To look for evidence of hepatitis E virus (HEV) exposure in HIV-infected patients with unexplained elevations of liver stiffness (LS).
MethodsCase–control study conducted in 31 HIV-infected patients with unexplained elevations of LS and in 31 HIV-controls with normal LS, matched by age, sex and CD4 cell-counts. Serum HEV antibodies were tested by two ELISA procedures and by Immunoblot. We defined exposure to HEV as the detection of serum HEV antibodies by at least one of the two ELISA assays, provided that it was confirmed by Immunoblot. A real-time PCR RNA assay was conducted in all plasma samples to identify subjects with active HEV infection.
ResultsExposure to HEV was demonstrated, according to the criteria used in this study, in 9 (29%) of the cases, whereas it was shown in 5 (16%) of the controls (p=.3). Serum HEV RNA was detected in none of the controls and in only in one case. This patient had a documented chronic hepatitis E with progression to cirrhosis.
ConclusionsHEV antibodies are frequently found in HIV-infected patients with unexplained liver disease.
Evaluar la existencia de exposición previa al virus de la hepatitis E (VHE) en pacientes infectados por el VIH con elevaciones inexplicadas de rigidez hepatica (RH).
MétodosEstudio caso-control realizado en 31 pacientes con infección por el VIH y elevaciones inexplicadas de RH y 31 controles infectados por el VIH con RH normal, apareados por edad, sexo y recuento de células CD4. Se investigó la presencia de anticuerpos en suero frente al VHE mediante dos técnicas de ELISA y por Inmunoblot. La exposición previa al VHE se definió como la detección de anticuerpos séricos mediante al menos una de las dos técnicas de ELISA que se confirmó posteriormente mediante Inmunoblot. En todos los pacientes se realizó una PCR en tiempo real para identificar a aquellos pacientes con infección activa por el VHE.
ResultadosSe demostró la presencia de exposición previa al VHE, de acuerdo a los criterios usados en el estudio, en 9 (29%) de los casos y en 5 (16%) de los controles (p=0.3). La PCR en tiempo real confirmó la presencia de RNA del VHE en el suero de uno de los casos y en ninguno de los controles. Este paciente presentó una hepatitis crónica por VHE documentada con progresión a cirrosis.
ConclusionesLos pacientes infectados por VIH con enfermedad hepática de origen inexplicado presentan una frecuencia elevada de anticuerpos frente al VHE.
Autochthonous hepatitis E virus (HEV) is an emerging infection in HIV patients in developed countries.1–4 Epidemiological data from Spain have suggested that HIV may be an independent risk factor for autochthonous HEV.5,6 In a retrospective study, HEV infection accounted for 4% acute liver abnormalities in HIV-infected individuals.7 Besides HEV-related unexplained acute elevations of liver enzymes, chronic HEV infection with rapid progression to cirrhosis has been reported in HIV-infected patients.8–10
Unexplained elevations of liver stiffness (LS) were found in 11% of the HIV-infected patients without hepatitis B virus (HBV) or hepatitis C virus (HCV) co-infection from our cohort.11 Liver histology proved several sort of liver damage virtually in all patients with unexplained elevations of LS.11 In those with normal values of LS, sequential examinations revealed that 7% of them developed persistent elevations of LS, which were mainly attributed to fatty liver disease.12 However, previous HEV infection was not routinely investigated in these patients. In fact, it has been hypothesized that subclinical hepatic steatosis or fibrosis could be a host risk factor for clinical disease expression in patients exposed to HEV.13
Our objective was to look for evidence of HEV exposure in HIV-infected patients with unexplained elevations of LS.
MethodsStudy design and patientsThis was a case-control study conducted in two hospitals from Southern Spain. We selected as cases all patients fulfilling criteria of abnormal LS of uncertain origin as previously described among those who attended both institutions.11,12 Briefly, we selected HIV-infected patients with a LS≥7.2kiloPascals (kPa) in two consecutive visits, without previous exposure to HBV or HCV as determined by a negative hepatitis B surface antigen, negative HCV antibodies and a negative serum DNA HBV and RNA HCV PCR assessment, and without evidence of other causes of liver disease. Forty-four patients fulfilled these criteria before 30th June 2011. Of them, 31 (70%) had an available frozen serum sample collected at the date of LS assessment and were included as cases in the study. These cases were paired with HIV-infected patients, without evidence of active HCV or HBV coinfection, selected from the same cohort with a LS<7.2kPa who were matched by age, sex, CD4 cell count and study center.
Laboratory proceduresAll plasma samples were tested for the presence of HEV-specific IgG antibodies using both the Wantai HEV-IgG ELISA kit (Beijing Wantai Biological Pharmacy Enterprise CO., LTD., Beijing, China) and the recomWell HEV IgG ELISA (Mikrogen GmbH, Neuried, Germany). Confirmatory testing was performed using the recomLine HEV IgM/IgG immunoassay (Mikrogen GmbH, Neuried, Germany). Samples were analyzed according to the manufacturer's instructions. We defined exposure to HEV as the detection of serum HEV antibodies by at least one of the two ELISA assays, provided that it was confirmed by Immunoblot. A real-time PCR RNA assay was conducted in all plasma samples to identify subjects with active HEV infection.
Statistical analysisContinuous variables are expressed as median (Q1–Q3). Categorical variables are presented as numbers (percentage). Continuous variables were compared using the Wilcoxon test whereas the frequencies were compared by the McNemar test. Data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Ethical aspectsThis study has been designed and performed according to the Helsinki declaration and was approved by the Ethics Committee of Hospital Universitario de Valme.
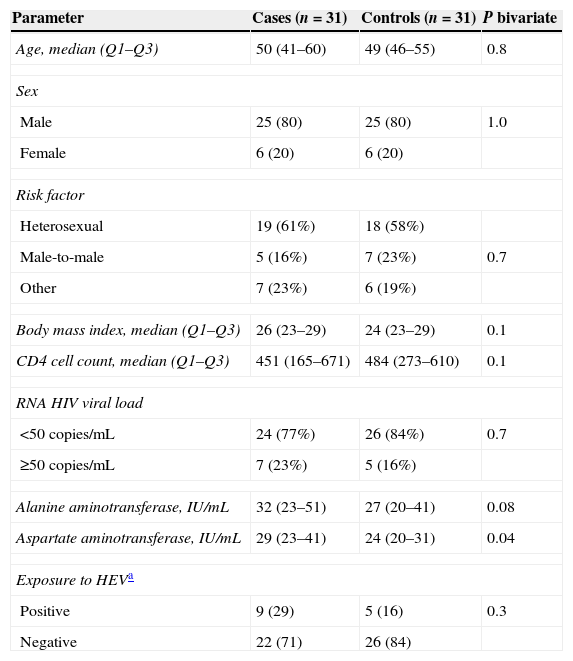
ResultsThe main features of the study population are depicted in Table 1. Among cases, 8 (26%) patients showed a LS≥14.6kPa and 9 (29%) had a LS≥9kPa but <14.6kPa. Twelve (39%) cases had elevated levels of liver enzymes whereas this was observed in 7 (22%) controls (p=0.3).
Features of the study population.
| Parameter | Cases (n=31) | Controls (n=31) | P bivariate |
|---|---|---|---|
| Age, median (Q1–Q3) | 50 (41–60) | 49 (46–55) | 0.8 |
| Sex | |||
| Male | 25 (80) | 25 (80) | 1.0 |
| Female | 6 (20) | 6 (20) | |
| Risk factor | |||
| Heterosexual | 19 (61%) | 18 (58%) | |
| Male-to-male | 5 (16%) | 7 (23%) | 0.7 |
| Other | 7 (23%) | 6 (19%) | |
| Body mass index, median (Q1–Q3) | 26 (23–29) | 24 (23–29) | 0.1 |
| CD4 cell count, median (Q1–Q3) | 451 (165–671) | 484 (273–610) | 0.1 |
| RNA HIV viral load | |||
| <50copies/mL | 24 (77%) | 26 (84%) | 0.7 |
| ≥50copies/mL | 7 (23%) | 5 (16%) | |
| Alanine aminotransferase, IU/mL | 32 (23–51) | 27 (20–41) | 0.08 |
| Aspartate aminotransferase, IU/mL | 29 (23–41) | 24 (20–31) | 0.04 |
| Exposure to HEVa | |||
| Positive | 9 (29) | 5 (16) | 0.3 |
| Negative | 22 (71) | 26 (84) | |
Exposure to HEV was demonstrated, according to the criteria used in this study, in 9 (29%) of the cases whereas it was shown in 5 (16%) of the controls (p=0.3) (Table 1). Serum HEV RNA was detected only in one case and in no control. This patient had a documented chronic hepatitis E with progression to cirrhosis.
DiscussionOur study suggests that previous exposure to HEV is higher among HIV-infected patients with unexplained elevations of LS than in the HIV population with normal values of LS. This finding supports the hypothesis that HEV infection may underlie some of the unexplained cases of liver disease in HIV-infected patients and may contribute to liver injury in this setting. Although no statistically significant differences have been observed in this study, this fact may have been a consequence of the limited power of the analyzed sample. However, the prevalence of HEV exposure was almost double in cases in comparison with controls. This datum suggests that significant differences might have been reached if a larger sample had been analyzed and warrants further studies to confirm this finding.
The seroprevalence of anti-HEV found in our HIV population with normal values of liver LS was high, namely 16% according to the criteria used in this study. This figure is higher than the seroprevalence reported in other HIV-cohorts, which have ranged from 1.5% to 11.2%5,14–21 and is also greater than the seroprevalence in the general population in Spain, which ranges between 0.6 and 7.3%.22 Additionally, the anti-HEV seroprevalence found in our study is slightly higher than the 14% seroprevalence reported in a recent study conducted in 448 HIV-infected individuals in Spain.6 Although variability in the strategy and procedures for serological testing could partly explain these differences, it is well documented that seroprevalence varies between countries and within countries,23 which may have accounted for these differences in a great extent. Further investigations should clarify if the high anti-HEV seroprevalence in HIV-infected patients with unexplained liver disease found in our study reflects a higher risk for HEV infection in this population or if, alternatively, HEV infection is a potential contributor to liver disease in this setting.
Our study has some limitations. First, as stated above, is the lack of power to find significant differences. However, identifying HIV-infected patients without hepatitis coinfection bearing unexplained liver disease is not easy, because reliable screening test as transient elastography is not routinely carried out in such a population. Because of this, we believe that these results are of interest, even without statistically significant difference, as they may prompt specific multicentric studies with larger sample sizes to be undertaken. The relevance that HEV may have as cause of unexplained liver disease in the HIV infection clearly justify these studies. Second, the serological diagnosis of HEV is not well standardized and lacks of a current widely accepted definition for HEV exposure. However, we have used several assays for the detection of anti-HEV antibodies and stringent criteria for minimizing variability in procedure results, including a confirmatory immunoblot test, also used by other experts.24 Additionally, the persistence of anti-HEV antibodies in the mid- and long-term is not completely known. Consequently, this and all previously published cross-sectional studies looking at the prevalence of anti-HEV antibodies may underestimate previous exposure to HEV due to loss of antibodies over time. Finally, although cases and controls were matched by relevant factors such as age, sex and CD4 count, the small sample size precluded us to include other matching factors as alcohol consumption or exposure to certain antiretroviral drugs as didanosine.
In summary, although this study should be considered preliminary, according to its results, previous HEV infection seems to be more frequent among HIV-infected patients with unexplained liver disease and it may be involved in the development of the latter disorder. On the contrary, active chronic hepatitis E is rare in this setting, but rapid progression to cirrhosis is a concern. Chronic hepatitis E should be considered in any HIV-infected patients with unexplained abnormal liver function tests, including abnormal LS, as effective treatment of HEV may induce reversal of liver injury.10
Conflict of interestNone.
FundingThis work was partly supported by the Red de Investigación en SIDA (ISCIII-RETIC RD06/006 and ISCIII-RETIC RD12/0017) and the Fundación Progreso y Salud de la Junta de Andalucía (grants references: PI: 0208/2009 and 0036/2010). JM is the recipient of a grant from the Servicio Andaluz de Salud de la Junta de Andalucía (B-0037). JAP is the recipient of an intensification grant from the Instituto de Salud Carlos III (grant number Programa-I3SNS). The Unit of Infectious Diseases and Microbiology, Hospital Universitario de Valme (Seville, Spain) has received human resources research support from Servicio Andaluz de Salud de la Junta de Andalucía (Reference B-0037).