Cytomegalovirus infection remains a major complication of solid organ transplantation. In 2005 the Spanish Transplantation Infection Study Group (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) developed consensus guidelines for the prevention and treatment of CMV infection in solid organ transplant recipients. Since then, numerous publications have clarified or questioned the aspects covered in the previous document. These aspects include the situations and populations who must receive prophylaxis and its duration, the selection of the best diagnosis and monitoring technique and the best therapeutic strategy. For these reasons, we have developed new consensus guidelines to include the latest recommendations on post-transplant CMV management based on new evidence available.
La infección por citomegalovirus (CMV) constituye una complicación importante en los pacientes sometidos a trasplante de órgano sólido (TOS). En el año 2005 el Grupo de Estudio de Infección en el Trasplante (GESITRA) de la Sociedad Española de Microbiología Clínica y Enfermedades Infecciosas (SEIMC) elaboró un documento de consenso para la profilaxis y el tratamiento de la infección por CMV en pacientes sometidos a TOS. Desde entonces han sido numerosas las publicaciones que o bien han aclarado, o bien han planteado nuevas dudas respecto a los aspectos tratados en el anterior documento. Entre estos aspectos se encuentran las situaciones y poblaciones que deben recibir profilaxis y su duración, la elección de la mejor técnica para el diagnóstico y monitorización y la elección de la mejor estrategia terapéutica. Todo ello justifica la necesidad de elaborar un nuevo documento de consenso que incluya las últimas recomendaciones en el manejo de la infección por CMV post-trasplante en base a las nuevas evidencias disponibles.
Cytomegalovirus (CMV) infection continues to be a major complication in solid-organ transplant (SOT) recipients. In these patients, CMV is a significant cause of morbidity and mortality associated with both invasive CMV disease and the modulating effects of CMV on the host immune system.
The first GESITRA-SEIMC consensus guidelines on prophylaxis and the treatment of CMV infection in solid-organ transplant patients were published in 2005.1 Although more information on this subject has become available since then, CMV infection continues to present unresolved problems in transplant recipients. For all these reasons, a new consensus document needed to be prepared based on available information in order to review and update the measures for the prevention, diagnosis and treatment of diseases induced by CMV infection.
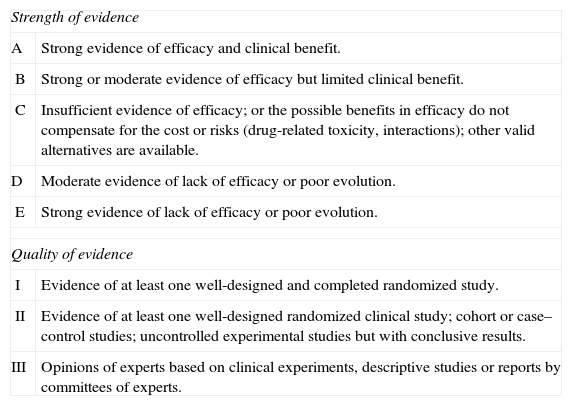
This document has been drafted in accordance with international recommendations on the preparation of consensus documents.2 The recommendations have been classified according to the American Centers for Disease Control (CDC) presented in Table 1.
Classification of recommendations in the document based on the strength and quality of the evidence analyzed.
| Strength of evidence | |
| A | Strong evidence of efficacy and clinical benefit. |
| B | Strong or moderate evidence of efficacy but limited clinical benefit. |
| C | Insufficient evidence of efficacy; or the possible benefits in efficacy do not compensate for the cost or risks (drug-related toxicity, interactions); other valid alternatives are available. |
| D | Moderate evidence of lack of efficacy or poor evolution. |
| E | Strong evidence of lack of efficacy or poor evolution. |
| Quality of evidence | |
| I | Evidence of at least one well-designed and completed randomized study. |
| II | Evidence of at least one well-designed randomized clinical study; cohort or case–control studies; uncontrolled experimental studies but with conclusive results. |
| III | Opinions of experts based on clinical experiments, descriptive studies or reports by committees of experts. |
The nomenclature used in relation to CMV infection varies enormously. We believe that it is important to standardize the definitions used, which have been recently revised.1,3,4
“Infection or replication” is defined as the isolation of the virus or the detection of viral proteins (antigenemia) or CMV DNA/mRNA in any body liquid or tissue. “Primary” infection occurs when CMV is detected in an individual who was previously CMV seronegative. “Persistent infection” refers to the detection of CMV in culture or by polymerase chain reaction (PCR) or antigenemia, over a prolonged period, in otherwise asymptomatic patients. “Recurrent infection” is the new detection of CMV at least 4 weeks after the control of first infection. Recurrent infection may result from the reactivation of a latent virus (endogenous) or re-infection (exogenous). “Reactivation” is defined as the detection of a CMV strain that is the same as the strain causing the original infection and “re-infection” refers to the detection of a different strain.
“Viremia” is defined as the isolation of CMV from the blood culture of a patient. “Antigenemia” consists of the direct detection of the CMV pp65 antigen in peripheral blood leukocytes, mainly neutrophils. “DNAemia” and “RNAemia” are defined as the detection of CMV DNA and RNA, respectively, in plasma, whole blood or leukocyte samples.
“CMV disease” is considered to exist when the infected patient displays symptoms or signs (viral syndrome or visceral involvement). “Viral syndrome” is defined as the presence of fever >38°C (for at least 2 days in a 4-day period), associated with the presence of leukopenia, thrombocytopenia or an increase in transaminases, coupled with the detection of CMV infection in blood. In hematopoietic progenitor transplants, the use of this term should be avoided since it may cause confusion.
CMV visceral involvement is exhibited by symptoms and signs in the affected organ. The most common visceral involvements are pneumonia, digestive disease, hepatitis, encephalitis, retinitis, nephritis, cystitis, myocarditis and pancreatitis. “Probable disease” is defined as the presence of clinical symptoms compatible with the presence of viral replication. Accurate diagnosis requires the presence of a clinical–analytical condition compatible with the presence of histological lesions in CMV-positive biopsies and/or cultures. PCR-based CMV detection in tissue samples is not considered to be a diagnosis. Virus blood and urine cultures for diagnosis of the disease have a limited role. Immunostaining increases the sensitivity of histological biopsy tests. The identification of inclusion bodies or viral antigens in biopsies by immunohistochemistry or in bronchoalveolar lavage (BAL) samples by immunocytochemistry can improve the predictive value of positive cultures. In the diagnosis of CMV-induced pneumonia, detection of the virus in BAL samples is accepted. However, positive cultures from BAL samples are not always correlated with disease. Various studies have suggested that quantitative evidence of nucleic acid in viral load in BAL samples can be helpful for predicting pneumonitis.5 In patients with hepatitis or gastrointestinal infection, diagnosis of CMV invasive disease must be confirmed by immunohistochemistry or DNA in situ hybridization.
For the diagnosis of central nervous system disease, CMV detection by culture or PCR in cerebrospinal fluid samples is accepted. The diagnosis of retinitis is based on the presence of typical lesions observed during ophthalmological examination. In these cases, the diagnostic value of viral load in blood or plasma or in other laboratory tests as predictors of ocular CMV disease is low, although they may be positive before or at the same time as diagnosis.
The presence of CMV in the urine of patients with renal dysfunction or micturition syndrome is insufficient for diagnosing organ disease.
“Universal prophylaxis” consists of administering an effective antiviral drug to prevent the development of CMV infection and/or disease in risk patients, if there are no clinical suspicion of and microbiological data indicating infection. “Pre-emptive treatment” or “pre-emptive therapy” consists of starting pre-emptive antiviral treatment in patients displaying asymptomatic CMV replication (detected by regular monitoring of blood DNA or viral antigenemia).
Diagnosis and virological monitoringSolid-organ transplant (SOT) patients must be monitored virologically in order to detect CMV infection. Although great progress has been made, especially in molecular tests, none of the techniques currently available are universally valid6,7; hence, each technique must be selected according to the specific clinical objective, patient characteristics, the technical specifications of the method and the possibilities of the laboratory.
Pre-transplant assessmentThe aim in this stage is to assess the risk of complications in the post-transplant period. Therefore, it is necessary to determine the immune status to CMV of both the donor and the recipient, since the latter conditions the risk of active CMV infection and disease post-transplant. For this purpose, a specific and sensitive serological method must be used, based on the detection of IgG-class antibodies (AII). Techniques that simultaneously detect IgM antibodies do not add sensitivity and in contrast may produce false positives or incorrect values.8 In general, commercial enzyme immunoassay techniques are reliable, although not all are equivalent and must be validated beforehand by the laboratory. As regards recent transfusions, the normal precautions must be taken in pre-transplant serological screening, due to the possibility of hemodilution or passive antibody transfer.
In CMV-seronegative recipients, serological tests must be repeated in intervals close to the transplant date.
Other virological tests (cultures, antigen detection, nucleic acid amplification) are not useful in this context and should not be used prior to transplant (EII), except in exceptional circumstances.
Virological diagnosis post-transplantVirological diagnosis post-transplant aims to detect both asymptomatic viral replication and CMV disease in transplanted patients. In this sense, serological tests have no use whatsoever in this period and must not be used to monitor these patients. In contrast, cultures, antigen detection and molecular methods are particularly useful in this period.6,7 As a general rule, quantitative techniques are recommended, given the known relationship between replication intensity, disease development and relapse risk.
Diagnosis of CMV diseaseViral syndrome is the most common clinical expression of CMV disease. Quantitative virological tests are required to diagnose this condition. Systematic monitoring with urine or saliva samples is not recommended since they have little value for predicting disease. Blood cultures (including rapid shell vial cultures) are not useful because they take a long time to produce results and due to their low sensitivity. These techniques are only useful either for obtaining strains for epidemiological characterization or for the performance of phenotypic resistance studies.
The antigenemia test detects the pp65 antigen of the virus in peripheral blood leukocytes by indirect immunofluorescence. This test has been shown to be useful for diagnosing CMV disease, particularly in viral syndrome cases9; evidence has been published indicating that sudden increases in antigenemia values predict the appearance of symptoms.10
The advantages of this test are that it is easy to perform and economical. However, it does have certain limitations, e.g. low antigen stability, which means samples must be processed within 6–8h, it cannot be used in patients with less than 1000 neutrophils/μL, and its lack of standardization, which means that results from different laboratories cannot be compared and makes it difficult to establish a universally valid reference value. Consequently, no threshold value can be recommended. This value must be established in each center and individual threshold values may even be established for each patient according to his/her risk specific factors.
When interpreting antigenemia values, consideration must be given to the type of transplant (lower values for pulmonary or intestinal transplants and higher values for cardiac transplants), as well as the immunosuppression regime used. Although a threshold value of 20–50 CMV+ cells/105 leukocytes generally correlates well with the presence of symptoms, in the case of digestive disorder or retinitis, lower or even negative antigenemia values may be recorded.
Molecular tests, especially those based on PCR techniques, are the main alternative to antigenemia for making a diagnosis, starting pre-emptive treatment and monitoring response to treatment.7,9 The results of these tests are not affected by spontaneous degradation of viral DNA and are therefore more sensitive and robust than antigenemia, and better for quantifying viral kinetics. In recent years, the technical advantages (sensitivity, speed, large linearity interval and reduced risk of contamination) of quantitative methods based on real-time PCR technology have resulted in more widespread use of these techniques for monitoring transplant patients. For this reason, although antigenemia is still acceptable, it would seem to be reasonable to recommend the use of these molecular methods (BIII).
Viral load values may be determined in both plasma and whole blood samples, and both are well correlated. However, it is much easier to determine these values in plasma. Whether using whole blood or plasma samples, the recommended anticoagulant is ethylenediaminotetraacetic acid (EDTA) as heparin interferes with PCR. Since the viral load values in whole blood are higher than those obtained in plasma, around 1 logarithmic unit,11 individual patients should always be monitored using the same type of sample (AII).
Since DNAemia is subject to substantial biological variability, three-to-five-fold increases in the initial value (0.5–0.7 logarithmic units) may not be significant.12
Although DNAemia is more robust, the variability between different PCR techniques or between different laboratories is very important. This has prevented its standardization and the determination of common cut-off points; hence, no recommendation can be made in this respect. For this reason, specific patients can be monitored using a specific technique and in the same laboratory (AII). Recently, an international reference standard was introduced similar to the one available for other viruses, and this may be a significant advance in standardization,13 reducing inter-laboratory variability. However, the introduction of this new standard will not eliminate differences in viral load, either among centers or among different types of SOT.
Diagnosis of focal diseaseAntigenemia or DNAemia values may be negative or low, especially in patients with gastrointestinal involvement or retinitis. Therefore, diagnosis should always be performed on tissue samples whenever possible.3 Qualitative PCR is not recommended for diagnosing the disease in organs as, except in the case of central nervous system involvement, accurate diagnoses cannot be made.3
Virological monitoring for pre-emptive treatmentMany transplant groups have used pre-emptive treatment as a preventive strategy instead of universal or risk factor-based prophylaxis. This strategy must only be performed in centers with viral load quantification methods. Although both antigenemia and DNAemia are useful for this purpose, DNAemia detected by PCR is recommended and efforts should be made to obtain the outcomes of these tests within 24h.
One weekly determination is recommended during the period of greatest risk. A value close to the cut-off point should enable closer patient monitoring.7 Due to the lack of standardization of either antigenemia or DNAemia, the establishment of a cut-off point for starting pre-emptive therapy is controversial. For this reason, the threshold values of these techniques must be determined by each transplant group and may be individualized for each patient.
Monitoring of treatment response and the appearance of relapsesExcept in exceptional circumstances, such as in cases of central nervous system disease, quantitative tests are necessary to monitor the response to treatment. Given the characteristics of diagnostic techniques, molecular methods seem to be more appropriate than antigenemia in this context, and testing at least weekly once is recommended during treatment. Increases in antigenemia levels 24–48h after the start of treatment do not necessarily mean that treatment has failed.7 The aim must be achieving negative antigenemia or negative DNAemia results at the end of the second or third week of treatment. An increase or maintenance in antigenemia or DNAemia levels during therapy may indicate resistance, although it may also be due to host-dependent factors.
Consensus recommendations- 1.
Patients undergoing SOT must be monitored virologically to detect CMV infection (AI).
- 2.
To determine CMV immune status, a serological method is recommended based on the detection of IgG class antibodies (AII). Except in exceptional circumstances, the performance of other virological tests (cultures, antigen detection, amplification of nucleic acids) is of little use in this context and should not be performed prior to transplant (EII).
- 3.
Serological tests are of no interest for monitoring CMV infection in the post-transplant period and must not be used (EII). To monitor CMV infection, quantitative techniques must be used, given the known relationship between replication intensity, disease development and relapse risk (AII).
- 4.
Systematic monitoring with urine or saliva cultures is not recommended for diagnosing CMV disease due to their limited value for predicting disease (EI). Blood cultures, including rapid shell vial cultures, are of no interest due to the time it takes to obtain results and their low sensitivity (EII).
- 5.
It has been shown that both pp65 antigenemia and molecular tests based on PCR techniques are useful for diagnosis, starting pre-emptive therapy and monitoring treatment response (AI). No recommendation can be made on the cut-off point for starting treatment. This cut-off point must be established at each center and may even be established individually for each patient according to his/her risk factors (CIII).
- 6.
Since PCR-based molecular techniques have certain advantages over antigenemia (greater sensitivity, quantification of viral kinetics, speed, less risk of contamination), the consensus panel recommends the use of molecular methods, although antigenemia is still acceptable (BIII).
- 7.
The viral load value may be determined from plasma or whole blood samples; individual patients must always be monitored using the same type of sample (AII).
- 8.
Pre-emptive treatment must only be used in centers with viral load quantification methods (AII). For this purpose, at least one weekly test is recommended during hospitalization and during the period of greatest risk (BIII).
- 9.
Quantitative tests must be carried out to monitor response to treatment, except in exceptional circumstances, for example in patients with central nervous system disease (BII). Molecular methods seem to be more appropriate than antigenemia in this context (BIII); viral load should be determined at least once per week during treatment (BIII).
Viral resistance depends on the existence of mutations in the CMV genome. Although most studies mention resistance to ganciclovir, resistance has also been described to any antiviral drug used for prophylaxis or treatment of CMV disease.14 The main risk factors favoring the appearance of resistance are the absence of pre-existing immunity to CMV (D+/R−), prolonged exposure to antiviral drugs, continued use of antiviral medication, especially at suboptimum concentrations, intermittent treatments, the presence of high viral loads and intense immunosuppression.
Although not all clinical findings during treatment may be attributed to viral resistance, the presence of resistant strains is often associated with invasive disease, progressive dysfunction, rejection of the transplanted organ and even a high mortality, that may affect up to 65% of patients.15–17
Guidelines from the resistance study. Available methodsDuring the first 2 weeks of treatment, antigenemia or DNAemia levels may increase in more than two thirds of patients, although this is not necessarily indicative of the presence of resistant strains. For this reason, resistance studies are not recommended in these cases.
Resistance to antiviral drugs must be suspected in the presence of progressive or stable viral loads (virological resistance) or if clinical symptoms persist 2 weeks after the start of appropriate antiviral treatment (clinical resistance). Clinical resistance is not necessarily accompanied by virological resistance since it may be due to the presence of factors related with the immune response of the recipient18 or because adequate levels of the antiviral drug at plasma and/or tissue level are not reached. Therefore, in patients displaying slow response to treatment it would be advisable (in centers where this is possible) to determine the plasma levels of ganciclovir and to study CMV-specific immunity.
The presence of resistance to antiviral medication may be confirmed by phenotypic or genotypic methods. Phenotypic methods measure the concentration of antiviral medication necessary to inhibit 50% of viral growth (CI50). These methods require the virus to be isolated beforehand; hence, the time required to obtain results is a limitation. Furthermore, the lack of standardization of such methods conditions the variability of results. These methods must only be used to study in vitro sensitivity to antiviral medication in order to characterize mutations not described previously, or to determine the combined effect of various antiviral drugs.
Genotypic methods are the most frequently used and consist of detecting genetic mutations associated with resistance. The selection method consists of amplifying specific regions of the viral genome, followed by sequencing. This technique may be performed using a CMV strain isolated in culture or, easier still, directly from clinical samples, since in this way results can be obtained in 2–3 days. To obtain results, viral load must be at least 1000 copies/ml. The main limitations of genotypic methods are that they do not provide quantitative results and that the results are difficult to interpret since irrelevant mutations may be detected that do not offer resistance to ganciclovir.19
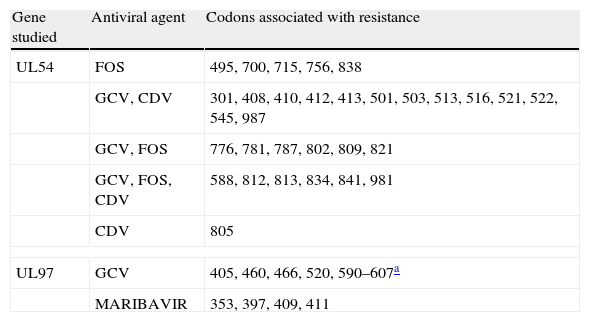
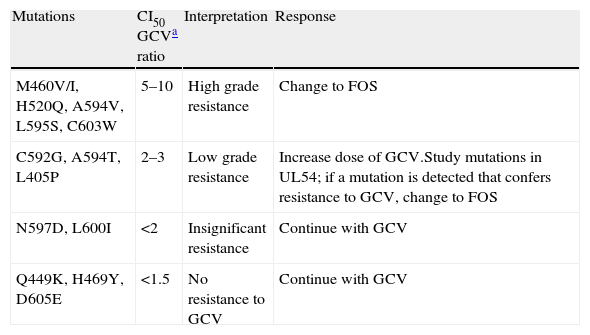
CMV resistance to antiviral agents is mainly due to mutations in the gene encoding the viral protein kinase responsible for initial phosphorylation of ganciclovir (UL97 gene), and less frequently for mutations in the gene encoding the viral DNA polymerase (UL54 gene). If mutations only appear in the UL97 gene, viruses are not sensitive to ganciclovir but they are sensitive to cidofovir and foscarnet. If mutations appear in the UL54 gene, there may be resistance to one or all antiviral agents (ganciclovir, cidofovir, foscarnet). Tables 2 and 3 show the most frequent resistance mutations to ganciclovir, as well as their interpretation and the recommended approach for each type of mutation.
Main mutations associated with the resistance of cytomegalovirus to antiviral agents.
| Gene studied | Antiviral agent | Codons associated with resistance |
| UL54 | FOS | 495, 700, 715, 756, 838 |
| GCV, CDV | 301, 408, 410, 412, 413, 501, 503, 513, 516, 521, 522, 545, 987 | |
| GCV, FOS | 776, 781, 787, 802, 809, 821 | |
| GCV, FOS, CDV | 588, 812, 813, 834, 841, 981 | |
| CDV | 805 | |
| UL97 | GCV | 405, 460, 466, 520, 590–607a |
| MARIBAVIR | 353, 397, 409, 411 | |
FOS: foscarnet, GCV: ganciclovir, CDV: cidofovir.
Different levels of resistance to GCV in mutations in the UL97 gene.
| Mutations | CI50 GCVa ratio | Interpretation | Response |
| M460V/I, H520Q, A594V, L595S, C603W | 5–10 | High grade resistance | Change to FOS |
| C592G, A594T, L405P | 2–3 | Low grade resistance | Increase dose of GCV.Study mutations in UL54; if a mutation is detected that confers resistance to GCV, change to FOS |
| N597D, L600I | <2 | Insignificant resistance | Continue with GCV |
| Q449K, H469Y, D605E | <1.5 | No resistance to GCV | Continue with GCV |
- 1.
Antigenemia or DNAemia levels often increase during the first 2 weeks of treatment. In such cases, resistance studies are not recommended (CIII).
- 2.
In patients who respond slowly to treatment, plasma ganciclovir levels should be determined (in centers where this is possible) and CMV-specific immunity studied (CIII).
- 3.
Resistance to antiviral medication may be confirmed using phenotypic or genotypic methods. Phenotypic methods are recommended for studying sensitivity in vitro, for characterizing mutations, or for determining the combined effect of various mutations. In other cases, genotypic methods are recommended (CIII).
Controlling CMV infection is a complex process that involves both innate and adaptive immune mechanisms.20,21 Natural killer (NK) cells play an important role in controlling primary and recurrent CMV infections, increasing in number in response to viral replication.22–24 However, T lymphocyte-mediated response plays a critical role in controlling CMV infection.20,21 CD8+ and CD4+ T-lymphocytes intervene decisively in resolving replication episodes20,21 through the recognition of a broad spectrum of viral proteins, including most notably the proteins pp65 and IE-1, which appear to generate dominant responses.25–27 Therefore, the monitoring of T-lymphocyte immune response to these proteins may be useful for identifying patients with a greater risk of developing viral replication episodes.28–40
As regards humoral immunity, it has been suggested that glycoprotein B (gB) and H (gH) neutralizing antibodies may reduce the risk and severity of viral primary infection.20,21,41–43 However, there is no consensus in this regard, since although hypogammaglobulinemia is associated with a greater risk of CMV infection in heart and lung transplant patients, the same does not occur in liver transplant patients.44,45 Humoral response to CMV enables the identification of transplant patients at greater risk of primary CMV infection (D+/R−), although there is no unanimity on its usefulness for predicting the development of the disease.46,47
Methods for quantifying and analyzing CMV-specific T cellsVarious methods are currently available for functional and phenotyping and quantification ex vivo of CMV-specific T lymphocytes. Most of these methods are used for experimental purposes. The methods that employ HLA peptide multimers determine the number of T-lymphocytes that recognize a specific viral epitope but do not provide information on their functional capacity. In contrast, other methods provide information on the functionality of lymphocytes based on the quantification of cytokine production after stimulation of T cells with CMV peptides or viral lysate. These methods include intracellular staining, which provides functional and quantitative information on the population of CMV-specific T lymphocytes since it allows IFNγ quantification to be combined with the expression of surface markers. The ELISPOT technique quantifies the number of individual T cells that release a specific cytokine (usually IFNγ or TNF-α) after stimulating them, although it does not distinguish between CD4+ and CD8+ T-lymphocytes. The QuantiFERON-CMV technique can be used to estimate the number of T lymphocytes compared with a limited number of immunogenic CMV epitopes presented by a broad spectrum of HLA specificities, through IFNγ quantification. None of these tests are standardized, with the exception of the recently commercialized QuantiFERON-CMV technique.48
T cell immunological monitoring strategiesImmunological monitoring of CMV-specific T lymphocyte response and virological monitoring of CMV infection could be used to individualize and optimize antiviral treatment in SOT patients.49 In high-risk (D+/R−) and intermediate risk (R+) patients, an inverse relationship has been reported between the peripheral levels of certain specificities of CD4+ and CD8+ T-lymphocytes (producers of IFNγ, TNF-α and IL-2 against CMV), with the consequent risk of developing CMV disease.28–40,48 Phenotyping of CMV-specific T-lymphocytes can also provide information on the risk of CMV replication and disease. In this sense, the expression of the PD-1 (programmed death-1) marker on the surface of CMV-specific CD4+ and CD8+ T-lymphocytes has been linked to a high risk of developing replication, viral syndrome and organ disease.50,51,52
Consensus recommendations- 1.
Various immunological markers are available for estimating, with variable degrees of precision, the risk of active CMV infection and disease within the scope of SOT. For now, none are universally accepted.
- 2.
The ideal method for monitoring immune response to CMV must not only be quantitative but must also offer information on the functionality and surface phenotype of CMV-specific CD4+ and CD8+ T-lymphocytes (CIII). It must also be easy to perform, fast, cost-effective and reproducible (CIII). Only the QuantiFERON-CMV method has been made commercially available, although its benefits are being evaluated.
- 3.
Although the monitoring of T-cell response to CMV is potentially useful for therapeutic management of CMV infection in SOT recipients (CIII), there is no informed clinical experience in this respect. For now, therapeutic intervention strategies based on the immunological monitoring of patients are not recommended.
CMV infection appears in between 30% and 80% of SOT recipients, although its incidence and the presence of symptomatic disease vary depending on the type of transplant, the presence of associated risk factors (Table 4) and the prevention strategies used.53
Risk factors of cytomegalovirus disease in solid-organ transplant patients.
| Primary infection in seronegative recipients (D+/R−) from |
▪ Transplanted organ |
▪ Blood derivatives |
| Factors favoring the reactivation of cytomegalovirus in recipients |
▪ Stress |
▪ Surgery |
▪ Intraoperative hypothermia |
▪ Sepsis or severe bacterial infections (pneumococcus or gram-negative bacteria) |
▪ Co-infections caused by other viruses |
▪ Herpes virus 6 (HHV6) |
▪ Herpes virus 7 (HHV7) |
| Factors favoring progression to CMV disease |
| Immunosuppressants |
▪ OKT3, anti-thymocyte globulins, anti-lymphocyte globulins |
▪ Mycophenolate, azathioprine |
▪ Methylprednisolone |
▪ Alemtuzumab |
▪ Viral load |
▪ Immunomodulation |
▪ Herpes virus 6 (HHV6) |
▪ Herpes virus 7 (HHV7) |
| Immunological factors favoring CMV infection |
▪ Mutations in TLR2 and TLR4 genes |
▪ Deficiency of mannose-binding lectin or genotype associated with low production of same |
| Factors that reduce CMV disease |
▪ Recipient immunity against CMV prior to transplant |
▪ Immunosuppressants: mTOR inhibitors (sirolimus and everolimus) |
▪ Anti-viral prophylaxis or with immunoglobulin |
▪ Pre-emptive antiviral treatments |
The period of maximum risk is between the first and sixth month post-transplant. Maximum incidence occurs between the second and third month. However, some factors may alter this chronology, either causing infection to start earlier, such as in the case of treatment with OKT3 monoclonal antibodies, or delaying infection, for example in patients receiving universal prophylaxis or pre-emptive treatment.53
In primary infection, the lack of specific immunity of the recipient leads to increased viral replication (increasing by 1.82 units/day), normally associated with the development of CMV disease.54 In reactivations, humoral immunity and cellular immunity of the recipient reduce the virus replication process (0.61 units per day)54 with the subsequent decline in the incidence and severity of the disease, which develops in between 10% and 20% of patients. In re-infections, in situ reactivation of CMV in the transplanted organ, in addition to producing the disease in up to 30% of patients, may prompt the onset of terminal disease in the transplanted organ.
In SOT recipients, the risk of CMV disease is the result of the balance between the amount of virus present or viral load and the humoral and cellular immunological response capacity of the recipient. Factors such as rejection55 or co-infections,56 which are accompanied by the production and secretion of cytokines triggering the inflammatory cascade, may stimulate latent CMV replication.57
Risk factorsImmunological status of the recipient and donorThe transplantation of a seropositive organ to a seronegative recipient (D+/R−) has been shown to be the main risk factor for CMV disease in all types of transplant.58–62 Seronegative recipients who receive seronegative transplanted organs (D−/R−) run a very low risk of developing infection unless they receive hemoderivative transfusions of unfiltered leukocytes from seropositive donors63,64 or exhibit primary infection.
Viral load and transplanted organThe degree of viral replication has been directly associated with the development of CMV disease,63,65 with primary infections being the most symptomatic and severe as they are normally accompanied by higher viral loads. Other factors that influence viral load are type of transplant, prophylaxis strategy and the net state of immunosuppression of the recipient.
As regards the type of transplanted organ, the onset of CMV disease is more frequent, and normally more severe, in intestine, pancreas and lung transplants than in liver, heart and kidney transplants. This greater incidence of CMV disease in intestinal and pancreatic transplants is probably due to the fact that both allografts have abundant lymphoid or macrophage tissue with high loads of latent or replicating CMV.58–60 For the same reason, multiple transplants (renal-pancreatic, cardiopulmonary) are riskier than single organ transplants.60,63
Immunosuppressive treatmentImmunosuppressants are used to prevent rejection delay and/or mitigate specific humoral and cellular immunological responses, permitting the uncontrolled replication of the latent virus. Immunosuppressants displaying this activity include methylprednisolone at high doses,65 anti-lymphocyte agents such as anti-lymphocyte (ALG) and anti-thymocyte (ATG) globulins, the monoclonal antibody OKT3 (currently not commercially available),66,67 and mycophenolate mofetil.68,69
The use of anti-lymphocyte antibodies for induction therapy, or to treat rejection, increases the rate of CMV infection three-to-four-fold, especially in seropositive patients.70 The mechanism may be related with fever and the release of tumor necrosis factor alpha, the depletion of helper T lymphocytes and the inversion of the CD4/CD8 coefficient.
The anti-CD25 monoclonal antibodies basiliximab and daclizumab (the latter is currently not commercially available) have not been associated with a higher risk of CMV infection or disease. However, it has been shown that alemtuzumab71,72 is a risk factor when used to treat rejection in renal-pancreatic or heart transplant recipients. Cyclosporin, tacrolimus and prednisone at conventional doses do not normally reactivate latent CMV, although they do reactivate replicating CMV.73 The use of mycophenolate in kidney transplant patients has significantly reduced the incidence of rejection, although it is accompanied by a greater risk of CMV disease, particularly in the intestinal tract.68,69 Results in other types of transplants are less conclusive.
The mTOR (mammalian target of rapamycin) inhibitors sirolimus and everolimus74–76 used in kidney and heart transplants have been associated with a lower incidence of CMV infection when compared with approaches that include cyclosporin or azathioprine.
Other factorsSome observational studies in liver and kidney transplant patients have shown that the reactivation and replication of other beta-herpes viruses, such as human herpes viruses type 6 and 7, are associated with CMV disease.77–79
Similarly, other factors such as donor age over 60 years,74 kidney transplanted from cadaver, female recipients, advanced age of recipients, re-transplant, the need for multiple transfusions and prophylaxis or short pre-emptive treatments, have been associated with a greater incidence of CMV infection.63
Moreover, in recipients of abdomen transplants, factors such as intraoperative hypothermia, stress associated with surgery or with critical situations and post-operattive bacterial infections have also been related to associate with greater CMV replication.80,81
Finally, recent evidence shows that some defects in the immune system of the host may be associated with greater risk of CMV infection. These situations include certain polymorphisms of Toll-like receptors 2 and 4 and certain deficiencies in the complement, cytokines, chemokines or mannose-binding lectin.4,18,63,82,83
Consensus recommendationsThe main risk factors of CMV disease depend on the serological condition of the donor and recipient, the type of organ transplanted and the degree of immunosuppression of the recipient once the organ has been transplanted.
- 1.
Transplantation from a seropositive donor to a seronegative recipient (D+/R−) is one of the main risk factors (AI).
- 2.
Intestine, pancreas and lung transplant recipients have a greater risk of CMV disease than other transplants (AI).
- 3.
Some immunosuppressants such as steroids65 and anti-lymphocyte antibodies used for induction treatment or to prevent rejection66,67 are associated with a greater incidence of CMV disease (AI).
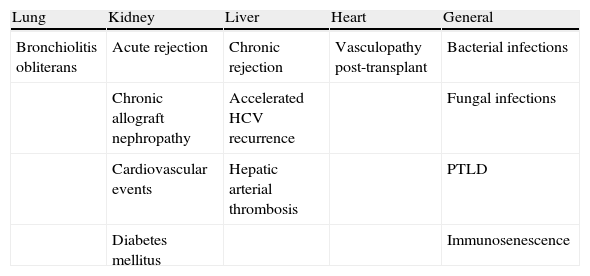
In addition to the direct effects produced by invasive organ infection, CMV produces a series of indirect effects that may be defined as those resulting from interaction of the virus with the host immune system and which are independent of the presence of high levels of viremia, and are probably related with the existence of low levels of viral load over prolonged periods. These indirect effects have been described in all types of SOT (Table 5) and include, among others, acute and chronic allograft rejection, atherosclerosis, post-transplant diabetes mellitus and increases in opportunistic infections. There is no consensus among the panel of experts regarding the demonstrated existence of a cause-effect relationship between CMV and indirect effects, since the evidence available until now only allows a relationship of greater or lesser association to be established between these indirect effects and CMV for each type of transplant.
Indirect effects of CMV infection described in each type of transplant.
| Lung | Kidney | Liver | Heart | General |
| Bronchiolitis obliterans | Acute rejection | Chronic rejection | Vasculopathy post-transplant | Bacterial infections |
| Chronic allograft nephropathy | Accelerated HCV recurrence | Fungal infections | ||
| Cardiovascular events | Hepatic arterial thrombosis | PTLD | ||
| Diabetes mellitus | Immunosenescence |
HCV: hepatitis C virus; PTLD: post-transplant lymphoproliferative disease.
In kidney transplant recipients, results from observational studies have associated both asymptomatic viremia and CMV disease with greater risk of acute allograft rejection84 (BII). However, the results of studies that have evaluated the association between CMV and chronic allograft nephropathy are controversial,85–87 hence no relationship of causality can be established between CMV and chronic allograft nephropathy. In these patients, CMV infection has been related with a greater risk of cardiovascular events. One observational and prospective study has reported a greater frequency of these events (myocardial infarction, cardiovascular disease and peripheral vasculopathy) in patients displaying CMV replication during the first year post-transplant.88 Another later retrospective review described greater frequency of cardiovascular diseases during the first 4 years post-transplant in patients with CMV infection compared with patients without infection after adjusting for traditional cardiovascular risk factors.89 Finally, some authors have suggested that CMV infection and disease are associated with a greater risk of post-transplant diabetes mellitus, although the design of these studies made it impossible to establish a direct causal relationship.90–92
Lung transplantThe main indirect effect of CMV described in lung transplant recipients has been the development of bronchiolitis obliterans syndrome (BOS). However, studies evaluating the causal relationship between CMV and BOS present contradictory results, probably due to differences in the definition of CMV infection, immunosuppression methods and prophylaxis strategies used.93–99 To conclude, based on data currently available, a definitive causal relationship cannot be established between CMV and the development of BOS. Similarly, the influence of different prophylaxis strategies to combat the development of CMV disease must be determined in future studies.
Liver transplantCMV infection has been related to chronic allograft rejection in liver transplant recipients, although the etiological role of the virus in rejection has yet to be determined.100–103 Similarly, available evidence on the role of CMV in the accelerated recurrence post-transplant of hepatitis C virus is contradictory.104–106 Finally, CMV donor-recipient serodiscordance has been related with a greater risk of liver artery thrombosis in such patients,107–110 although the design of these studies made it impossible to establish a definitive causal relationship.
Heart transplantIn heart transplant recipients, CMV has been related with a greater risk of allograft vasculopathy. Findings supporting this relationship have mainly been published in studies comparing different prophylaxis strategies, reporting that patients who did not receive prophylaxis or who received pre-emptive treatment were more at risk of developing CMV than those who received universal prophylaxis.111–113
General indirect effectsCMV has been related with a greater risk of bacterial and fungal infections, with evidence to support that this risk can be reduced through the use of prophylaxis strategies to combat the virus.114–118 CMV has also been related with a greater risk of lymphoproliferative disease post-transplant, although most existing evidence in this respect has been reported in retrospective studies.119 Finally, the replication of CMV has been associated in a cross-sectional study with the appearance of immunosenescence through the proliferation of CD27/CD28 CMV-specific CD8+ T cells,120 although these results need to be confirmed in prospective studies.
Consensus recommendations- 1.
CMV has been associated with the appearance of different indirect effects, including acute and chronic rejections (known as chronic allograft nephropathy in renal transplantation, allograft vasculopathy in cardiac transplantation and bronchiolitis obliterans syndrome in lung transplantation), increases in the number of opportunistic bacterial and fungal infections, lymphoproliferative disease post-transplant, cardiovascular disease and diabetes mellitus.
- 2.
The panel of experts considers that an association relationship exists between CMV infection and acute rejection in kidney transplant recipients (BII), as well as an increase in opportunistic infections, lymphoproliferative disease post-transplant and CMV infection (CIII).
- 3.
However, this panel did not reach a consensus on the role of CMV in the other indirect effects.
The two main strategies for preventing CMV disease are universal prophylaxis and pre-emptive therapy. Universal prophylaxis consists of administering effective antiviral medication to all patients at risk, even in the absence of clinical suspicion and microbiological data of infection. Pre-emptive treatment consists of starting antiviral treatment in patients showing asymptomatic CMV replication, detected by regularly monitoring the amplification of nucleic acids or viral antigenemia in blood. Each of these strategies has advantages and disadvantages. Universal prophylaxis has the advantage of potentially preventing the reactivation of other herpes viruses, as well as preventing indirect effects, and the need to obtain repeated samples in order to quantify viral load or antigenemia. However, prolonged exposure to antiviral drugs may increase the risk of resistance and toxicity related with antiviral treatment. Universal prophylaxis has also been related with the late CMV disease, probably due to defective development of specific cellular immunity to the virus.121 Pre-emptive therapy can reduce the cost and toxicity of antiviral medication, however this strategy depends on the availability of adequate logistics at each transplant center.
Universal prophylaxisThe advantages of universal prophylaxis have been demonstrated in clinical trials comparing this strategy with non-prophylaxis or placebo. In these trials, the administration of prophylaxis has been associated with a 58–80% reduction in the incidence of CMV disease.122–124 The drugs evaluated for this strategy were acyclovir, valaciclovir, oral and intravenous ganciclovir and valganciclovir. In the first studies, acyclovir was inferior to ganciclovir.125,126 In another controlled trial performed with kidney transplant recipients, valaciclovir administered for 90 days was associated with a decrease in the incidence of CMV disease and a delay in the onset of this disease.127 A later randomized, controlled and multicenter trial with kidney, liver, pancreas and heart transplant recipients compared oral ganciclovir and valganciclovir and reported comparable efficacy in the prevention of CMV disease in D+/R− patients.121 Adverse effects were similar in both groups, although a higher incidence of invasive CMV disease in organs was observed in the subgroup of liver transplant patients receiving valganciclovir.121
Oral ganciclovir is currently not commercially available, in spite of achieving good outcomes and widespread use in recent years. Thus, although different studies have analyzed the role of oral ganciclovir, this drug is not included in any of the final recommendations of the consensus panel.
Pre-emptive therapyDifferent comparative studies have contrasted the efficacy of pre-emptive therapy with non-treatment or placebo. These approaches have been analyzed jointly in three meta-analyses.122–124 In these studies, the incidence of CMV disease was reduced by an average of 70%; pre-emptive therapy was as effective as universal prophylaxis and its cost was similar.122
Comparison between universal prophylaxis and pre-emptive therapyNo clinical trials have been carried out with SOT recipients to compare universal prophylaxis and pre-emptive treatment in the prevention of CMV disease. In non-randomized studies comparing prophylaxis and pre-emptive therapy, no differences in efficacy were observed. The authors of a meta-analysis of 17 randomized studies carried out previously in 2006 in high-risk kidney and liver transplant recipients concluded that compared with placebo, both strategies reduced the frequency of CMV disease by 80% and 72%, respectively, as well as the frequency of rejection. However, since only prophylaxis reduced the frequency of bacterial and fungal infections (51%) and mortality (38%), the aforementioned authors preferred prophylaxis,124 even though most of the studies included in the meta-analysis were small and open and individually reported no benefit in mortality. In contrast, a greater frequency of leukopenia has been reported in patients receiving prophylaxis compared with those undergoing pre-emptive treatment.128 Small et al. performed another meta-analysis, which included 17 prophylaxis studies and 9 pre-emptive treatment studies, but did not observe any differences in efficacy for the prevention of CMV disease.123
The most comprehensive comparative study of prophylaxis and pre-emptive treatment has been carried out in kidney transplant recipients.129 In this study, no differences were observed in terms of the efficacy of both strategies, including the subgroup of high-risk patients (D+/R−), with no reported differences in cost between either form of treatment. Another comparative study, also performed in kidney transplant patients, compared prophylaxis with valganciclovir and pre-emptive treatments with intravenous ganciclovir in 148 kidney transplant recipients, and reported a greater frequency of CMV infection in the pre-emptive treatment group (51% vs. 18%). Allograft survival after 4 years was lower in the group of patients displaying CMV infection and receiving pre-emptive treatment, and no differences in mortality were observed.130
General recommendations- 1.
Both universal prophylaxis and pre-emptive therapy are useful strategies for preventing CMV disease (AI).
- 2.
In D+/R− patients, both universal prophylaxis and pre-emptive therapy can be used (Table 7), although universal prophylaxis is particularly recommended in high-risk transplants (lung, intestine, pancreas, pancreas-kidney transplantations). Most of the panel would also recommend universal prophylaxis in D+/R− patients receiving other organ transplants if rigorous compliance with the applicable virological monitoring protocol cannot be guaranteed. The recommended duration of prophylaxis is 3–6 months (AI) but this decision will depend on the degree of immunosuppression and the type of organ transplanted. In lung transplants, the duration of prophylaxis will be 6–12 months post-transplant. Due to the risk of late disease, pre-emptive therapy is recommended for a period of 3–6 months after the end of universal prophylaxis (CIII).
- 3.
In R+ patients, pre-emptive therapy (BII) is recommended except in high-risk transplants (lung, intestine) and at centers where it is difficult to monitor viral load or antigenemia; in the latter case, universal prophylaxis is recommended for 3 months (CIII).
- 4.
D−/R− patients are considered to be low risk, and therefore anti-CMV prophylaxis is not recommended in this population (DI). In these patients, leukocyte-depleted blood products from seronegative donors must be used and patients should be monitored using conventional virological monitoring techniques. When a primary infection is detected, standard treatment must be applied (see section on treatment) (BII).
- 5.
If universal prophylaxis is selected, in both seropositive and seronegative patients, neither viral load nor pp65 antigenemia has to be monitored during prophylaxis because the risk of disease during this period is very low (BII). In these cases, viremia or antigenemia must only be determined if the patient develops symptoms indicative of CMV disease during this period. One unresolved aspect is the action to take once universal prophylaxis has ended. This panel considers that the patient progress must be monitored through monthly prospective evaluations of pp65 antigenemia or viral load (CIII).
- 6.
Whenever a pre-emptive therapy strategy is selected, each center is recommended to establish and validate its own practice protocols. Treatment should be started taking into account the characteristics of each patient, and antiviral therapy may begin after the first positive antigenemia or viral load test is obtained in accordance with the cut-off points established in each center (CIII).
- 7.
Pre-emptive therapy may be prolonged until a negative antigenemia or viral load result is obtained. However, in D+/R− or high-risk transplant patients it is advisable to obtain two consecutive negative results (CIII).
- 8.
For prophylaxis in patients receiving combined transplants (e.g. pancreas-kidney transplants), the instructions for higher-risk transplants should be followed (CIII).
- 9.
In deferred transplant patients (e.g. kidney transplants in heart transplant recipients), the prophylaxis instructions followed in the previous transplant should be followed (CIII).
- 10.
In patients with severe post-transplant kidney failure (creatinine clearance <10ml/min) the use of ganciclovir or valganciclovir is not recommended because the kidney is the only elimination route. In these patients, the start of prophylaxis or pre-emptive treatment should be delayed until the creatinine clearance is greater than 10ml/min or up to a maximum of 15 days post-transplant (CIII).
- 11.
Since exposure to valganciclovir and ganciclovir depends entirely on elimination in urine, the administered dose must be adjusted strictly according to creatinine clearance (Table 6).
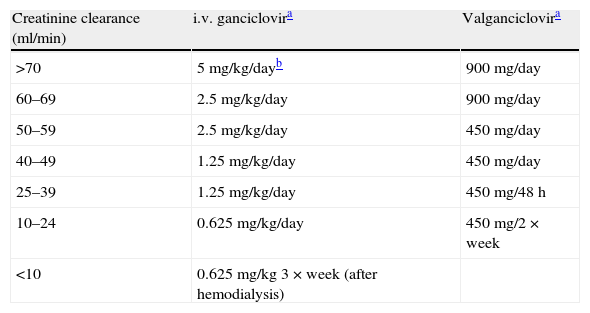
Table 6.Recommended doses in prophylaxis and treatment (including pre-emptive treatment) with intravenous ganciclovir and valganciclovir.
Creatinine clearance (ml/min) i.v. ganciclovira Valganciclovira >70 5mg/kg/dayb 900mg/day 60–69 2.5mg/kg/day 900mg/day 50–59 2.5mg/kg/day 450mg/day 40–49 1.25mg/kg/day 450mg/day 25–39 1.25mg/kg/day 450mg/48h 10–24 0.625mg/kg/day 450mg/2×week <10 0.625mg/kg 3×week (after hemodialysis) i.v: intravenous.
Table 7.Summary of recommendations for the prevention of CMV in solid-organ transplants.
Type of transplant D+/R− patients Other patients Liver PPX: valganciclovir (900mg/d) for 3–6 months (AIII), i.v. ganciclovir (5mg/kg/d) (AI) followed by pre-emptive treatment up to 3 months after end of PPX (AII), or:PT: valganciclovir (900mg/12h) for 14 days, checking negativization of viremia and monitoring every 1–2 weeks, according to risk, during the first 4 months (AII). In R+ patients, either PPX (valganciclovir, 900mg/d 3 months (BII), or i.v. ganciclovir, 5mg/kg/d (AI), followed by valganciclovir, 900mg/d) or PT with valganciclovir, 900mg/12h (AII), or i.v. ganciclovir, 5mg/kg/12h (AI), for 14 days and subsequent monitoring.In D−/R− patients, leukocyte-depleted blood products and products from seronegative donors can be used. Treatment in case of primary infection. Kidney PPX: valganciclovir (900mg/d) (AI) for 6 months post-transplant (AI). Alternatives: oral valaciclovir (2g/6h) (AI), or i.v. ganciclovir (5mg/kg/d) (if not tolerated orally), for a maximum of 3 months post-transplant (AI). In R+ patients, PT is recommended with valganciclovir (900mg/12h) or i.v. ganciclovir (5mg/kg/12h) for 14–21 days and monitoring (BI). Alternative: PPX with valganciclovir (900mg/d), valaciclovir (2g/6h) or i.v. ganciclovir for 3 months (AII).In D−/R− patients, leukocyte-depleted blood products and products from seronegative donors can be used. Treatment in case of primary infection.In treatment with anti-lymphocyte antibodies: i.v. ganciclovir (5mg/kg/d) for at least 14 days (BI) or valganciclovir for 3 months (BI). Heart PPX: i.v. ganciclovir (5mg/kg/day) or valganciclovir (900mg/day) for 3–6 months (AI). Patients receiving induction with anti-lymphocyte antibodies (except basiliximab) or exhibiting steroid-resistant rejection must receive ganciclovir (5mg/kg/day) for at least 14 days (BI) or valganciclovir (900mg/d) for 3 months (CIII).In R+ patients: PPX or PT. Monitoring of CMV in non-prophylaxis patients. If monitoring results are positive, i.v. ganciclovir (5mg/kg/12h) or valganciclovir (900mg/12h) for 2–4 weeks (BII).In some situations, CMV-specific gammaglobulin may be considered (BII).Exclude hypogammaglobulinemia in patients with relapsing CMV disease (BIII). Lung PPX: i.v. ganciclovir (5mg/kg/24h) until tolerated orally and then valganciclovir (900mg/d) for 6–12 months (AII).Anti-CMV gammaglobulin in association with i.v. ganciclovir may benefit high-risk patients (BII).At the end of PPX, monitor patients and start PT with valganciclovir (900mg/12h) or i.v. ganciclovir (5mg/kg/12h IV) (AII). PPX: i.v. ganciclovir (5mg/kg/12h) until tolerated orally and then valganciclovir (900mg/d) until month six (AII).Anti-CMV gammaglobulin in association with i.v. ganciclovir may benefit high-risk patients (BII).In the case of treatment with anti-lymphocyte antibodies or steroids at doses above 10mg/kg/day, treatment with valganciclovir must be restarted at doses of 900mg/day for a maximum of 3 months (BIII). Pancreas and pancreas-kidney PPX: valganciclovir (900mg/12h) for 3 months (C-II). In the presence of other associated risk factors (co-infections, anti-rejection treatment, co-morbidity) consider increasing prophylaxis to 6 months (CIII).Then PT with valganciclovir (900mg/12h) or ganciclovir (5mg/kg/12h) (CIII). In R+ patients who have received anti-lymphocyte antibodies or high doses of steroids as rejection treatment, pre-emptive therapy is recommended (CIII) with valganciclovir (900mg/12h) or ganciclovir (5mg/kg/12h).In D−/R− patients, leukocyte-depleted blood products and products from seronegative donors can be used. Treatment in case of primary infection.In patients who have received anti-lymphocyte antibodies for more than 3 days in the induction phase, treatment with valganciclovir (900mg/day) for 3 months is recommended (CIII).In patients with acute rejection and receiving anti-lymphocyte antibodies or steroids at high doses, valganciclovir (900mg/day) for 1–3 months is recommended (CIII). Intestine PPX: i.v. ganciclovir (5mg/kg/12h) or valganciclovir (900mg/d) for a minimum of 6 months; treatment may be prolonged until lymphocyte counts=CD4+>200 cells/ml (CIII).Anti-gammaglobulin may be considered (150mg/kg) in weeks 0, 2, 4, 6 and 8, followed by 100mg/kg in weeks 12 and 16 (CIII). In R+ patients, PPX with i.v. ganciclovir (5mg/kg/day) or valganciclovir (900mg/day) for between 3 and 6 months post-transplant (CIII).In patients receiving anti-lymphocyte antibodies or presenting cortico-resistant rejection, start prophylaxis with i.v. ganciclovir or valganciclovir for between 1 and 3 months (CIII). Recommended doses of antiviral agents for normal renal function (creatinine clearance >70ml/min) and neutrophil counts >1000/μl.
D+/R−: positive donor/negative recipient; R+: positive recipient; PPX: universal prophylaxis; PT: pre-emptive treatment; i.v.: intravenous.
The use of anti-lymphocyte antibodies in induction treatment or as anti-rejection therapy is associated with an increase in the risk of CMV disease.66,70,72 The effect of antiviral prophylaxis in patients receiving antiviral medication has been demonstrated in different trials with SOT recipients. Two of these trials, which compared the administration of intravenous ganciclovir with no treatment in kidney transplant recipients receiving anti-lymphocyte antibodies, reported the protective effect of ganciclovir.131,132
RecommendationsIn patients receiving anti-lymphocyte or monoclonal antibodies such as OKT3 or alemtuzumab, prophylaxis must be used with antiviral drugs (AI). No conclusive data are available on the duration, optimum dose and drug that must be used, and may vary according to the characteristics of individual patients (e.g. CMV serostatus) and type of transplanted organ. In this guideline, both intravenous ganciclovir and valganciclovir were used.
This strategy may also be considered to treat rejection with high doses of steroids (CIII).
Liver transplantUniversal prophylaxisIn liver transplant patients, universal prophylaxis with different drugs is effective for preventing CMV disease. In a randomized double-blind trial with 55 liver transplant recipients, prophylaxis with oral acyclovir (800mg/6h) for 28 days compared with placebo reduced the frequency of CMV disease (25% vs. 52%, P=.05).133 In another randomized trial that included 304 liver transplant recipients, 46 of whom were high-risk patients (15%), prophylaxis with oral ganciclovir (1g/8h) for 90 days compared with placebo reduced the frequency of CMV disease during the first 6 months post-transplant (5% vs. 19%) and also in high-risk recipients (15% vs. 44%).134 In another randomized trial with 64 high-risk liver transplant recipients (D+/R−), prophylaxis with oral ganciclovir (1g/8h) was as effective as intravenous ganciclovir (6mg/kg/day, 5 days per week until day +100), and the frequency of CMV disease was 9% and 12.5%, respectively.135 In 219 CMV-seropositive liver transplant recipients, oral ganciclovir (1g/8h) was more effective than acyclovir (800mg/6h) in reducing CMV disease (1% vs. 7%)126 during the first 100 days post-transplant.
The incidence of CMV disease in high-risk liver transplant patients (D+/R) receiving prophylaxis with valganciclovir for 100 days was 9%.136
Finally, the efficacy and safety of valganciclovir (900mg/day×100 days) were compared with oral ganciclovir (1g/8h×100 days) in a randomized double-blind study in 364 high-risk SOT recipients (D+/R−), of whom 177 were liver transplant recipients, and who represented the largest group of patients in the study (48%). In liver transplant recipients, the results were unfavorable for valganciclovir due to the greater frequency of CMV disease (19% vs. 12% in the first 6 months) and neutropenia (8% vs. 3%).121 Similar results were described in a retrospective study comparing valganciclovir (900mg/day) with oral ganciclovir (1g/8h) and intravenous valganciclovir (6mg/kg/day) in 66 high-risk liver transplant recipients (D+/R−). CMV disease was four times more frequent in the group receiving valganciclovir (22%) than in the one receiving oral ganciclovir (6%) or intravenous ganciclovir (4.5%).137 A recent meta-analysis evaluated the efficacy of valganciclovir for preventing CMV disease in solid-organ transplant recipients and concluded that it is no more effective than standard treatment, accompanied by a greater risk of neutropenia, late disease and invasive CMV disease, which, coupled with its higher cost, has prevented this drug from being recommended as first-line medication for prophylaxis or pre-emptive treatment in these patients.138
Due to these results, guidelines on the use of valganciclovir in prophylaxis established in technical data sheets do not include liver transplant recipients, or adult or pediatric patients. However, valganciclovir has been used in these patients since oral ganciclovir was withdrawn from the market.
Pre-emptive treatmentDifferent studies have shown that pre-emptive treatment effectively prevents CMV disease in liver transplant recipients. One meta-analysis consisting of 10 randomized trials (including four liver transplant trials) with 476 patients showed that pre-emptive treatment was effective compared with placebo or no prevention.128 In another study carried out on 69 liver transplant recipients, pre-emptive treatment with oral ganciclovir (1g/8h for 8 weeks) monitored by PCR and compared with placebo reduced the overall frequency of CMV disease (12% vs. 3%, P<.05) and in the subgroup of D+/R− recipients (36% vs. 0%, P<.05).139 Another trial in 72 liver transplant recipients compared the efficacy of pre-emptive treatment with oral ganciclovir (2g/8h×2 weeks followed by 1g/8h×4 weeks) vs. intravenous ganciclovir (5mg/kg/12h×7 days) in 22 patients with positive antigenemia. The incidence of disease was similar in the group receiving oral ganciclovir vs. intravenous ganciclovir (0% vs. 11%, P=ns) and zero in 50 patients with negative antigenemia.140
The efficacy of valganciclovir (900mg/12h) in pre-emptive treatment was evaluated in 36 high-risk liver transplant recipients, guided in the first phase by antigenemia (cut-off point ≥1 cell with characteristic immunofluorescence per 2×105 leukocytes) and PCR in a second phase (DNA≥15 copies/ml), and the frequency of CMV disease was null.141 At the same center, a cohort of 216 liver transplant patients was studied to determine the frequency of CMV disease, rejection, bacterial infections, fungal infections, recurrence of hepatitis C, re-transplant and short and long-term survival (6 months to 5 years), including patients who received pre-emptive treatment and those who did not (58 vs. 158), and no differences were reported.142
Viremia relapse is frequent after pre-emptive treatment and frequencies of between 8% and 40% have been described in high-risk patients.141,143 This viremia is normally resolved after new treatment. Recently, BenMarzouk et al. studied 21 high-risk liver transplant patients (D+/R−) undergoing pre-emptive treatment and presenting episodes of viral replication. None of these patients displayed symptoms associated with CMV. All the patients also developed specific immunity to CMV.144
The duration of pre-emptive treatment has not been established. Most studies apply treatments for between 2 and 3 weeks. Diaz-Pedroche et al., in a study with high-risk SOT recipients, reported that pre-emptive treatment with valganciclovir (900mg/12h) reduced basal viral load by 78% and 98% after 7 and 14 days of treatment, respectively.143 In seropositive recipients, shorter treatment periods may be effective. In one study of 58 CMV-seropositive liver transplant recipients, pre-emptive treatment was administered for 7 days, resulting in only one probable case of disease, and relapse of the infection in 20% of the patients.145
Consensus recommendationsIn liver transplant patients, CMV disease prevention strategies must be designed according to serostatus.
- 1.
In R+ recipients, prophylaxis and pre-emptive treatments are two recommendable strategies (AI). Most of the panel recommends pre-emptive treatment.
- 2.
In high-risk recipients (D+/R−), both strategies are equally recommendable. Most of the panel recommends universal prophylaxis if rigorous compliance with the applicable virological monitoring protocol is not possible. If prophylaxis is selected, pre-emptive treatment is recommended for at least 3 months after prophylaxis (AII).
- 3.
If prophylaxis is selected, and since oral ganciclovir has been withdrawn from the market, the best option available is valganciclovir at doses of 900mg/24h in adult patients with normal renal function and during the first 3 and 6 months post-transplant (AIII). The duration (i.e. 3 or 6 months) can be determined according to the degree of immunosuppression of the patient, including the use of anti-lymphocyte antibodies. It is recommended to start prophylaxis on day +10 post-transplant; if ganciclovir cannot be administered orally, it should be administered intravenously at doses of 5mg/kg/day until the patient is able to tolerate oral medication (AI).
- 4.
If pre-emptive treatment is chosen, valganciclovir is recommended at doses of 900mg/12h (in patients with normal renal function) for 2 weeks, checking for suppression of viremia (AII). During the first 4 months post-transplant, viremia should be monitored weekly in high-risk patients and every 2 weeks in other patients (AII).
- 5.
The selection test for monitoring viremia is quantitative PCR; each center must establish the logistics required to perform the test and the viral load at which to start treatment. The alternative is pp65 antigenemia and each center must establish its own cut-off point, although, as a guide, the level recommended by the panel is ≥2 cells×105 leukocytes (BII).
Universal prophylaxis with acyclovir,146 valaciclovir,127 oral ganciclovir130 or valganciclovir121 in D+/R− kidney transplant patients can be used to effectively reduce the incidence of CMV disease, at least during the period it is administered (normally 100 days). All these antiviral drugs reduce the frequency of the disease to below 15% in this population. Valganciclovir is currently the most frequently used drug due to its better bioavailability and ease of administration.
In previous efficacy trials, more than 25% of D+/R− patients developed late CMV disease 12 months after the suspension of universal prophylaxis administered for 100 days.121 Later, and in spite of the controversy surrounding its methodology,147,148 the results of the IMPACT study have shown that prolonging anti-CMV prophylaxis for 200 days in D+/R− kidney transplant patients can reduce the incidence of late CMV disease by 16%.149 This reduction corresponded mainly to a decrease in the incidence of viral syndrome (in this study, 83 of the 85 patients [96.7%] diagnosed with CMV disease corresponded to viral syndrome, the definition of which differs from that established in this consensus paper). Universal prophylaxis for 200 days was associated with a decrease in the incidence of opportunistic infection – but not rejection – in these patients.
In CMV-seropositive recipients there is much less evidence of the efficacy of universal prophylaxis, although some studies have reported significant reductions in CMV infection and disease with both oral ganciclovir and valganciclovir.130,150 Moreover, it has been shown that in patients requiring the administration of anti-lymphocyte antibodies (ATG/ALG/OKT3), universal prophylaxis with both intravenous ganciclovir and valganciclovir significantly reduces the frequency of CMV disease in kidney transplant patients.132,143
Pre-emptive treatmentIt has been shown that antigenemia or PCR-monitored pre-emptive treatment with oral ganciclovir151 or valganciclovir143,152 is effective in kidney transplant recipients. However, it has been suggested that patients receiving pre-emptive treatment may be at greater risk of acute rejection 12 months after transplant.153 This effect could be related to the higher degree of viremia observed in patients receiving pre-emptive treatment compared with those receiving universal prophylaxis.154
Recommendations for kidney transplantsDifferent studies published in recent years have shown that both pre-emptive treatment and universal prophylaxis can reduce the incidence of CMV disease in kidney transplant recipients.130,150 A number of meta-analyses have reported that universal prophylaxis can also reduce the incidence of rejection, opportunistic infection and death in this population.124
- 1.
In D+/R− patients, universal prophylaxis is recommended with valganciclovir (900mg/day) (AI) for a maximum of 6 months post-transplant (AI). Alternatively, intravenous ganciclovir (5mg/kg/day) (AI) or oral valaciclovir (2g/6h) (AI) could be used, especially in patients with severe leukopenia, for 3 months (AI). In cases of oral intolerance, ganciclovir can be administered intravenously at doses of 5mg/kg/day until the treatment is completed (AI). Pre-emptive therapy may be an alternative to universal prophylaxis in centers with adequate infrastructure to guarantee the monitoring of patients. In recipients treated with anti-lymphocyte antibodies, valganciclovir is recommended for 3 months (BI) or intravenous ganciclovir (5mg/kg/day) for at least 14 days (BI).
- 2.
In CMV-seropositive recipients, pre-emptive treatment is recommended with valganciclovir (900mg/12h) or intravenous ganciclovir (5mg/kg/12h), in case of oral intolerance, for 14–21 days or until antigenemia or viral load of CMV are negative or undetectable (BII). Another alternative is universal prophylaxis with oral valganciclovir (450–900mg/day), valaciclovir (2g/6h) or intravenous ganciclovir (5mg/kg/day) (if it cannot be administered orally) for a maximum of 3 months (AII).
In lung transplant patients, it is difficult to make recommendations because most studies are cohort studies and very few controlled randomized studies have been carried out in these patients. In general, these studies have shown that prophylaxis, either with intravenous or oral ganciclovir, or with valganciclovir, delays the onset of CMV disease. However, late CMV disease has been reported even after prophylaxis maintained during the first 6 months post-transplant.155–158
In lung transplants, the only studies that have reported a decrease in the incidence of CMV disease to levels close to the annual level of 5% based their strategies on universal prophylaxis for 6 months followed by pre-emptive treatment for 1 year. Zamora et al.159 determined the safety and efficacy of valganciclovir in 90 lung transplant recipients who survived for more than 30 days and who also received anti-CMV gammaglobulin. In these patients, prophylaxis was used in combination with valganciclovir (450mg/12h) for 180, 270 or 365 days, followed by pre-emptive treatment. The results of this group were compared with a historical cohort of 140 patients who received high doses of acyclovir after intravenous ganciclovir plus specific gammaglobulin. CMV disease was significantly lower in the group that received valganciclovir (2.2% compared with 20%; P<.01), and no differences were observed when the duration of prophylaxis was increased to more than 180 days, although in 32% of patients the drug had to be withdrawn due to the appearance of leukopenia.
Later, a multicenter study carried out in Spain160 included 66 lung transplant recipients who received universal prophylaxis with valganciclovir (900mg/day for 120 days) followed by pre-emptive treatment in patients displaying significant infection. These patients were compared with a historical group of patients who received prophylaxis with oral ganciclovir using the same strategy, where the incidence of CMV disease in the group receiving valganciclovir was 7.9% compared with 16% in the group receiving oral ganciclovir. However, the use of valganciclovir was associated with a greater risk of leukopenia and withdrawal due to adverse effects.
Jaksch et al.161 compared a group of 15 high-risk patients (D+/R−) who received valganciclovir (900mg/day) for 3 months, compared with another group receiving valganciclovir for 1 year. Results for the latter group showed a decrease in the incidence of viremia (75% vs. 33%), CMV disease (44% vs. 13%) and acute rejection (26% vs. 5%). However, and in spite of these results, the publication of the study by Zamora et al.159 has prompted most guidelines to recommend prophylaxis for 6 months.
Valentine et al.98 published a study that included 90 lung transplant recipients who received valganciclovir at 900mg/day in an unspecified manner, and reported an incidence of CMV-induced pneumonitis of 2% over an average treatment period of 4 years. However, these authors did not observe any decrease in the incidence of bronchiolitis obliterans after 5 years, which was 50% and similar to that reported in most published series.
Recently, Palmer et al.162 performed a multicenter, randomized, double-blind and controlled trial with placebo to compare the efficacy of prolonged prophylaxis with valganciclovir (900mg/day) for 12 months compared with 3 months. These authors reported a significant reduction in the incidence of CMV disease (4% vs. 32%; P<.001), CMV infection (10% vs. 64%; P<.001) and viral load upon diagnosis of CMV disease (3200 copies/ml vs. 110,000 copies/ml; P=.009). The incidence of acute rejection, opportunistic infections, adverse effects and ganciclovir resistance mutations was similar in both groups. However, in spite of the randomized and double-blind nature of this study, these results must be interpreted with caution, since in the comparison group valganciclovir was administered for 3 months, while normal practice in most transplant groups is 6 months. More importantly, the group treated for 1 year was only monitored for a period of 30 days.
Although the role of hyperimmune gammaglobulin has been examined in different studies with contradictory results,163,164 recently published studies seem to demonstrate that a combination of specific gammaglobulin with intravenous ganciclovir reduces the incidence of CMV disease. Valantine et al.165 reported a lower incidence of CMV disease, acute rejection and chronic evolution after 12 and 24 months in patients who received a combination of these drugs compared with those who only received ganciclovir. Similarly, other studies with historical controls have also reported favorable results with combined treatment.166
Pre-emptive treatmentIn lung transplant recipients, the efficacy and safety of pre-emptive treatment have not been studied and therefore, cannot be recommended (DII). Although various studies carried out in this population seem to demonstrate that this strategy could be valid if used sequentially with universal prophylaxis (BII),159,160 the use of pre-emptive treatment entails a risk for the patient because the first infection diagnosed could be a serious infection, such as pneumonitis.160
Recommendations for lung transplant- 1.
Universal prophylaxis is the best strategy for preventing CMV disease in lung transplant recipients (AII). Intravenous ganciclovir is recommended in doses of 5mg/kg/24h until it can be tolerated orally, followed by valganciclovir at a dose of 900mg/day for 6 months post-transplant (AII). In D+/R− recipients, it can be increased to 12 months in patients who are difficult to monitor (AII).
- 2.
Evidence exists to suggest benefits in the use of specific gammaglobulin against CMV. This panel considers that this gammaglobulin can be used according to the characteristics of the patient (e.g. clinical evolution or immunosuppression status) (BII).
- 3.
Antigenemia or viral load should be monitored by PCR every 2 weeks or otherwise during every medical examination in order to exclude breakthrough viremia which could imply a greater risk of later appearance of ganciclovir-resistant CMV. If a breakthrough viremia is diagnosed, the dose of valganciclovir must be increased to 900mg/12h and viral load must be monitored weekly to reduce the dose to 900mg/day after negativization (AII). After prophylaxis has been completed, pre-emptive treatment should be started in the event of a significant infection with valganciclovir (900mg/12h) provided that there is no high-grade viremia, in which case treatment must be started with intravenous ganciclovir (5mg/kg/12h IV) (AII). In D+/R− patients or in patients with higher levels of immunosuppression, pre-emptive treatment could also be considered provided that there is no evidence of viral replication. Patients must be monitored in each medical examination until the second year post-transplant, except D+/R− patients who must be monitored at least every 2 weeks until the ninth month post-transplant. If these patients cannot be monitored, prophylaxis may be extended until 1 year post-transplant.
- 4.
Whenever immunosuppression has to be increased, viral load should be monitored by quantitative PCR. The frequency of monitoring must be determined according to the characteristics of the patient, and must be at least once per month (CIII).
- 5.
In the case of treatment with anti-lymphocyte antibodies or with steroids at doses above 10mg/kg/day, treatment with valganciclovir should be restarted at a dose of 900mg/day for a maximum of 3 months (BIII).
Intestine transplants are associated with a high risk of CMV infection, probably as a consequence of the abundance of lymphoid tissue and the high level of immunosuppression to which these patients are subjected. Unlike other types of transplants, little information is available on anti-CMV prophylaxis in intestine transplant recipients. No randomized studies on antiviral prophylaxis have been carried out in these patients. Different management protocols exist that all employ universal prophylaxis with both ganciclovir and valganciclovir for between 3 and 6 months. Specific gammaglobulin is also used by some centers, for variable treatment durations.167,168 Despite the lack of information in this respect, valganciclovir seems to be well tolerated orally by intestine transplant recipients.169
In a Spanish group administered alemtuzumab for induction therapy, CD4+ T-lymphocyte counts were monitored; this monitoring strategy was used as a criterion for increasing anti-CMV prophylaxis to more than 6 months post-transplant in patients with lymphocyte counts below 100 cells/mm3.170
Pre-emptive treatmentThis prophylaxis strategy has not been studied in depth in intestine transplant recipients. In this group of patients, due to their high risk and the high morbidity and mortality associated with CMV disease, pre-emptive therapy is currently not recommended (DIII).
Use of specific anti-cytomegalovirus gammaglobulinIn a recent meta-analysis that reviewed the efficacy of gammaglobulin for preventing CMV disease in solid-organ transplant recipients, studies with intestine transplant patients were not included.171
Specific anti-CMV gammaglobulin is widely used in many intestine transplant groups in the USA, at a dose of 150mg/kg in weeks 0, 2, 4, 6 and 8, followed by 100mg/kg in weeks 12 and 16 (C-III).
Recommendations for intestine transplant- 1.
In D+/R− recipients, the use of universal prophylaxis with intravenous ganciclovir (5mg/kg/day) or valganciclovir (900mg/day) is recommended for a minimum of 6 months (especially if alemtuzumab is used for induction) and may be prolonged until CD4+ T-lymphocyte counts are higher than 100 cells/mm3. The committee of experts considers that the use of specific anti-CMV gammaglobulin (150mg/kg in weeks 0, 2, 4, 6 and 8 followed by 100mg/kg in weeks 12 and 16) may be considered, taking into account the characteristics of the patient and his/her clinical evolution or immunosuppression status (CIII).
- 2.
In seropositive recipients, universal prophylaxis is recommended with intravenous ganciclovir (5mg/kg/day) or valganciclovir (900mg/day) for a duration of between 3 and 6 months post-transplant (CIII).
- 3.
In patients receiving induction treatments or anti-rejection treatment with anti-lymphocyte antibodies, or who display corticosteroid-resistant rejection, prophylaxis should be started with intravenous ganciclovir or valganciclovir for between 1 and 3 months (CIII).
From a practical standpoint, most pancreas transplant recipients will be treated as high-risk patients due to the high percentage of treatments that employ induction therapy with anti-lymphocyte antibodies in these patients. In pancreas-kidney transplants, prophylaxis with ganciclovir has been shown to reduce the incidence of CMV infection by 50%, as well as the rate of acute rejection by 20%, compared with non-therapy or acyclovir.172 Although the required duration of prophylaxis has not been established, the results of different studies propose durations of between 3 and 6 months. Becker et al. showed that prophylaxis for 3 months with acyclovir or oral ganciclovir was followed by CMV disease in 13.4% of cases, and was significantly associated with acute rejection and the existence of co-infections.173 Moreover, 6-month prophylaxis has been shown to be more cost-effective than 3-month prophylaxis in a cohort of kidney and pancreas-liver transplant recipients, reducing the incidence of CMV disease from 24.4% to 12%.174 In this sense, López-Medrano et al. have reported an incidence of CMV disease of 33% in seronegative recipients who received 3-month prophylaxis with ganciclovir.175
Pre-emptive treatmentIn one study in which only pre-emptive treatment was used, the incidence of CMV infection was 75%176 with a tendency for a shorter survival of pancreas transplants. In another retrospective study, universal 3-month prophylaxis was shown to be more effective than pre-emptive therapy for reducing the rate of CMV disease (6.9% versus 23%, P<.05).175
Recommendations for pancreas-kidney transplants- 1.
In D+/R− patients, valganciclovir is recommended (900mg/day) for 3 months (CII). If other associated risk factors (co-infection, anti-rejection treatment, comorbidity) are observed in these patients, the duration of prophylaxis may be increased to 6 months (CIII).
- 2.
In patients who have received anti-lymphocyte antibodies for more than 3 days in the induction phase, the use of valganciclovir is recommended at 900mg/day for 3 months (CIII).
- 3.
In patients with acute rejection and receiving anti-lymphocyte antibodies or steroids at high doses, the use of valganciclovir is recommended at 900mg/day for 1–3 months (CIII).
- 4.
In patients who have received universal prophylaxis, the presence of CMV infection must be monitored by antigenemia or PCR on a weekly basis for the following 3 months, and monthly until 1 year post-transplant. In the case of active replication, valganciclovir is recommended at 900mg/12h or ganciclovir at 5mg/kg/12h for at least 2 weeks and until one or two negative antigenemia or PCR results are obtained once or twice a week (CIII).
- 5.
In R+ patients not receiving anti-lymphocyte antibody treatment or high doses of steroids to treat rejection, pre-emptive therapy is recommended (CIII).
Few studies have compared different prophylaxis strategies in heart transplant recipients. The use of intravenous ganciclovir has been compared with placebo in D+/R− patients, showing the benefits of prophylaxis administered for 6 weeks and also for 14 days after each rejection episode.177 However, when prophylaxis was received by D+/R− patients for 4 weeks, outcomes were not superior to placebo.178
In addition to intravenous strategies, prophylaxis may be effective if administered orally in high-risk patients (D+/R−). In this sense, good outcomes have been reported with valganciclovir (900mg/day) compared with oral ganciclovir (3g/day) during the first 100 days post-transplant.121 This study included 56 heart transplant recipients and the proportion of patients with CMV disease was 6% vs. 10%. These good outcomes have also been reported by other authors, with some groups maintaining prophylaxis with valganciclovir (450–900mg/day) for 6 months.179,180 However, the benefits of universal prophylaxis have been undermined by the appearance of late CMV disease in both D+/R− and D+/R+ patients181,182 without any clinical risk factors having been identified for the appearance of this complication.
Universal prophylaxis is also recommended in patients receiving anti-lymphocyte antibodies. Aguado et al. carried out a prospective randomized open study with 31 seropositive patients who received induction with OKT3 for 14 days.183 Administration of full doses of intravenous ganciclovir for 14 days was more effective than anti-CMV gammaglobulin in the prevention of disease, although no differences were observed in the incidence of infection. Similarly, in a retrospective study which included 115 patients treated with OKT3 and receiving oral acyclovir and anti-CMV gammaglobulin, 14 days of treatment with intravenous ganciclovir reduced the incidence of CMV disease in D+/R− patients, albeit without statistical significance.184
Two (2) retrospective studies have been carried out with heart-lung transplant recipients, reporting good outcomes by combining the administration of anti-CMV gammaglobulin and intravenous ganciclovir for the first 21–28 days post-transplant,165,185 with a significant reduction in the disease, associated death and vascular allograft disease.
In R+ patients, some transplant groups recommend universal prophylaxis with intravenous ganciclovir for 4 weeks (5mg/kg/12h for 2 weeks followed by 6mg/kg/day for another 2 weeks) based on the results of a prospective study.178 However, in another prospective study universal prophylaxis (5mg/kg 3 times per week for 6 weeks) did not reduce the incidence of CMV disease in such patients. Finally, a recent study in seropositive patients compared retrospectively177 the use of high doses of ganciclovir (10mg/kg/day for 2 weeks, followed by 5mg/kg/day for another 2 weeks) with low doses (5mg/kg/day for 4 weeks), without recording significant differences in the incidence of infection, disease and acute rejection.186
Pre-emptive treatmentIn CMV-seropositive patients who do not receive anti-lymphocyte antibodies, preventive measures are normally based on pre-emptive treatment, which some authors have even successfully applied in high-risk patients.187 A recently published study included 37 heart transplant recipients with positive antigenemia who received intravenous ganciclovir or anti-CMV gammaglobulin without developing the disease.188 Other authors have also published favorable outcomes when administering intravenous ganciclovir in such patients.189–191
Favorable outcomes have also been reported with valganciclovir in pre-emptive treatment, although the studies published have only included small numbers of heart transplant recipients.152,192–194
Recommendations for heart transplants- 1.
D+/R− patients must receive universal prophylaxis (AI). For this purpose, intravenous ganciclovir (5mg/kg/day) or valganciclovir (900mg/day) may be administered for 3–6 months (AI). Pre-emptive therapy may be an alternative to universal prophylaxis in centers with an adequate infrastructure to guarantee patient monitoring.
- 2.
Patients treated with anti-lymphocyte antibodies (excluding basiliximab) or showing steroid-resistant rejection must receive ganciclovir (5mg/kg/day) for at least 14 days (BI) or valganciclovir (900mg/day) for 3 months (CIII).
- 3.
There is no ideal preventive treatment for R+ patients. Quantitative sequential monitoring of CMV is recommended in non-prophylaxis patients. If monitoring is positive, intravenous ganciclovir (5mg/kg/12h) or valganciclovir (900mg/12h) should be administered for 2–4 weeks (BII).
- 4.
In patients with CMV disease relapse, the absence of hypogammaglobulinemia must be confirmed (BIII).
- 5.
In some situations (e.g. clinical evolution or immunosuppression) the use of specific anti-CMV gammaglobulin may be considered (BII).
Ganciclovir has been the standard recommended treatment for CMV disease in SOT recipients for more than 15 years. More recently, valganciclovir has been shown to have an efficacy similar to intravenous ganciclovir.195 Valganciclovir is a esterified derivative of ganciclovir that is rapidly hydrolyzed after absorption in the intestine, forming ganciclovir as an active metabolite, with a bioavailability of 60% after oral administration.196,197 Recently, the VICTOR study, an international, multicenter, randomized trial, showed that valganciclovir was as effective and safe as intravenous ganciclovir in the treatment of CMV disease in a population of 321 SOT recipients.198 Although this study has some limitations (most of the patients included in the study were kidney transplant recipients and it did not include patients with serious CMV disease or pediatric patients), its results support the recommendation of valganciclovir for the treatment of CMV disease, at least in selected patients, since during long-term monitoring no significant differences were observed in clinical recurrence and virological recurrence between the valganciclovir group and the intravenous ganciclovir group.199
Oral ganciclovir cannot be used to treat patients with CMV disease because it is no longer commercially available. Other antiviral drugs that can be taken orally, such as acyclovir or valaciclovir, must also not be used to treat CMV disease.200
In mild or moderate CMV disease, the recommended drug for first-line treatment is valganciclovir (oral doses of 900mg every 12h) or intravenous ganciclovir (doses of 5mg/kg every 12h).198 Intravenous ganciclovir must be used in patients with severe or potentially fatal CMV disease and when valganciclovir is poorly tolerated or inadequately absorbed since current information on the efficacy of oral treatment in such patients is limited.198
Another potential therapeutic strategy is sequential therapy, i.e. treatment with intravenous ganciclovir followed by valganciclovir once the patients condition starts to improve. In a Spanish pilot study, this strategy provided effective therapy with adequate exposure to drugs, reducing treatment costs and avoiding prolonged hospitalization, thus resulting in greater comfort for the patients.201
Adequate doses of both valganciclovir and ganciclovir must be administered as doses below therapeutic levels may lead to therapeutic failure and promote the development of resistance,202 while doses above the therapeutic levels may lead to the appearance of toxic effects.16,203 During treatment, renal function must be monitored with frequent tests of the glomerular filtration rate by either direct quantification or estimation.204 Doses and dose intervals must be adjusted according to creatinine clearance values, as shown in Table 6.
Reducing ganciclovir or valganciclovir doses based on secondary effects, such as leukopenia must be avoided whenever possible. Before reducing these doses, consideration must be given to reducing the doses of other drugs such as mycophenolic acid derivatives (mycophenolate mofetil and sodium mycophenolate), mTOR inhibitors (sirolimus and everolimus), azathioprine and trimethoprim/sulfamethoxazole. In severe leukopenia, particularly when absolute neutrophil counts are lower than 1000μL−1, the use of a granulocyte colony-stimulating factor (G-CSF) may be considered.200
The optimum duration of treatment of CMV disease must be determined on an individual basis and guided by clinical monitoring and virological monitoring. Antigenemia or quantitative PCR must be determined weekly in order to monitor treatment response and the development of any resistance to ganciclovir.198,205 Treatment must be maintained until a negative viral load or antigenemia results are obtained. Nevertheless, in high-risk patients it would be recommendable to obtain two consecutive negative results at 1-week intervals to ensure elimination of the virus. In any case, the minimum duration of treatment must not be less than 2 weeks.198,199,205 This therapeutic strategy minimizes the risks of developing resistance and recurrence of CMV disease.199,206,207
Sometimes, certain forms of invasive tissue disease are not accompanied by detectable viremia. These are so-called forms of “compartmented disease” in which serial PCR as a tool for guiding treatment is of limited use.208 This is especially evident in forms of local reactivation in intestinal lymphoid tissue or in different forms of central nervous system disease.
In some transplant centers, secondary prophylaxis is used with valganciclovir at doses of 900mg/day after treatment has been completed, for between one and three months199,205 according to the presence of CMV infection recurrence risk factors (primary CMV infection, high basal viral load, persistence of viremia at the beginning of secondary prophylaxis, multiorgan disease, high-risk organs and increases in immunosuppression due to rejection63,199,207). During secondary prophylaxis, viral load must also be monitored at unspecified intervals, although tests must clearly be performed more frequently in patients with a higher risk of relapse.
In cases of severe and compartmented disease, longer treatment periods are recommended with clinical monitoring focusing on the detection of specific expressions of the disease. In patients suffering from a recurrence of CMV disease, secondary prophylaxis must be prolonged.200
Consensus recommendations- 1.
In mild or moderate CMV disease, the recommended first-line treatment drugs are valganciclovir (900mg/12h) or intravenous ganciclovir (5mg/kg every 12h) (AI). In clinical situations that require the patient to be hospitalized, intravenous ganciclovir is recommended (AII).
- 2.
In patients with severe, potentially fatal disease, and when valganciclovir is poorly tolerated or cannot be adequately absorbed, intravenous ganciclovir must be used (AII).
- 3.
An alternative therapeutic strategy is sequential therapy, i.e. starting treatment with intravenous ganciclovir and then changing to valganciclovir once the patient starts to improve (BII). This strategy may be considered in patients displaying clinical improvement, oral tolerance and a decrease in viral load or antigenemia in control tests.
- 4.
Oral ganciclovir, acyclovir or valaciclovir must not be used to treat CMV disease (DIII).
- 5.
During treatment, renal function must be monitored and the glomerular filtration rate must be determined frequently through direct quantification or calculated. Doses and dose intervals must be adjusted according to creatinine clearance values (BII).
- 6.
Reducing doses of ganciclovir or valganciclovir, based on secondary effects such as leukopenia, must be avoided whenever possible. Consideration must be given at first to the possibility of reducing doses of other myelotoxic drugs such as, mycophenolic acid derivatives, mTOR inhibitors, azathioprine and trimethoprim/sulfamethoxazole (BIII).
- 7.
In severe leukopenia (neutrophil counts<1000/μL), the use of granulocyte colony-stimulating factor (G-CSF) may be considered (BIII).
- 8.
The optimum duration of treatment must be determined on an individual basis and guided by clinical monitoring and virological monitoring. Antigenemia or quantitative PCR must be determined on a weekly basis in order to monitor treatment response and the development of any resistance (AII).
- 9.
Treatment must be maintained until the detected virus has been eliminated, confirmed by negative antigenemia or quantitative PCR results for CMV. However, in high-risk patients it is recommended that two consecutive negative results at one-week intervals should be confirmed to ensure elimination of the virus. In any case, the minimum duration of treatment must never be less than two weeks in viral syndrome cases or less than 4 weeks in patients with organ disease (BIII).
- 10.
In some cases (primary CMV infection, high basal viral load, persistence of viremia at the start of secondary prophylaxis, multiorgan disease, high-risk organ transplant and increases in immunosuppression due to rejection), secondary prophylaxis with valganciclovir at 900mg/day may be considered, although the risk/benefit ratio of this treatment has not been entirely clarified (CII). If used, the virus must be monitored in blood, at unspecified intervals, in order to detect any resistance (BIII). In this sense, treatment with valganciclovir may be preferable (BIII),
- 11.
The duration of secondary prophylaxis will be one to three months, according to the existence of risk factors for the recurrence of CMV infection (BIII).
- 12.
In forms of “compartmentalized disease”, which compromise organs without detectable viremia, the response to treatment will be guided by clinical response and histological response (BIII).
- 13.
In patients with severe disease and compartmentalized disease, longer treatment periods are recommended (BIII).
- 14.
In cases of recurrence of CMV disease, secondary prophylaxis, after re-treatment, must be prolonged (BIII).
- 15.
For the treatment of patients receiving combined transplants (e.g. pancreas-kidney), the instructions for the treatment of higher-risk transplants must be followed (CIII).
- 16.
In deferred transplant patients (e.g. kidney transplants in heart transplant recipients) the treatment instructions for the last transplant performed should be followed (CIII).
There are currently no controlled clinical trials that indicate the best alternative treatment in the event of evidence of ganciclovir-resistant CMV. Therapeutic decisions must be based on the genotype analysis of the UL97 and UL54 genes, patient immune state and disease severity. Reducing immunosuppressive therapy may be effective in some patients, particularly in those not subject to prolonged exposure to antiviral agents. However, depending on the severity of CMV disease, empirical treatment may be necessary until the results of the genotype analysis are available. These alternative empirical treatments include increasing the dose of ganciclovir (to more than 10mg/kg twice per day for normal renal function) in patients with mild disease, combining ganciclovir and foscarnet209 or administering foscarnet separately to patients with severe CMV disease.
If genotype analysis reveals the presence of a mutation in the UL97 gene related to a high degree of resistance (M460V/I, A594V, H520Q, L595S, C603W), the most appropriate approach is to change to foscarnet. However, if this is related to a low degree of resistance, treatment can be continued with high doses of ganciclovir, provided that renal function is stable.
Patients exhibiting a mutation in the UL54 gene, generally associated with resistance to ganciclovir and cidofovir, should change to foscarnet since crossed resistance to this drug is rare.210 The mutations responsible for resistance to foscarnet usually only appear in patients taking foscarnet.
Consensus recommendations- 1.
Mutations in the UL97 kinase gene and the pol UL54 polymerase gene may confer resistance to ganciclovir (BII).
- 2.
Mutations in the UL54 polymerase gene may be isolated or occur in combination with mutations in the UL97 kinase gene and may confer resistance to cidofovir and/or foscarnet or crossed resistance to ganciclovir, foscarnet and cidofovir (BIII).
- 3.
Risk factors for the development of resistance are: (a) seronegative recipients receiving organs from CMV-seropositive donors; (b) pancreas and lung transplant recipients; (c) high-level viral replication; (d) intense concomitant immunosuppressive treatment; and (e) prolonged exposure or suboptimum levels of ganciclovir (BII).
- 4.
Resistance to antiviral medication must be suspected when viral load or clinical progression of CMV disease increases despite adequate exposure to treatment two weeks after persistence is detected (BIII).
- 5.
When resistance to ganciclovir is demonstrated, immunosuppressive treatment should be reduced as much as possible (BIII).
- 6.
In the presence of severe disease or resistance risk factors, empirical treatment must be started until the results of the genotype resistance study are obtained (BIII).
- 7.
Alternative antiviral treatment, in the presence of severe disease and ganciclovir resistance risk factors, will consist of replacing ganciclovir with foscarnet (60mg/kg every 8h or 90mg/kg every 12h). As an alternative, foscarnet can be added to prior treatment with ganciclovir, either at standard or reduced doses (BIII). In patients exhibiting less severe disease and in the absence of risk factors, the dose of ganciclovir may be increased to above the standard dose, up to 10mg/kg every 12h, provided that renal function is maintained within normal limits (BIII).
- 8.
If genotype tests reveal a high-resistance mutation in the UL97 kinase gene (M460V/I, A594V, L595S, C603W, H520Q), ganciclovir must be replaced with foscarnet (60mg/kg every 8h or 90mg/kg every 12h) provided that this has not been done empirically beforehand (BIII). In the case of mutations that confer lower resistance, treatment can continue with ganciclovir at doses of up to 10mg/kg every 12h, provided that renal function is maintained within normal limits (BIII). The scope of the genetic study should be broadened in the case of mutations in the pol UL54 gene. If mutation in this gene is confirmed, treatment must be changed to foscarnet (BIII).
- 9.
The use of cidofovir as an alternative to ganciclovir is not recommended unless the absence of mutations in the pol UL54 gene is confirmed and the disease is not severe from a clinical standpoint (BIII).
There is currently insufficient evidence regarding the role of alternative treatments for CMV disease. A series of drugs have been used as potentially effective agents for treating CMV. These include leflunomide211 and artesunate,212 which have been shown to have anti-CMV effects, probably associated with an alteration in the physiology of host cells that hinder viral replication. However, data justifying the use of these drugs are still scarce. Different studies with sirolimus213 and everolimus have reported a lower incidence of CMV disease,74,76,214 prompting proposals for the use of these drugs to treat patients with ganciclovir-resistant CMV infection. The recent anti-viral drug maribavir is a good alternative against ganciclovir-resistant strains. This drug has good oral bioavailability and does not show hematological, renal or hepatic toxicity. Since it inhibits UL97 (viral kinase), it may interfere in the phosphorylation of ganciclovir, and must therefore not be used in combination with this drug. However, it does not affect the activity of foscarnet or cidofovir and may be administered in combination with these drugs. A phase II trial performed on patients subjected to hematopoietic progenitor transplant demonstrated the efficacy of maribavir in anti-CMV prophylaxis. However, phase II trials carried out to confirm this effect were interrupted after it was determined that the results with maribavir were similar to those obtained with placebo.200 As a result, at the time of publishing this consensus document its clinical development had been suspended. In spite of these results, maribavir has been used in isolated cases as rescue therapy in patients with multi-resistant CMV infection. Although the existence of crossed resistance between maribavir and currently available medication has not been reported,215 resistance with this drug has been described elsewhere.216
In addition to these commercially available drugs, other antiviral medication is currently in the research phase, such as CMX-001, an esterified derivative of cidofovir that shares a similar action mechanism to that of cidofovir, albeit with lower toxicity208; hence, it may be a therapeutic alternative in the future.
Immunologically based therapiesPassive immunotherapy, with administration of specific anti-CMV immunoglobulin,217 has been used in an attempt to improve the immune status of the host against the virus. Similarly, the adoptive transfer of CMV-specific T-cells, either directly from the donor or after their activation and expansion ex vivo, has been evaluated in both the prevention of viral replication and in the treatment of CMV disease, with encouraging results.218,219 Consequently, this therapy is currently being evaluated in clinical trials for both the prevention and treatment of resistant CMV infection.220,221–226 The efficacy of the adoptive transfer of T-lymphocytes is not only influenced not only by the functionality of infused cells, but also by their state of differentiation, proliferative capacity and longevity. Although ‘HPT’ (‘hematopoietic progenitor transplant’) has shown that lower differentiation of transferred cells is associated with greater protection against CMV, data available in this respect in patients undergoing SOT are still scarce.227,228
Prophylactic vaccinationIn recent decades, various vaccines have been developed against CMV. These vaccines have been shown to be safe and immunogenic in phase I preclinical or clinical trial, although none have yet been evaluated in phase II clinical trials.21,229 The attenuated live virus vaccine, the “Towne strain”, has been shown to minimize the severity of CMV disease in CMV-seronegative patients receiving kidney transplants from CMV-seropositive donors, although it does not prevent primary infection.230,231 Prototype vaccines that use replicating recombinant viruses or replicating vectors as immunogens generate B and T responses of variable magnitude,232–239 although data are currently not available on their efficacy for preventing CMV infection. The recombinant subunit vaccine gB, administered with the adjuvant MF59, generates a neutralizing antibody response comparable to that observed after natural infection. It has been shown that this vaccine provides protection against primary infection in 50% of vaccinated subjects.235,236 A bivalent DNA vaccine (VCL-CB01) has been developed recently containing two gB and pp65-encoding plasmids, respectively; its innocuity and immunogenicity have been demonstrated in a phase I clinical trial in both healthy CMV-seropositive subjects and CMV-seronegative patients.
Consensus recommendations- 1.
There is insufficient evidence on the role of adjuvant treatments such as leflunomide, artesunate, sirolimus and everolimus in the treatment of ganciclovir-resistant CMV infection (BIII).
- 2.
Passive immunotherapy with anti-CMV immunoglobulin and the adoptive transfer of T cells could be effective alternatives for treating active infection or CMV disease in organs, although there is insufficient evidence of their role in SOT patients (BIII).
- 3.
The usefulness of maribavir for the management of ganciclovir-resistant CMV is unknown (CIII).
- 4.
The adoptive transfer of T cells in HPT seems to be an effective alternative for treating active CMV infection or disease in organs and refractory to antiviral treatment in HPT. There is no informed experience in SOT.
- 5.
There are no anti-CMV vaccines licensed for clinical use. The priority objective of the use (prophylactic or therapeutic) of a vaccine against CMV in SOT is to minimize the risk of viremia and organ disease. Until new data become available, no recommendations can be made on the type and time of vaccination.
The importance of CMV infection has decreased in children receiving solid-organ transplants, mainly due to the availability of sensitive techniques for the diagnosis of this disease, the development of prevention strategies and the possibility of introducing effective antiviral treatments. However, in some types of transplants, such as lung transplants, CMV infection remains an important risk factor of mortality or re-transplant in D+/R− patients.240 The specific peculiarities of CMV infection in children are explained below, although this panel recommends reading the whole document in order to obtain full information on each section.
CMV disease risk factorsAs in the adult population, the main risk factor for the development of CMV disease post-transplant in children is the absence of specific immunity against the virus in the pre-transplant period. This scenario is more likely in children than in adults, and may occur in up to 60–70% of cases. Furthermore, this risk is greater in the patients receiving organs from seropositive donors (D+/R). Other possible donor-recipient combinations, such as D−/R+ or D+/R+, are considered to be medium risk and, in the case of D−/R−, low risk.241
When interpreting the serostatus of pediatric donors and recipients aged under 18 months, it must be remembered that positive results may indicate the presence of maternal IgG-CMV transferred passively during pregnancy. Therefore, in order not to underestimate the risk for these patients, these shall be considered to be positive in the case of donors and negative in the case of recipients.
Diagnostic methodsCMV infection and disease in children are managed in a similar way to adults. Since the amount of blood obtained by venipuncture in children may be limited, the use of PCR techniques is normally preferred over antigenemia. However, a cut-off value has not yet been established for the start of antiviral treatment.242
Prevention of CMV disease in pediatric patientsNo randomized studies have yet been carried out to compare the efficacy of prophylaxis over pre-emptive treatment in children.243 Most transplant centers normally use universal prophylaxis strategies with ganciclovir at doses of 5mg/kg/day, although some transplant programs start with ganciclovir doses of 5mg/kg/12h during the first two weeks. The use of prolonged 12-week prophylaxis treatments with ganciclovir has also been used safely in some patients.244 Although some studies have shown that valganciclovir is an effective and safe drug, information on its use for prophylaxis in pediatric patients is very scarce.245,246 The high variability of ganciclovir in plasma levels reported in children may justify the need to monitor these levels, especially in high-risk patients.247,248
The duration of prophylaxis has not been clearly defined, although this is normally between 3 and 6 months; prophylaxis tends to be applied for longer periods in high-risk patients, e.g. lung or intestine transplant recipients.244
The role of immunoglobulin used on its own or in combination with antiviral medication has not been defined. Different trials in children receiving liver249,250 or lung251 transplants have not reported any benefits in its use when compared with antiviral drugs.
Treatment of CMV disease in pediatric patientsLimited evidence is currently available to make firm recommendations on the treatment of CMV disease in children.200 In these patients, the proposed treatment is intravenous ganciclovir at a dose of 5mg/kg/12h until negative PCR or antigenemia results are achieved, the duration of treatment being equivalent to that in adults in cases of both viral syndrome and organ disease. The strategy of initial treatment followed by secondary prophylaxis is recommended by some experts.252 Since it has not been validated by studies with pediatric patients, no recommendations can be made for this population group regarding the use of valganciclovir to treat CMV disease. In patients with CMV-induced pneumonitis and enteritis, as well as in cases of hypogammaglobulinemia, the use of specific anti-CMV immunoglobulin is recommended.
The onset of ganciclovir-resistant CMV disease has been described anecdotally.253 As in adults, different agents are available for treatment of this condition, including foscarnet and cidofovir,200 although their use is limited due to their potential nephrotoxicity. Other agents such as maribavir, leflunomide or artesunate are currently being researched.
Consensus recommendations- 1.
Due to the difficulty in characterizing the serostatus of donors and recipients aged under 18 months, it must be assumed that children in this age group present a greater risk. Thus, if serological tests for CMV in donors aged under 18 months are positive, these patients must be treated as seropositive, even if these antibodies originate from the mother. Similarly, seropositive recipients included in this age group will be treated as seronegative since anti-CMV antibodies may be of maternal origin (AIII).
- 2.
Both PCR and pp65 antigenemia techniques have been shown to be effective for diagnosing and monitoring CMV infection in children (AII).
- 3.
Prophylaxis is recommended rather than early treatment (AIII). Most experts recommend the use of intravenous ganciclovir (5mg/kg/12h) for between 2 and 4 weeks, followed by valganciclovir (dose=7×body surface area in m2×creatinine clearance) until the end of 3–6 months’ prophylaxis (AII). Creatinine clearance is recommended using the modified Schwartz formula [k×height (cm)/serum creatinine (mg/dl)], where k is 0.33 for newborns, 0.45 for children aged between 4 months and 2 years, 0.55 for male patients aged between 2 and 13 and female patients aged between 2 and 16, and 0.7 for male patients aged between 13 and 16. The maximum creatinine clearance value applicable to this formula is 150ml/min/1.73m2; hence, even if the clearance value exceeds this level, the aforementioned maximum value will be used to calculate the dose.
- 4.
In the treatment of CMV disease, ganciclovir is recommended (5mg/kg/12h) until a negative PCR or pp65 antigenemia result is obtained on a weekly basis (AII). The total duration of treatment in both viral syndrome and organ disease will be the same as that established for adults. Anti-CMV immunoglobulin is recommended in cases of pneumonitis or enteritis, as well as in patients with hypogammaglobulinemia (BIII). The efficacy of valganciclovir has not been established in this population. Some experts consider that the treatment period can be completed by replacing intravenous ganciclovir with oral treatment in some older children and adolescents (BIII).
Dr. J. Torre-Cisneros has received fees for speaking or consulting, as well as research grants from Roche Farma S.A.; Dr. M.C. Fariñas declares no conflict of interest. Dr. J.J. Castón declares no conflict of interest; Dr. J.M. Aguado has received fees from Roche Farma S.A. as a speaker and for educational activities; Dr. S. Cantisan has received an unrestricted research grant from Roche Farma S.A.; Dr. J. Carratalá has received payment for speaking from Roche, Pfizer, Astellas, Gilead, Cephalon, Schering-Plough, Astra-Zeneca, Merck and Novartis; Dr. C. Cervera declares no conflict of interest; Dr. J.M. Cisneros declares no conflict of interest; Dr. E. Cordero declares no conflict of interest; Dr. M.G. Crespo-Leiro has received a fee for speaking from Roche Farma for educational activities; Dr. J. Fortún has received fees for speaking and reimbursement of expenses for attending a symposium from Roche Farma S.A.; Dr. E. Frauca declares no conflict of interest; Dr. J. Gavaldá declares no conflict of interest; Dr. S. Gil-Vernet declares no conflict of interest; Dr. M. Gurguí declares no conflict of interest; Dr. O. Len declares no conflict of interest; Dr. C. Lumbreras declares no conflict of interest; Dr. M.A. Marcos declares no conflict of interest; Dr. P. Martín-Dávila declares no conflict of interest; Dr. V. Monforte has received fees for speaking and educational activities and funds for research; Dr. M. Montejo declares no conflict of interest; Dr. A. Moreno declares no conflict of interest; Dra. P. Muñoz declares no conflict of interest; Dr. D. Navarro has received a fee for speaking; Dr. A. Pahissa declares no conflict of interest; Dr. J.L. Pérez declares no conflict of interest; Dr. A. Rodriguez-Bernot declares no conflict of interest; Dr. J. Rumbao declares no conflict of interest; Dr. R. San Juan declares no conflict of interest; Dr. F. Santos declares no conflict of interest; Dr. E. Varo declares no conflict of interest; Dr. F. Zurbano declares no conflict of interest.
The Consensus Conference was organized by the Spanish Transplantation Infection Study Group (Grupo de Estudio de la Infección en el Transplante – GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clinica – SEIMC). It was supported by the Spanish Ministry of Science and Innovation, the Carlos III Health Institute – co-financed by ERDF (European Regional Development Fund) “A way to achieve Europe”, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008). GESITRA has received an unrestricted grant from Roche Farma S.A.
Thanks to Mayte Aldudo for administrative support.