal timing of ARTRecommendations
- •
ART should always be started during treatment of tuberculosis, irrespective of the CD4+ T-lymphocyte count, since it reduces the risk of death (A-I). The optimal time for initiating ART depends on the CD4+ T-lymphocyte count.
- •
If the CD4+ T-lymphocyte count is <50cells/μL, ART should be started as soon as possible, after verifying tolerance to anti-tuberculosis treatment, but not later than the first 2 weeks (A-I).
- •
If the CD4+ T-lymphocyte count is >50cells/μL, initiation of ART can be delayed until the intense phase of anti-tuberculosis treatment has been completed (8 weeks). This approach reduces the risk of adverse effects and the development of immune reconstitution inflammatory syndrome (IRIS) without compromising survival (A-I).
Drug interactions constitute the main difficulty when attempting to treat tuberculosis and HIV infection simultaneously.
Recommendations
- •
Choice of NRTI. No significant interactions have been identified between anti-tuberculosis drugs and NRTI, neither is there evidence of toxicity between the two. Therefore, ABC, TDF, 3TC, and FTC can be used in these patients with no added risks (A-I).
- •
Choice of the third drug. Since most experience and the best results have been obtained with EFV, this is the antiretroviral drug of choice (A-I). The dose of EFV is standard for all patients (600mg/d), irrespective of body weight and with no need to increase to 800mg/d (A-I).
- •
Alternative third drugs. Alternative regimens can contain NVP at regular doses (A-II), RAL at 800mg/12h (A-II), and MVC at 600mg/12h in the absence of potent CYP3A4 inhibitors (A-III).
- •
Drugs that cannot be used. The other NNRTI (RPV and ETR), PI (whether boosted or not with RTV), and EVG should not be co-administered with rifampicin. In the exceptional case of a PI being the only option for ART, rifampicin should be replaced by rifabutin and the corresponding adjustment in drug doses should be made (A-II).
IRIS is a frequent complication, especially in patients with a very low CD4+ T-lymphocyte count and when ART is initiated very early with respect to anti-tuberculosis treatment.
Recommendations
- •
If the patient develops IRIS, neither ART nor anti-tuberculosis medication should be interrupted (A-III).
- •
The symptoms of IRIS can by managed by adding non-steroidal anti-inflammatory drugs in mild to moderate cases (A-III) or corticosteroids in moderate to severe forms (A-II).
- (c)
Renal insufficiency
The GESIDA/PNS consensus document on diagnosis, prevention, and treatment of renal disorders in HIV-infected patients should be consulted. The 2 major areas in HIV infection and kidney disorders are as follows:
HIV-associated kidney diseaseRecommendations
- •
The main therapeutic strategies in HIV-infected patients with kidney disease are ART (A-II), angiotensin blockers (B-III), angiotensin enzyme-converting inhibitors or angiotensin II receptor antagonists, either in monotherapy or in combination, at increasing doses to reduce proteinuria values to 1g/d and to achieve blood pressure <130/80mmHg.
- •
Corticosteroids should only be used when there is no improvement in kidney function parameters with ART and angiotensin blockers (C-III).
- •
The indications for renal replacement therapy with dialysis or transplant in these patients are similar to those of other chronic kidney diseases in the general population (A-III).
Recommendations
- •
It is necessary to adjust the dose of NRTI, except for ABC (A-II).
- •
No dose adjustment is required for NNRTI, PI, ENF, or RAL (A-II).
- •
The dose of MVC should be adjusted if it is used in combination with potent CYP3A4 inhibitors such as PI (except TPV/r), ketoconazole, itraconazole, clarithromycin, and telithromycin (A-II).
- •
Co-formulations of antiretroviral drugs are not advised in patients with significant renal insufficiency. In these cases, antiretroviral drugs should be administered separately and the appropriate adjustments made.
- •
In patients with renal insufficiency (any stage), kidney function should be closely monitored and nephrotoxic drugs avoided (A-III).
- •
In patients with advanced chronic renal insufficiency, the dose should be adjusted according to the recommendations of the summary of product characteristics, taking into account possible drug interactions, which are more common and more dangerous in this situation (A-II).
- (d)
Liver disease (HCV, HBV, cirrhosis)
Recommendations
- •
Patients co-infected with HCV should initiate ART irrespective of their CD4+ T-lymphocyte count (A-II).
- •
Patients co-infected with HBV for whom treatment of HBV infection is indicated should initiate ART containing TDF (A-I).
- •
In patients with >500 CD4+/μL who need treatment for HCV infection, it is preferable to delay initiation of ART until treatment of HCV has finished (A-II).
The choice of antiretroviral drugs must be made taking into account potential liver toxicity, presence of cirrhosis, HBV co-infection, and the need for simultaneous treatment of HCV.
Recommendations
- •
Any antiretroviral drug can be used in patients with chronic liver disease and normal liver function, including patients with cirrhosis (Child–Pugh, class A) (A-I), although it seems reasonable to avoid didanosine (A-III).
- •
In the case of cirrhosis with hepatocellular insufficiency (Child B or C), the dose of antiretroviral drugs should be adjusted, ideally by measuring plasma concentrations (B-II). If this is not possible, it must be remembered that the therapeutic margin of PI is greater than that of EFV in this setting (A-III). The third drugs of choice in these patients are FPV/r at doses adjusted to the Child–Pugh stage (A-I) and RAL, for which no dose adjustment is necessary (A-III).
- •
The combination of RVB with didanosine, d4T, or ZDV should be avoided (A-I).
- •
If pegylated interferon and EFV are administered simultaneously, the onset of adverse effects in the CNS should be closely monitored (A-II).
- •
If a patient who requires ART is going to initiate treatment with telaprevir, the drugs to be administered are ABC, 3TC, FTC, TDF, RAL, ATV/R, ETR, RPV, and EFV (in the case of EFV, the dose of telaprevir should be increased to 1.125mg/8h). If TDF is used, the patient should be closely monitored for toxicity; if RPV is used, the QT interval should be monitored on the ECG (A-I).
- •
If boceprevir is used in a patient who requires ART, it can be administered with TDF, ABC, 3TC, FTC, RAL, RPV, and ETR (A-I). If ETR is chosen, HIV PVL should be closely monitored. In patients with undetectable HIV PVL and no suspected resistance with the ART used, ATV/r can be administered (B-I), again, with close monitoring of HIV PVL.
- (e)
Cancer
The reader is referred to the GESIDA documents on cancer in HIV-infected patients.
Recommendations
- •
ART is an essential component of the treatment of HIV-infected patients with Kaposi sarcoma or non-Hodgkin lymphoma (A-II).
- •
Patients with other types of cancer who are not receiving ART should initiate therapy as soon as possible (A-III).
- •
Given its pharmacological characteristics, excellent tolerance, and minimal interactions, RAL should be the antiretroviral drug of choice, where possible, in patients receiving chemotherapy (A-III).
Antonio Antela has carried out consultancy work for Abbvie, Bristol-Myers Squibb, Gilead Sciences and Janssen-Cilag; he has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme and ViiV Healthcare, as well as payments for developing educational material for Boehringer Ingelheim, Gilead Sciences and ViiV Healthcare.
José R. Arribas has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck, Tobira and ViiV Healthcare; he has received grants for clinical research from Janssen, MSD and Gilead; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Víctor Asensi has carried out consultancy work for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmith Kline and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Bristol-MyersSquibb, Gilead Sciences, Janssen, Merck, Boehringer-Ingelheim, GlaxoSmith Kline and ViiV Healthcare; he has received payments for the development of educational presentations for Bristol-Myers Squibb.
Juan Berenguer has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen Cilag, Janssen Therapeutics, Merck Sharp & Dohme and ViiV Healthcare; he has received grants for clinical research from Bristol-Myers Squibb, Merck Sharp & Dohme and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Janssen-Cilag, Merck Sharp & Dohme and ViiV Healthcare.
José R. Blanco has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Janssen, Merck and ViiV Healthcare, as well as payments for the development of educational presentations for Gilead Sciences and Bristol-Myers Squibb.
Bonaventura Clotet has carried out consultancy work or has participated in clinic trials or giving talks with financial rewards from the following pharmaceutical companies: BMS, Abbvie, Gilead, Janssen, Merck (MSD) and ViiV Healthcare.
Pere Domingo has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; he has received grants for clinical research from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare, and has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare.
M. Jose Galindo has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, and Merck; has received grants for clinical research from Abbvie, Boehringer Ingelheim, Glaxo, and Janssen, and has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche, has collaborated in the preparation of educational materials for Janssen, Pfizer, ViiV, Glaxo and Abbvie.
José M. Gatell has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Merck and ViiV Healthcare; he has received grants for clinical research from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Merck and ViiV Healthcare.
Juan González has carried out consultancy work in the pharmaceutical companies of Abbott Laboratories, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbott Laboratories, AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Jose Antonio Iribarren has carried out consultancy work in the pharmaceutical companies, Gilead Sciences and Janssen-Cilag; he has received grants for clinical research from the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Basque Country Government, FIPSE and FISS, grants to attend Congresses from Abbvie, Gilead, Janssen-Cilag and ViiV, and has participated in educational activities, giving talks, or symposia sponsored by Abbvie, Bristol-Myers Squibb, Gilead, Merck, Novartis, Janssen, Pfizer and ViiV.
Jaime Locutura has carried out consultancy work for Abbvie, Bristol-Myers Squibb, Gilead Sciences and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme and ViiV Healthcare, as well as payments for developing educational material for Abbvie, Boehringer Ingelheim and ViiV Healthcare.
José López Aldeguer has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen and ViiV Healthcare; he has received grants for clinical research from Bristol-Myers Squibb, ViiV Healthcare and Merck; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Juan Carlos López Bernaldo de Quirós has carried out consultancy work for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Janssen, Merck-Sharp & Dome, Roche Pharmaceuticals and ViiV Healthcare, and has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Janssen, Merck-Sharp & Dome, Roche Pharmaceuticals and ViiV Healthcare. He has also received payments for developing educational material for Boehringer Ingelheim, Gilead Sciences and ViiV Healthcare.
Fernando Lozano has carried out consultancy work for Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare, and has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, GlaxoSmithKline, Janssen, Merck-Sharp & Dome, Pfizer, Roche Pharmaceuticals and ViiV Healthcare.
Josep Mallolas has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Janssen, Roche and ViiV Healthcare; he has received grants for clinical research from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Roche, Merck and ViiV Healthcare.
Esteban Martínez has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare; he has received monetary payments for giving talks from the pharmaceutical companies of Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck Sharp & Dohme, Theratechnologies, Tibotec, and ViiV Healthcare, as well as payments for developing material for educational presentations for Abbvie, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, and ViiV Healthcare.
Celia Miralles has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she has received monetary payments for giving talks from the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare, as well as payments for developing educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
José M. Miró has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Merck, Novartis and Sanofi; he has received grants for clinical research from Cubist, Novartis, Merck, Health Research Fund (FIS) of the Carlos III Institute Madrid (Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (Madrid)), Foundation for Research and Prevention of AIDS in Spain (FIPSE, Madrid) (Fundación para la Investigación y Prevención del Sida en España), Ministry of Health, Social Security and Equality (MSSSI, Madrid), National Institutes of Health (NIH, Bethesda, MA, USA) and NEAT, and has received monetary payments for giving talks from Novartis and ViiV Healthcare.
Santiago Moreno has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche, he has received grants for clinical research from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche, and has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and Roche.
Rosario Palacios has carried out consultancy work in the pharmaceutical company, Boehringer Ingelheim, and has received monetary payments for giving talks from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, ViiV Healthcare and Roche.
María J. Pérez Elías has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-MyersSquibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; she has received grants for clinical research from the pharmaceutical companies, Abbvie, Gilead Sciences, ViiV Healthcare, and Janssen; she has received monetary payments for giving talks from Abbvie, Bristol-MyersSquibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payments for developing educational presentations for Abbvie, Bristol-Myers Squibb, Janssen, Merck and ViiV Healthcare.
Juan A. Pineda has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Pfizer, Schering-Plough and ViiV Healthcare, has received grants for clinical research from Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Schering-Plough and ViiV Healthcare, and has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Glaxo Smith Kline, Gilead Sciences, Janssen, Merck, Roche, Schering-Plough and ViiV Healthcare
Daniel Podzamczer has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he has received grants for clinical research from Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, and ViiV Healthcare, he has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Glaxo Smith Kline, Merck and ViiV Healthcare.
Rosa Polo declares to have no conflicts of interests.
Joaquin Portilla has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, he has received grants for clinical research from Abbvie, Janssen, Merck and ViiV Healthcare, and has received monetary payments for giving talks from Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, Roche and ViiV Healthcare.
Federico Pulido has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbott Laboratories, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Esteban Ribera has carried out assessments or has received grants for research or teaching in relation to HIV infection from the following pharmaceutical companies: Abbvie, Boehringer Ingelheim, BMS, Ferrer International, Gilead, GSK, Janssen-Cilag, MSD, Pfizer, Roche Farma, Schering Plough and ViiV.
Melchor Riera has received grants for travel to meetings and Congresses from Janssen and Gilead Sciences and financial support to the Department for teaching or research work from the pharmaceutical companies, Abbvie and Bristol-Myers Squibb.
Antonio Rivero has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare; he has received grants for clinical research from the pharmaceutical companies, Abbvie, Gilead Sciences, Merck and ViiV Healthcare; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare, as well as payments for developing educational presentations for Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare.
Rafael Rubio has carried out consultancy work in the pharmaceutical companies, Abbvie, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Glaxo Smith-Kline, Janssen, Merck and ViiV Healthcare; he has received grants for clinical research from Abbott Laboratories, Gilead, Janssen and Roche; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Glaxo Smith-Kline, Janssen, Merck, Roche and ViiV Healthcare.
Jesús Santos has carried out consultancy work in the pharmaceutical companies, Bristol-Myers Squibb, Gilead Sciences and Janssen; he has received monetary payments for giving talks from Bristol-Myers Squibb, MSD, Janssen and Gilead Sciences, and has received payments for the development of educational presentations for Bristol-Myers Squibb, Gilead Sciences, Janssen and MSD.
Jesús Sanz has carried out consultancy work in the pharmaceutical companies, Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim; he has received monetary payments for giving talks from Abbvie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, ViiV Healthcare and Boehringer Ingelheim, and has received payments for the development of educational presentations for ViiV Healthcare.
Montse Tuset has received grants for clinical research from the pharmaceutical companies, Bristol-Myers Squibb, Gilead Sciences, Merck and Janssen; and has received monetary payments for giving talks from Abbvie, Merck, Gilead and ViiV Healthcare.
Francesc Vidal has received monetary payments for giving talks from Abbvie, Merck and Gilead Sciences.
The Board of Directors of GeSIDA and the AIDS National Plan are grateful for the contributions and opinions of: David Alonso, Marisa Álvarez, Piedad Arazo, Ivan Bernardo, Esther Cabrero, Manuel Cotarelo, Manuel Crespo, Adrián Curran, Federico García, Henar Hevia, Juan Emilio Losa, and Nuria Sánchez.
Writing Committee: Juan Berenguer1, Rosa Polo2, Fernando Lozano3, José López Aldeguer4, Antonio Antela5, José Ramón Arribas6, Víctor Asensi7, José Ramón Blanco8, Bonaventura Clotet9, Pere Domingo10, María José Galindo11, José María Gatell12, Juan González-García6, José Antonio Iribarren13, Jaime Locutura14, Juan Carlos López1, Josep Mallolas12, Esteban Martínez12, Celia Miralles15, José M. Miró12, Santiago Moreno16, Rosario Palacios17, María Jesús Pérez Elías16, Juan Antonio Pineda3, Daniel Podzamczer18, Joaquín Portilla19, Federico Pulido20, Esteban Ribera21, Melchor Riera22, Rafael Rubio20, Jesús Santos17, Jesús Sanz23, Montserrat Tuset12, Francesc Vidal24 and Antonio Rivero25
1Hospital General Universitario Gregorio Marañón, Madrid; 2Secretaría del Plan Nacional sobre el Sida, Ministerio de Sanidad, Servicios Sociales e Igualdad; 3Hospital Universitario Virgen de Valme, Sevilla; 4Hospital Universitario La Fe, IIS La Fe, Valencia; 5Hospital Clínico Universitario, Santiago de Compostela; 6Hospital Universitario La Paz, IdiPAZ. Madrid; 7Hospital Universitario Central de Asturias, Oviedo; 8Hospital San Pedro, Logroño; 9Hospital Germans Trias i Pujol, Badalona; 10Hospital de la Santa Creu i Sant Pau, Barcelona; 11Hospital Clínico Universitario, Valencia; 12Hospital Clínic/IDIBAPS, Universidad de Barcelona, Barcelona; 13Hospital Universitario Donostia, San Sebastián; 14Hospital Universitario, Burgos; 15Complejo Hospitalario Xeral, Vigo; 16Hospital Ramón y Cajal, IRYCIS, Madrid; 17Hospital Universitario Virgen de la Victoria, Málaga; 18Hospital Universitari de Bellvitge, L’Hospitalet, Barcelona; 19Hospital General Universitario, Alicante; 20Hospital Doce de Octubre, Madrid; 21Hospital Vall d’Hebron, Barcelona; 22Hospital Son Espases, Palma de Mallorca; 23Hospital Universitario de la Princesa, Madrid; 24Hospital Universitario Joan XXIII, Tarragona; 25Hospital Reina Sofía, Córdoba
See writing committee in Appendix A.