Solid organ transplant (SOT) recipients are at greater risk than the general population for complications and mortality from influenza infection. We have conducted a systematic review to assess the management and prevention of influenza infection in SOT recipients.
Recommendations are provided about the procurement of organs from donors with influenza infection. We highlight the importance of the possibility of influenza infection in any SOT recipient presenting upper or lower respiratory symptoms, including pneumonia. The importance of early antiviral treatment of SOT recipients with suspected or confirmed influenza infection and the necessity of annual influenza vaccination are emphasized. The microbiological techniques for diagnosis of influenza infection are reviewed. Guidelines for the use of antiviral prophylaxis are provided. Recommendations for household contacts of SOT recipients with influenza infection and health care workers are also included. Antiviral dose adjustment guidelines are presented for cases of impaired renal function and for pediatric populations.
Los receptores de un trasplante de órgano sólido (TOS) presentan un riesgo mayor de complicaciones y una mortalidad más alta de la infección por el virus de la gripe que la población general. Hemos realizado una revisión sistemática para determinar el abordaje de la infección por virus de la gripe en receptores de TOS.
Se realizan recomendaciones sobre la obtención de órganos de donantes con infección por virus de la gripe. Se destaca la importancia de mantener un alto nivel de sospecha de infección gripal en cualquier portador de TOS que consulte por clínica de infección de vías respiratorias altas o bajas, incluida la neumonía. Se resalta la importancia de iniciar tratamiento antiviral precoz en todos los portadores de TOS con sospecha o confirmación de infección por virus de la gripe, así como la importancia de que anualmente reciban la vacuna frente a este virus. Se revisan las diferentes técnicas microbiológicas para la detección de la infección por virus de la gripe. Se aportan directrices para el empleo de profilaxis antiviral. Se incluyen recomendaciones para los sujetos que conviven estrechamente con portadores de TOS infectados por virus de la gripe y para los trabajadores sanitarios. Se incluyen recomendaciones sobre el ajuste de dosis de antivirales en sujetos con deterioro de la función renal y para la población pediátrica.
Worldwide, 40,000 organ transplants are performed annually, with very high success rates. In Spain, 3706 organ transplantations were performed in 2011. Renal transplants were the most common, followed by liver, heart, lung, and others, including dual organ, pancreatic, and intestinal transplantation.
Solid organ transplant (SOT) recipients are at greater risk for complications and mortality from influenza infection than those in the general population due to their immunocompromised status. Compared with the immunocompetent population, patients who have received transplants might have a greater viral burden and shed virus for longer periods of time. After the 2009 influenza pandemic, important information regarding influenza infection has been published in the SOT setting. Based on this, experienced SOT researchers and clinicians, with expertise in adults and pediatrics infectious diseases, nephrology, cardiology and surgery, have developed and implemented this consensus document in collaboration with several Spanish scientific societies, the Spanish National Transplant Organization and the research study networks.
The target populations of this document are adults and children receiving SOT, organ donor and recipient candidates, health-care workers and SOT household contacts. The intended guideline audience includes physicians involved in the care of SOT recipients (including primary care physicians), transplant coordinators and other health-care workers attending SOT recipients. Here we report a consensus from a public health policy perspective with the objective of assessing the available overall evidences and to propose recommendations on the following key questions:
- 1.
How should we proceed when the donor has suspected or confirmed influenza infection?
- 2.
When is influenza chemoprophylaxis indicated for SOT recipients?
- 3.
What should be recommended regarding influenza vaccination for SOT recipients?
- 4.
What recommendations can be given to household contacts of SOT recipients?
- 5.
What should be done to avoid nosocomial transmission of influenza among SOT recipients?
- 6.
When should influenza infection be suspected in SOT recipients?
- 7.
What are the prognostic factors for influenza in solid-organ transplant recipients?
- 8.
If influenza infection is suspected in a SOT recipient, which microbiological studies should be performed?
- 9.
When should a SOT recipient with suspicion of influenza be treated with antivirals?
- 10.
What other therapeutic measures should be adopted in solid-organ transplant recipients with influenza infection?
- 11.
When should antibiotic treatment be administered to SOT recipients with influenza infection?
- 12.
How should neuraminidase inhibitors be used in solid-organ transplant recipients with renal function impairment?
- 13.
What are the special recommendations regarding influenza in SOT pediatric recipients?
Several areas related to infections in SOT recipients that are unresolved and controversial are also discussed, including emerging issues such as donor-derived infection, impact of influenza infection in SOT recipients, time and extension of antiviral therapy, drug-resistant infections, and timing of influenza vaccination after transplantation, among others.
MethodsWe conducted a systematic review to assess the management of influenza infection in SOT recipients. Data for this document were identified by search of Pubmed and references from relevant articles using the search terms “transplant*” and “influenza”. The criteria for search included articles in English that involved human participants. We selected and revised a total of 949 articles from 1971 to October 2012.
Evidence level based on the available literature is given for each recommendation to assess the strength of the evidence for risks and benefits of the procedure. This article was written in accordance with international recommendations on consensus statements (Table 1) and the recommendations of the Appraisal of Guidelines for Research and Evaluation II (AGREE II). The authors met twice for discussing the consensus and to achieve formal recommendations. The coordinators and authors had agreed on the content and conclusions. The consensus statement was sent to the 96 members of GESITRA for external revision of the manuscript. The board of directors of GESITRA will designate the coordinators for updating the statements within 5 years. The full version of the consensus document of this executive summary is available at1.
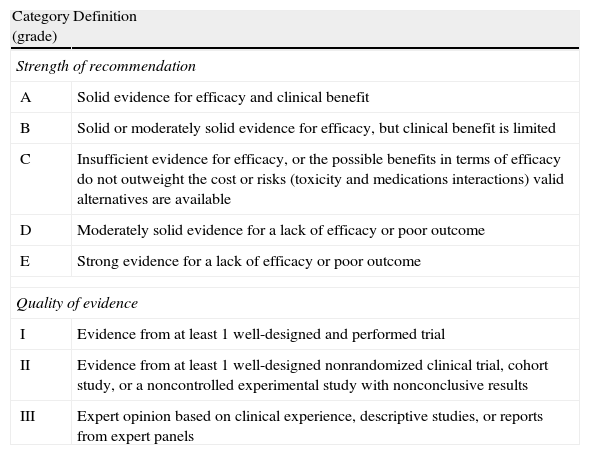
Classification of the recommendations of this consensus document, based on the strength and quality of the evidence analyzed.
| Category (grade) | Definition |
| Strength of recommendation | |
| A | Solid evidence for efficacy and clinical benefit |
| B | Solid or moderately solid evidence for efficacy, but clinical benefit is limited |
| C | Insufficient evidence for efficacy, or the possible benefits in terms of efficacy do not outweight the cost or risks (toxicity and medications interactions) valid alternatives are available |
| D | Moderately solid evidence for a lack of efficacy or poor outcome |
| E | Strong evidence for a lack of efficacy or poor outcome |
| Quality of evidence | |
| I | Evidence from at least 1 well-designed and performed trial |
| II | Evidence from at least 1 well-designed nonrandomized clinical trial, cohort study, or a noncontrolled experimental study with nonconclusive results |
| III | Expert opinion based on clinical experience, descriptive studies, or reports from expert panels |
- 1.
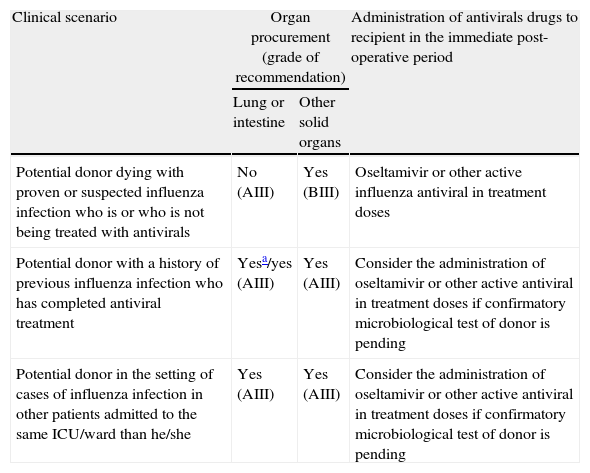
If feasible, potential donors with upper or lower respiratory tract infection symptoms should be microbiologically tested to rule out influenza infection if deceased during the annual influenza epidemic, especially in cases of lung transplantation (BIII).
- 2.
Subjects who died in the context of confirmed or suspected influenza infection with or without having received antiviral treatment can be considered as donors for solid organ transplantation provided that the recipient is prophylactically treated with neuraminidase inhibitors. This recommendation is not applicable for lung or intestine transplantation (BIII).
- 3.
Subjects who died in the context of confirmed or suspected influenza infection should be ruled out as donors for lung or intestine transplantation independently of the treatment provided (AIII).
- 4.
Transplantation from a living donor with influenza infection should be postponed if feasible (AIII).
- 5.
All potential candidates for solid organ transplantation should receive inactivated influenza vaccine annually (AI).
Guidelines for the management of potential donors for solid organ transplantation with suspected or confirmed influenza infection.
| Clinical scenario | Organ procurement (grade of recommendation) | Administration of antivirals drugs to recipient in the immediate post-operative period | |
| Lung or intestine | Other solid organs | ||
| Potential donor dying with proven or suspected influenza infection who is or who is not being treated with antivirals | No (AIII) | Yes (BIII) | Oseltamivir or other active influenza antiviral in treatment doses |
| Potential donor with a history of previous influenza infection who has completed antiviral treatment | Yesa/yes (AIII) | Yes (AIII) | Consider the administration of oseltamivir or other active antiviral in treatment doses if confirmatory microbiological test of donor is pending |
| Potential donor in the setting of cases of influenza infection in other patients admitted to the same ICU/ward than he/she | Yes (AIII) | Yes (AIII) | Consider the administration of oseltamivir or other active antiviral in treatment doses if confirmatory microbiological test of donor is pending |
- 6.
Preexposure and post-exposure antiviral chemoprophylaxis should not routinely be used in solid organ transplant recipients, and might be only reserved for selected cases such as patients who are severely immunosuppressed and at high risk for influenza-related complications (BIII). For rest of the cases, solid organ transplant patients with known exposure to influenza should be advised to seek care if they experience symptoms of influenza infection in order to initiate early antiviral treatment (AII).
- 7.
As infection can occur during chemoprophylaxis, patients should seek medical evaluation if they develop fever or respiratory illness suggestive of influenza infection. In these cases the possibility of an infection with a resistant virus should be considered (BIII).
- 8.
Oseltamivir or zanamivir can be recommended for antiviral chemoprophylaxis of influenza A (H1N1), influenza A (H3N2), or influenza B viral infection (AI).
- 9.
Postexposure chemoprophylaxis should be administered for a total of 10 days after the most recent influenza contact.
- 10.
The duration of pre-exposure chemoprophylaxis should correspond to the entirety of the flu season (BIII). There are no data regarding the safety and tolerability of chemoprophylaxis for more than 6 weeks.
- 11.
Seasonal inactivated influenza vaccination is strongly recommended every year in solid organ transplant recipients (AII).
- 12.
Influenza vaccine can be given after the first month of the transplant (BII).
- 13.
More evidence is required for recommending intradermal vaccination, a second booster dose and high-dose influenza vaccine (CIII).
- 14.
Influenza vaccination is indicated in organ transplant children older than six months. Children younger than 9 years require two doses of the influenza vaccine in cases of not having received a previous vaccine (AII).
- 15.
The live attenuated influenza vaccine is not recommended for transplant patients (EIII).
- 16.
The pneumococcal vaccine is recommended for solid organ transplant recipients (AII).
- 17.
All the persons, including children older than 6 months, who live with or care for solid organ transplant recipients should receive the influenza vaccine every year (AI).
- 18.
These persons should receive the trivalent inactivated vaccine unless contraindicated due to severe adverse reactions (AI).
- 19.
In cases of antecedent of severe adverse reaction to trivalent inactivated vaccine, the live attenuated influenza vaccine can be administered. As a precautionary measure, contacts of solid organ transplant recipients receiving the live attenuated vaccine should avoid providing care for severely immunosuppressed patients for 7 days after vaccination. In case of householders, they should minimize the exposure of respiratory secretions (AI).
- 20.
To avoid influenza transmission, in cases of recipient's household contacts with upper respiratory disease during influenza season, good hand hygiene with water and soap or an alcohol-based hand rub should be encouraged. Linens, eating utensils, and dishes belonging to those who are sick should not be shared without washing thoroughly first. Exposure to respiratory secretions should be minimized by the use of correct cough etiquette and tissues (AI).
- 21.
Household contacts of a solid organ transplant recipient should avoid contact with people who are known to have influenza (BII).
- 22.
In cases of household contact with influenza like illness, solid organ transplant recipients should be advised to seek care and start antiviral therapy if developing respiratory symptoms or fever (AIII).
- 23.
Exceptionally, in selected cases of severely immunosuppressed organ transplant recipients at high risk of influenza infection when a household contact is suspected of having influenza disease, all the family members can be administered prophylaxis with oseltamivir or zanamivir (AII).
- 24.
All the health-care workers attending solid organ transplant recipients should receive influenza vaccine annually (AI).
- 25.
All the health-care workers and visitors to the hospital should strictly comply with hand hygiene recommendations and cough etiquette (AII).
- 26.
All the visitors and health-care workers presenting symptoms of upper or lower respiratory tract infection should avoid entering wards where solid organ transplant recipients are admitted (AIII).
- 27.
Influenza should be considered in the differential diagnosis for fever and/or respiratory symptoms in any hospitalized solid-organ transplant recipient during seasonal epidemic (AII).
- 28.
Solid organ transplant recipients admitted to a hospital with suspected or confirmed influenza virus infection should be placed in a private room where standard and droplet precautions should be adopted (AIII).
- 29.
Wearing a surgical mask and other measures (for example correct hand hygiene) to avoid droplet transmission are recommended when entering the room or cubicle of a patient with confirmed or suspected influenza infection (AI).
- 30.
Wearing a particulate respirator, a non-sterile long sleeved gown and gloves is recommended when performing aerosol-generating procedures (AII).
- 31.
Antiviral prophylaxis with oseltamivir or other neuraminidase inhibitors may be considered for solid organ transplant recipients sharing the hospital room with patients with confirmed influenza infection, regardless of the influenza vaccination status (especially in the case of a lung transplant recipients or severely immunosuppressed solid organ transplant recipients). Early recognition of illness and prompt initiation of treatment is an alternative to antiviral prophylaxis after suspected exposure of solid organ transplant recipients (except for lung transplant recipients or severely immunosuppressed solid organ transplant recipients) (BII).
- 32.
If two or more cases of influenza infection are confirmed in subjects admitted to different rooms of the same ward (outbreak), prophylaxis could be considered for all the solid organ transplant recipients admitted to that ward. In this context, the measures to avoid transmission are especially emphasized (AII).
- 33.
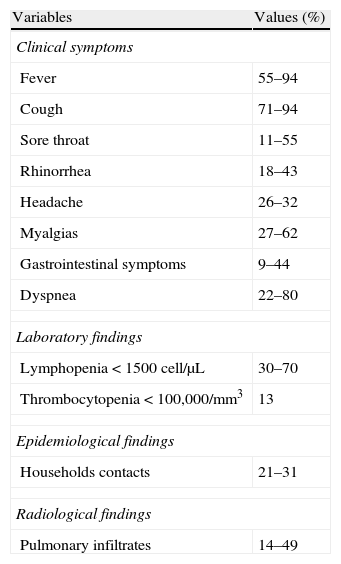
Influenza infection should be clinically suspected in all the solid organ transplant recipients presenting with flu-like symptoms such as fever, rhinorrhea, headache, cough, sore throat, myalgias and dyspnea in an epidemiological setting, (AII) especially in the presence of household contacts with influenza symptoms (AII).
- 34.
Moreover, influenza should be considered in the differential diagnosis of every case of pneumonia occurring in solid organ transplant recipients during the influenza season, (AII) particularly in patients with flu-like symptoms and bilateral pulmonary infiltrates (AII).
Reported clinical symptoms, laboratory data and radiological findings in SOT recipients with influenza infection.
| Variables | Values (%) |
| Clinical symptoms | |
| Fever | 55–94 |
| Cough | 71–94 |
| Sore throat | 11–55 |
| Rhinorrhea | 18–43 |
| Headache | 26–32 |
| Myalgias | 27–62 |
| Gastrointestinal symptoms | 9–44 |
| Dyspnea | 22–80 |
| Laboratory findings | |
| Lymphopenia<1500cell/μL | 30–70 |
| Thrombocytopenia<100,000/mm3 | 13 |
| Epidemiological findings | |
| Households contacts | 21–31 |
| Radiological findings | |
| Pulmonary infiltrates | 14–49 |
- 35.
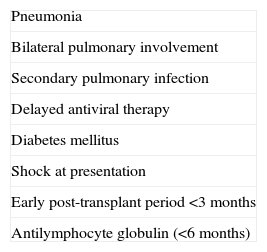
Influenza appears to be most severe in the early post-transplant period (<3 months) in solid organ transplant recipients (AII).
- 36.
The most significant factors associated with a worse outcome are diabetes mellitus, septic shock at presentation, pneumonia and secondary/concomitant pulmonary infection (AII).
- 37.
Early antiviral therapy is associated with better outcomes, and is the only prognostic factor that may be modified by medical intervention (AII).
- 38.
The use of antilymphocyte globulin has also been associated with poor outcomes in solid organ transplant recipients with influenza infection (AII).
- 39.
Influenza infection may also cause indirect effects including allograft rejection (AII).
Prognostic factors of influenza infection in SOT recipients associated with poorest outcome.
| Pneumonia |
| Bilateral pulmonary involvement |
| Secondary pulmonary infection |
| Delayed antiviral therapy |
| Diabetes mellitus |
| Shock at presentation |
| Early post-transplant period <3 months |
| Antilymphocyte globulin (<6 months) |
- 40.
It is recommended to confirm a diagnosis by specific testing when influenza infection is suspected in a transplant patient (AII).
- 41.
Specimens should be collected as soon as possible after illness onset, preferently within the first 48h after the onset of symptoms (AII).
- 42.
Appropriate samples include nasal and oropharyngeal swabs, both placed in a single viral transport medium. If there is clinical or radiological evidence of lower respiratory tract involvement, bronchoalveolar lavage should be considered (AII).
- 43.
Respiratory specimens should be refrigerated at 4°C pending testing and should be tested for influenza as soon as possible after collection (AII).
- 44.
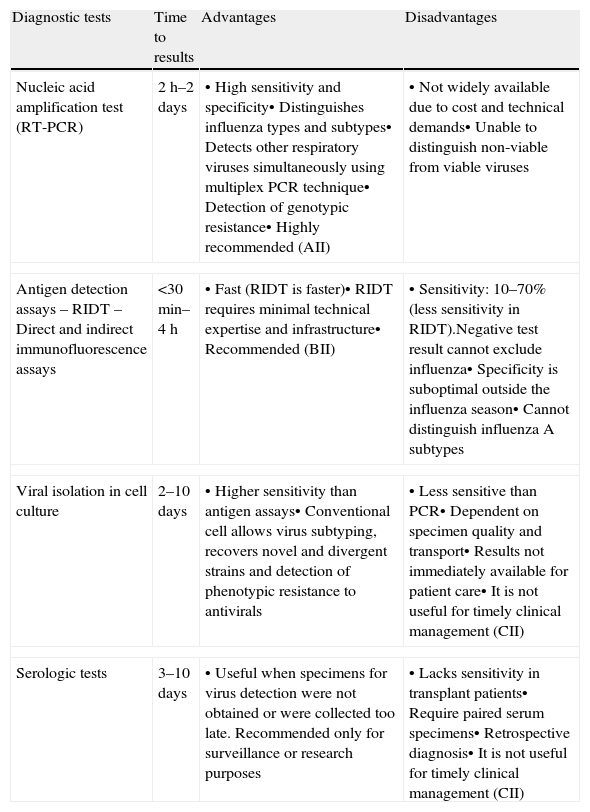
Reverse transcriptase polymerase chain reaction (RT-PCR) is the most sensitive and specific testing modality for influenza and it is useful for differentiating quickly between influenza types and subtypes; if RT-PCR is not available, rapid antigen detection assays may be used, although due to the lower sensitivity of this method as compared to RT-PCR, a negative test result does not rule out influenza virus infection that should be confirmed (AII).
- 45.
During influenza season, in patients with lower respiratory tract disease, both influenza and bacterial etiologies should be considered, regardless of a positive microbiological result in any of them (AII).
- 46.
In any patient undergoing treatment who fails to have an appropriate clinical response within 3–5 days of initiating antiviral therapy or who has a relapsing course despite ongoing therapy, the possibility of antiviral resistance should be considered (AII).
Comparison of diagnostic techniques for influenza virus infection.
| Diagnostic tests | Time to results | Advantages | Disadvantages |
| Nucleic acid amplification test (RT-PCR) | 2h–2 days | • High sensitivity and specificity• Distinguishes influenza types and subtypes• Detects other respiratory viruses simultaneously using multiplex PCR technique• Detection of genotypic resistance• Highly recommended (AII) | • Not widely available due to cost and technical demands• Unable to distinguish non-viable from viable viruses |
| Antigen detection assays– RIDT– Direct and indirect immunofluorescence assays | <30min–4h | • Fast (RIDT is faster)• RIDT requires minimal technical expertise and infrastructure• Recommended (BII) | • Sensitivity: 10–70% (less sensitivity in RIDT).Negative test result cannot exclude influenza• Specificity is suboptimal outside the influenza season• Cannot distinguish influenza A subtypes |
| Viral isolation in cell culture | 2–10 days | • Higher sensitivity than antigen assays• Conventional cell allows virus subtyping, recovers novel and divergent strains and detection of phenotypic resistance to antivirals | • Less sensitive than PCR• Dependent on specimen quality and transport• Results not immediately available for patient care• It is not useful for timely clinical management (CII) |
| Serologic tests | 3–10 days | • Useful when specimens for virus detection were not obtained or were collected too late. Recommended only for surveillance or research purposes | • Lacks sensitivity in transplant patients• Require paired serum specimens• Retrospective diagnosis• It is not useful for timely clinical management (CII) |
RIDT, rapid influenza diagnostic tests.
- 47.
Adequate antiviral therapy should be initiated, as soon as possible, in all the solid organ transplant recipients with suspected influenza infection, (AII) independently of the severity of illness and duration of symptoms (AII) and without waiting for diagnostic results (AII).
- 48.
Early antiviral treatment should be especially recommended in solid organ transplant recipients with influenza pneumonia, (AII) as the presence of this complication is an important factor for poor outcome (AII).
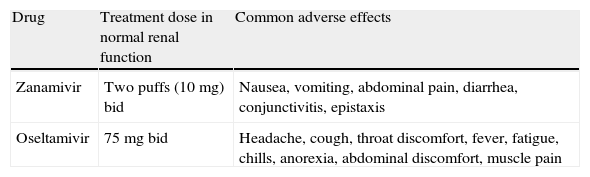
Recommended doses and adverse effects of neuraminidase inhibitors for treating influenza.
| Drug | Treatment dose in normal renal function | Common adverse effects |
| Zanamivir | Two puffs (10mg) bid | Nausea, vomiting, abdominal pain, diarrhea, conjunctivitis, epistaxis |
| Oseltamivir | 75mg bid | Headache, cough, throat discomfort, fever, fatigue, chills, anorexia, abdominal discomfort, muscle pain |
bid, twice daily.
- 49.
Drugs containing salicylates should be avoided in children and adolescents with influenza infection because of the risk of Reye's syndrome (EIII).
- 50.
Immunomodulatory drugs such as statins and macrolides have not demonstrated any benefit in treating influenza infection (CIII).
- 51.
High doses of glucocorticosteroids should be avoided in patients with influenza infection because of the risk of opportunistic infections, prolonged viral shedding, severe disease and death (EII).
- 52.
Secondary/concomitant bacterial infections in SOT recipients with influenza should be suspected in patients with pneumonia, shock, purulent sputum and leukocytosis, especially in diabetic patients, thus antibiotic treatment should be administered in this setting (AIII).
- 53.
Inhaled zanamivir has very limited systemic bioavailability and dose adjustment is not required in patients with renal function impairment (AIII).
- 54.
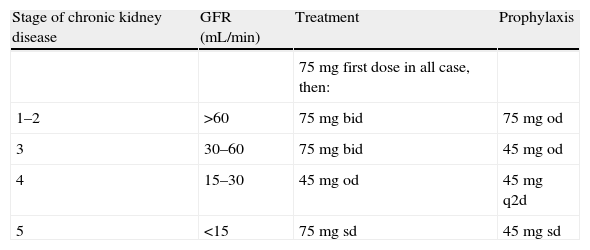
A dosage reduction of oseltamivir is recommended only for patients with a glomerular filtration rate under 30mL/min (AIII).
- 55.
It is recommended to administer 75mg of oral oseltamivir for the first dose in all patients with renal function impairment and then adjust the doses according to renal function (AIII).
- 56.
Oseltamivir does not affect pharmacokinetics of cyclosporine, mycophenolate or tacrolimus (AII).
- 57.
A 30mg dose of oseltamivir given once weekly in chronic ambulatory peritoneal dialysis or after alternate sessions in hemodialysis patients provides sufficient exposure to oseltamivir to allow safe and effective treatment and prophylaxis for influenza infection (AII).
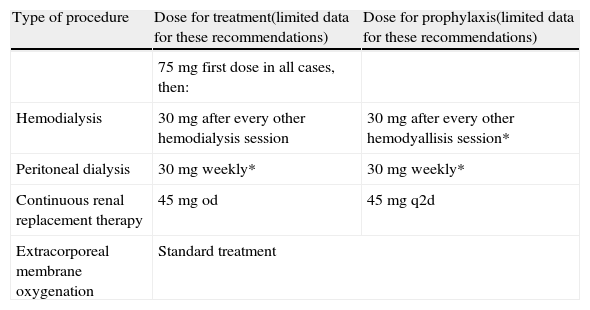
- 58.
A regimen of either 30mg daily or 75mg every 48h is recommended for patients on continuous renal replacement therapies (AII).
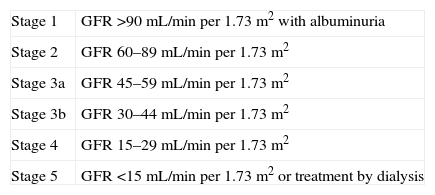
Kidney Disease outcomes Quality Initiative (K/DOQI) stages of chronic kidney disease.
| Stage 1 | GFR >90mL/min per 1.73m2 with albuminuria |
| Stage 2 | GFR 60–89mL/min per 1.73m2 |
| Stage 3a | GFR 45–59mL/min per 1.73m2 |
| Stage 3b | GFR 30–44mL/min per 1.73m2 |
| Stage 4 | GFR 15–29mL/min per 1.73m2 |
| Stage 5 | GFR <15mL/min per 1.73m2 or treatment by dialysis |
Dosage of oseltamivir in patients with renal impairment.
| Stage of chronic kidney disease | GFR (mL/min) | Treatment | Prophylaxis |
| 75 mg first dose in all case, then: | |||
| 1–2 | >60 | 75mg bid | 75mg od |
| 3 | 30–60 | 75mg bid | 45mg od |
| 4 | 15–30 | 45mg od | 45mg q2d |
| 5 | <15 | 75mg sd | 45mg sd |
bid, twice daily; od, once daily; q2d, every other day; sd, single dose.
Oseltamivir doses for patients on renal replacement therapies.
| Type of procedure | Dose for treatment(limited data for these recommendations) | Dose for prophylaxis(limited data for these recommendations) |
| 75 mg first dose in all cases, then: | ||
| Hemodialysis | 30mg after every other hemodialysis session | 30mg after every other hemodyallisis session* |
| Peritoneal dialysis | 30mg weekly* | 30mg weekly* |
| Continuous renal replacement therapy | 45mg od | 45mg q2d |
| Extracorporeal membrane oxygenation | Standard treatment | |
od, once daily; q2d, every other day.
*Dose administered after the session.
- 59.
Influenza vaccine is approved for solid organ transplant infant recipients and recommended for children older than 6 months of age (AII).
- 60.
The pediatric solid organ transplant recipients of 6–12 months of age should be a priority group for vaccination, given the paucity of data on the use of antiviral agents in infants <12 months of age (AIII).
- 61.
In children younger than 9 years of age with no previous influenza vaccination is recommended the administration of two doses of influenza vaccine, 1 month apart (AII).
- 62.
All household and close contacts of pediatric solid organ transplant recipients should also be vaccinated (AII).
- 63.
The adherence to the infection control measures (use of masks, compliance with respiratory isolation) may be difficult in very young children. Viral shedding may be more prolonged and copious in young children compared to adults. Thus, the young solid organ transplant recipient is expected to shed virus for an even more prolonged period, and be more likely than adults to spread the virus to close contacts and to the environment (AI).
- 64.
The use of antiviral agents in the treatment of influenza infection in children is similar to that in adults (AI).
- 65.
Adequate antiviral therapy should be initiated as early as possible in all pediatric solid organ transplant recipients with suspected influenza infection (AII) independently of the severity of illness and duration of symptoms (AIII) and without waiting for diagnostic results.
- 66.
There is concern regarding the safety of the use of oseltamivir in infants younger than 1 year of age due to data extracted from animal studies at doses higher than those used in children. While more clinical data become available, we recommend the use of oseltamivir in children under 1 year of age with dosing based on the body weight (CIII).
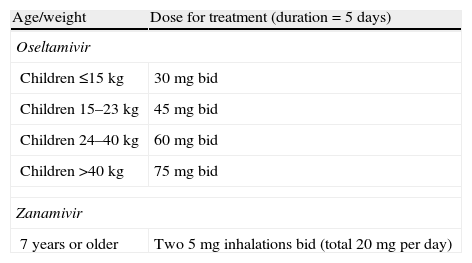
Dose recommendations for children 12 months of age or older.
| Age/weight | Dose for treatment (duration=5 days) |
| Oseltamivir | |
| Children ≤15kg | 30mg bid |
| Children 15–23kg | 45mg bid |
| Children 24–40kg | 60mg bid |
| Children >40kg | 75mg bid |
| Zanamivir | |
| 7 years or older | Two 5mg inhalations bid (total 20mg per day) |
Note: Adjustments recommended for patients with a glomerular filtration rate under 30mL/min. For these patients, the treatment dose is reduced to a once daily dose.
bid, twice daily.
The authors have no conflict of interest to declare.
This document has been reviewed and is supported by the Spanish Society of Transplantation (SET), the National Transplant Organization (ONT) of the Ministry of Health of Spain, the Spanish Society of Nephrology (SEN), the Section of Heart Failure and Heart Transplantation of the Spanish Society of Cardiology (SEC) and the Spanish Society of Liver Transplantation (SETH).
Coordinators of the document. These authors contributed equally to this work.