Acinetobacter baumannii is one of the six most important multidrug-resistant (MDR) microorganisms isolated in hospitalised patients worldwide, having an extraordinary capacity to spread to different areas.1 In the last three decades A. baumannii has acquired resistance to antibiotics including carbapenems and even polymyxins, representing a challenge for achieving effective antibacterial treatment.1,2 In the global priority list of antibiotic-resistant bacteria of the World Health Organisation, A. baumannii is considered the most critical pathogen.3 Knowledge of the epidemiology and antibacterial susceptibility profile of A. baumannii is still incomplete in many parts of the world including Africa. Here, we report a fatal pulmonary infection by MDR A. baumannii in Maputo, Mozambique.
In 2014, a woman in her 20s, with HIV infection on antiretroviral treatment for the preceding 12 months, was admitted to the Maputo Central Hospital with cough, dyspnoea and seizures of acute presentation. Physical examination revealed: a Glasgow score of 15/15, temperature 38.2°C, blood pressure 180/120mmHg, heart rate 100bpm, and respiratory rate 24rpm. Thick and thin smear tests for malaria were negative. Laboratory analyses during hospitalisation showed anaemia (haematocrit 24.9% and haemoglobin 8.3g/dL), leukopenia (white blood cell count 2.9×109/L), elevated transaminases (AST 157IU and ALT 726IU), and kidney failure (maximum creatinine and urea levels were 363μM/L and 29.4μM/L respectively); the estimated glomerular filtration (Cockcroft-Gault Equation) was 18.8mL/min. Chest X-ray was performed and only a cardiomegaly was reported. The nadir CD4 count was 192cells/mL. Sputum Gram stain and blood culture were not performed. The patient received penicillin, cotrimoxazole, and oxygen but died on day 14 of hospitalisation. Pre-mortem clinical diagnoses were: HIV/AIDS, Kaposi's sarcoma, dilated cardiomyopathy, kidney failure, and pulmonary hypertension. The patient was not intubated or in mechanical ventilation.
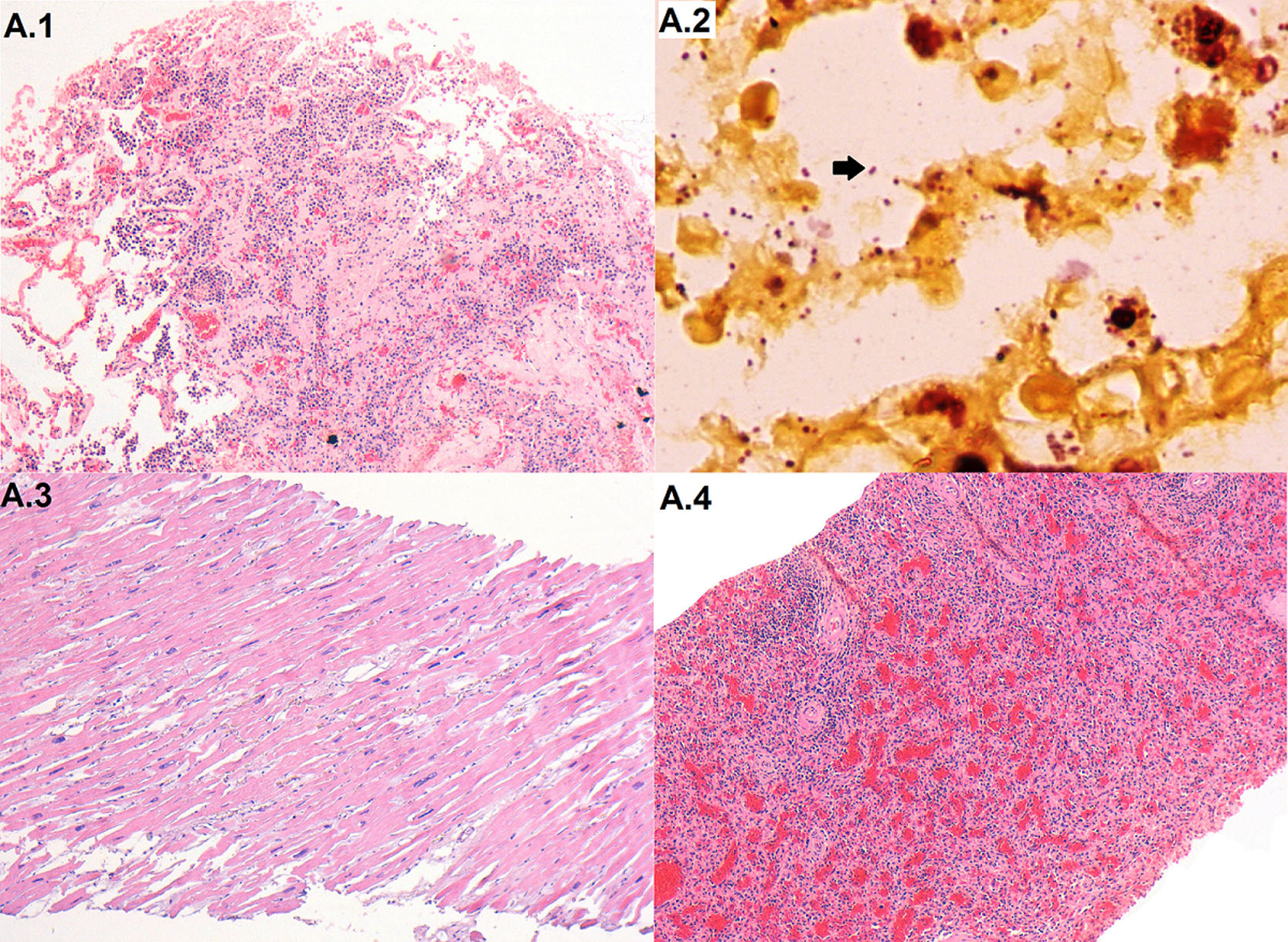
The case was included in the CaDMIA project, a validation study of a minimally invasive autopsy (MIA) protocol against the complete diagnostic autopsy (CDA).4,5 A universal screening for several key pathogens was conducted, and microbiological analysis were performed according to the histopathological findings.4,5 The autopsy revealed a severe pyogenic pneumonia (Fig. 1). Serum samples tested positive for antibodies against HIV with a viral load of 182copies/mL. Lung samples resulted negative for tuberculosis, Cryptococcus, Toxoplasma gondii, Pneumocystis jirovecii and respiratory viruses by PCR testing. A. baumannii was isolated from brain, lung and liver samples. Gram negative bacilli were visible in the Gram stain of histological lung samples (Fig. 1), brain, and liver. A. baumannii was also identified by 16S rRNA PCR in plasma, brain, lung, liver and cerebrospinal fluid samples. The cause of death was assigned to fatal pneumonia caused by a MDR A. baumannii infection, following a previously described algorithm.6 Clinical diagnosis of Kaposi's sarcoma and dilated cardiomyopathy were not confirmed at autopsy.
Fatal MDR A. baumannii infection: microbiological and pathological results of post-mortem samples.
Histological images of relevant findings. (A.1) Lung with pyogenic pneumonia (haematoxylin and eosin, 100×); (A.2) Gram negative bacteria (A. baumannii) pneumonia (arrow) infecting the lung (gram stain, 1000×); (A.3) heart with hypertrophy (haematoxylin and eosin, 100×); (A.4) spleen with lymphocytic depletion and congestion (haematoxylin and eosin, 100×).
Antibiotic susceptibility tests were performed and interpreted according to the EUCAST guidelines (version 7.0,2017; http://www.eucast.org) which consider A. baumannii intrinsically resistant to penicillins and cephalosporins. In addition, the strain was resistant to the following antibiotics: ciprofloxacin, levofloxacin, trimethoprim-sulphamethoxazole, and gentamicin; showing intermediate resistance to meropenem (4μg/mL) and susceptibility to amikacin, tobramycin, imipenem and colistin. The MIC of tigecycline was 1μg/mL. Multi-Locus Sequence Typing following the Pasteur scheme (https://pubmlst.org/abaumannii/) identified all the A. baumannii isolates as belonging to international clone II and sequence type 2(ST2).
Few data are available in the literature regarding A. baumannii in Africa. A recent report analysed 65 strains from 5 different countries and found a high prevalence of MDR strains.7 International clone II/ST2 isolates belong to one of the major clonal lineages associated with the spread of MDR A. baumannii worldwide, but in Africa they have only been reported in Algeria and Kenya.8,9 Two recent studies10,11 (one of them conducted at the Maputo Central Hospital) reported non-MDR A. baumannii in Mozambique, whereas, to our knowledge, this is the first report of a MDR A. baumannii strain in this country. The patient had several known risk factors for acquiring A. baumannii infection such as severe immunosuppression and having been hospitalised for two weeks. However, the final cause of death was only identified after a complete diagnostic autopsy and in depth microbiological studies were carried out. Diagnostic autopsies are rarely performed in sub-Saharan Africa due to, among others, the lack of resources and trained pathologists. We show that a standardised minimally invasive sampling procedure can provide accurate identification of a pathogen causing death. This method may improve the capacity of the current surveillance methods to detect bacterial infections and associated antimicrobial resistance. Our report highlights the utility of post-mortem investigations for accurate determination of cause of death and the need for microbiological surveillance to tackle the growing problem of nosocomial MDR infections in low-income countries.
Availability of data and materialAll relevant data are within the paper. Any additional data use and transfer is monitored by ISGlobal Data Management and Biostatistics Unit (contact e-mail: ubioesdm@isglobal.org).
Ethics approval and consent to participateThe study protocol received approval from the National Mozambican Ethics Committee (ref.342/CNBS/13) and the Ethics Committee of the Hospital Clinic of Barcelona (Spain; approved, File 2013/8677). The MIA and CDA procedures were only conducted after verbal informed consent was provided by the relatives.
FundingThe CaDMIA research project (Validation of the minimally invasive autopsy tool for cause of death investigation in developing countries) was funded by the Bill & Melinda Gates Foundation (Global Health grant numbers OPP1067522) and by the Spanish Instituto de Salud Carlos III (FIS, PI12/00757). Data analysis has been supported by the CaDMIA plus research project, funded by the Bill & Melinda Gates Foundation (Global health grant numbers OPP1128001) and the Spanish Instituto de Salud Carlos III (Acciones CIBER). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya (http://cerca.cat/en/suma/). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CaDMIA bacterial study group: Juan Carlos Hurtado, Francesc Marco, Mamudo R. Ismail, Paola Castillo, Marta Marí-Almirall, Ignasi Roca, Dercio Jordao, Lucilia Lovane, Cesaltina Lorenzoni, Mireia Navarro, Isaac Casas, Inacio Mandomando, Anelsio Cossa, Ariadna Sanz, Quique Bassat, Jaume Ordi, Clara Menéndez, Jordi Vila, Carla Carrilho and Miguel J. Martínez.