Chlamydia trachomatis is considered a public health problem due to the high prevalence in sexually active women and men. The distribution of genital Chlamydia genotypes among Mexican men is unknown.
ObjectiveTo assess the prevalence of Chlamydia genotypes in men with infertile women as sexual partners.
MethodsA total of 659 urine samples were collected from men whose sexual partners were infertile women; the identifying Chlamydia infection was by means of a real-time nucleic acid amplification test (qPCR). OmpA gene PCR-RFLP and sequencing were used to confirm the genotypes of C. trachomatis. The association of genotypes with age, spermatic parameters and gynecological data of sexual partners was further analyzed.
ResultsForty-nine urine samples were positive infection (7.4%). The Chlamydia infection was significantly associated with teratozoospermia, azoospermia, hypospermia, and oligozoospermia. Five genotypes (F 51%; 12.2% to D; 12.2% to E; 6.1% to L2 and 4.1% Ia) were correctly identified. None genotypes identified in this comparative study were positively associated with changes in some of the spermatic values because all of them typically produce some considerable damage to these cells.
ConclusionsThe F genotype was the most frequent genotype identified in infertile men from Mexico City and all genotypes play an important role in the seminal alteration of Mexican men whose female partners are infertile.
Chlamydia trachomatis se considera un problema de salud pública debido a la alta prevalencia en mujeres y hombres sexualmente activos. Se desconoce la distribución de los genotipos genitales de Chlamydia entre los hombres mexicanos.
ObjetivoEvaluar la prevalencia de los genotipos de Chlamydia en hombres con mujeres infértiles como parejas sexuales.
MétodosSe recogieron 659 muestras de orina de hombres cuyas parejas sexuales eran mujeres infértiles; la identificación de la infección por Chlamydia se realizó mediante una prueba de amplificación de ácido nucleico en tiempo real (qPCR). Se utilizaron la PCR-RFLP y la secuenciación del gen OmpA para confirmar los genotipos de C. trachomatis. Se analizó en mayor profundidad la asociación de los genotipos con la edad, los parámetros espermáticos y los datos ginecológicos de las parejas sexuales.
ResultadosCuarenta y nueve muestras de orina dieron positivo para la infección (7,4 %). La infección por Chlamydia se asoció significativamente con la teratozoospermia, la azoospermia, la hipospermia y la oligozoospermia. Se identificaron correctamente cinco genotipos (F 51 %; 12,2 % para D; 12,2 % para E; 6,1 % para L2 y 4,1 % Ia). Ninguno de los genotipos identificados en este estudio comparativo se asoció positivamente con cambios en algunos de los valores espermáticos porque todos ellos suelen producir algún daño considerable en estas células.
ConclusionesEl genotipo F fue el más frecuente identificado en hombres infértiles de Ciudad de México y todos los genotipos desempeñan un papel importante en la alteración seminal de los hombres mexicanos cuyas parejas femeninas son infértiles.
Chlamydia trachomatis (CT) is a common sexually transmitted infections, considered a public health problem due to the high frequency of active infection in women and men and its consequences on reproductive health. Currently, around 131 million people are infected by CT worldwide each year.1 CT produces several sequels to the infection including urethritis, mucopurulent cervicitis, and endometritis.1 In this way, CT typically produces pelvic inflammatory diseases (PID), infertility (due to tubal occlusion), chronic pelvic pain in women, while in men CT infection causes non-gonococcal urethritis, epididymitis, and orchitis.1,2 In Mexico, the infection prevalence among women is reported to be 4.3%–19%,3,4 while in men it is 3.6%–31.9%.5,6
Currently, 19 serovars of CT are universally recognized, all with well-established profiles.7 These serovars can be identified by means of the specific serologic reaction to the major outer membrane protein (MOMP). This key protein is coded by the ompA gene.7–10 In the past two decades, several developed countries throughout the modern world have carefully carried out researcher regarding specific genotypes of CT that typically affect their local community.4,7–10 In 2011 in Mexico City, eight genotypes of CT were reported to cause active infection in infertile women.9 The most frequent genotype was genotype F.9 In 2018, in Jalisco State, Casillas-Vega et al.4 identified eight genotypes of CT. The E, F and D genotypes were detected the most.4 On the other hand, in Mexico, CT genotypes causing male infections are completely unknown. Our aim was to identify the apparent frequency of CT genotypes in males whose sexual partners were infertile female patients in the Mexican Perinatology Institute.
Material and methodsPatientsMale participants from January to December 2018, whose sexual partners had attended the National Institute of Perinatology from Mexico City due to infertility were recruited for this study. The gynecological data of women and spermatic parameters of men were obtained from their medical records. This study was approved by the ethics committee of the National Institute of Perinatology with ID number 2017-0-112.
Specimen collection and testingUrine samples were obtained from each participant in order to perform the direct detection of CT. The detection of this pathogen was performed using the real-time nucleic acid amplification test on the Abbott Real-time CT/NG assay (Catalog number, Abbot Molecular Inc., Des Plaines, IL, USA). The amplification and automatic detection were carried out on the Abbott m2000 System. All specimens were tested giving to the manufacturer's instructions.
Amplification of the ompA specific geneThe DNA of positive samples to CT were recovered and properly processed to amplify the ompA specific gene by means of the nested PCR assay. The primers described by Yang et al.11 were used. The first 1142bp fragment was recovered and used to obtain the second fragment of 879bp. This second fragment was used to identify the CT genotype by mean of RFLP assay. The PCR assays were realized in a PT100 thermal cycler (MJ Research, USA). All primers and conditions of PCR assays were described previously.9,11
RFLP analysisThe fragment of 879bp was submitted to restriction endonuclease AluI digestion (Thermo Scientific™) for 10h at 37°C; 20μl of reaction mixture contained 2U of AluI, 8μl of the purified fragment, and 2μl restriction endonuclease buffer were used according to provider instruction. Restriction profiles were analyzed by 12% polyacrylamide gel electrophoresis at 80v/cm for 2h, in alignment with previously published work.9,11
Genomic analysisThe purified ompA gene was analyzed in the Biology Institute of the National University of Mexico by sequencing with the ABI PRISM sequencer (Applied Biosystems). The obtained nucleotide sequences of ompA amplicon were carefully analyzed with the Chromas software, identified by Blast (NCBI) and aligned with ClustalW and Multalin (France) programs.
Statistical analysesThe analyses were performed using IBM SPSS Statistics for Windows, Version 24.0 (IBM Corp., Armonk, NY, USA). Fisher's test was performed to search for a significant association between the categorical variables (Type of infertility, Teratozoospermia, Azoospermia, Asthenozoospermia, Fallopian tube occlusion, etc.) and Chlamydia infection. A p-value of less than 0.05 was considered statistically significant.
ResultsSix hundred fifty-nine urine samples were analyzed for identifying C. trachomatis DNA. Forty-nine samples (7.4%) were positive for CT. The average age of participants with a positive sample to Chlamydia infection was 32.6 years old, while the age range of study patients was 20–67 years. Table 1 shows the seminal values and gynecological data of the sexual couple. The age with a notable prevalence of Chlamydia infection under 26 years old (p<0.03). A significant association between Chlamydia infection, morphology, and the number of spermatozoa (p<0.002) as well as in the volume of semen (p<0.005) was observed. In addition, of the twelve patients with azoospermia, six of them showed CT DNA [95% CI: 7.52 (3.99–14.2); p<0.001].
Spermatobioscopy parameters from men with Chlamydia trachomatis infection and the gynecological data relationship from their sexual partners.
| Clinical data | n | Chlamydia trachomatis | RR (95% CI) | p< | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Age of the male | |||||
| 20–25 | 50 | 42 | 8 | 2.38 (1.18–4.79) | 0.03 |
| 26–30 | 112 | 100 | 12 | 1.58 (0.85–2.94) | NS |
| 31–35 | 241 | 227 | 14 | 0.70 (0.38–1.26) | NS |
| 36–40 | 158 | 148 | 10 | 0.81 (0.42–1.59) | NS |
| >40 | 98 | 93 | 5 | 0.65 (0.27–1.60) | NS |
| Teratozoospermia | |||||
| Yes | 270 | 226 | 44 | 12.68 (5.09–31.6) | 0.002 |
| No | 389 | 384 | 5 | ||
| Asthenozoospermia | |||||
| Yes | 49 | 44 | 5 | 1.42 (0.59–3.40) | NS |
| No | 610 | 566 | 44 | ||
| Azoospermia | |||||
| Yes | 12 | 6 | 6 | 7.52 (3.99–14.20) | 0.002 |
| No | 647 | 604 | 43 | ||
| Hypospermia | |||||
| Yes | 19 | 13 | 6 | 4.70 (2.28–9.68) | 0.005 |
| No | 640 | 597 | 43 | ||
| Oligozoospermia | |||||
| Yes | 45 | 35 | 10 | 3.50 (1.87–6.54) | 0.002 |
| No | 614 | 575 | 39 | ||
| Age of the female | |||||
| 20–25 | 44 | 39 | 5 | 1.59 (0.66–3.80) | NS |
| 26–30 | 178 | 162 | 16 | 1.31 (0.74–2.32) | NS |
| 31–35 | 289 | 267 | 22 | 1.04 (0.61–1.79) | NS |
| 36–40 | 133 | 127 | 6 | 0.55 (0.24–1.27) | NS |
| Over 40 | 15 | 15 | 0 | ||
| Type of infertility | |||||
| Primary | 434 | 398 | 36 | 1.44 (0.78–2.65) | NS |
| Secondary | 225 | 212 | 13 | ||
| Ectopic pregnancies | |||||
| Yes | 39 | 38 | 1 | 0.33 (0.05–2.34) | NS |
| No | 620 | 572 | 48 | ||
| Abortion | |||||
| Yes | 138 | 129 | 9 | 0.85 (0.42–1.71) | NS |
| No | 521 | 481 | 40 | ||
| Male infertility | |||||
| Yes | 287 | 244 | 43 | 9.29 (4.01–21.5) | 0.001 |
| No | 372 | 366 | 6 | ||
| Fallopian tube obstruction | |||||
| Yes | 173 | 160 | 13 | 1.01 (0.55–1.87) | NS |
| No | 486 | 450 | 36 | ||
| Endocrine-ovarian factor | |||||
| Yes | 377 | 332 | 45 | 8.42 (3.06–23.13) | 0.001 |
| No | 282 | 278 | 4 | ||
| Chlamydia infection of the women | |||||
| Yes | 11 | 0 | 11 | 17.1 (12.5–23.2) | 0.001 |
| No | 648 | 610 | 38 | ||
RR=relative risk; NS=no significant.
On the other hand, there were no significant differences in the type of infertility, Fallopian tube obstruction presence, and ectopic pregnancy and abortion history data in the sexual partners of Chlamydia-positive men. Masculine infertility was observed in 87.8% of women whose sexual partners showed Chlamydia infection (Table 1) and 90.5% of these women showed a significant association with endocrine disorders [95% CI: 8.42 (3.06–23.13), p<0.001]. The Chlamydia infection concordance between couples was 22.4% with a significant relative risk of 17. 1 (95% CI=12.5–23.2; p<0.001).
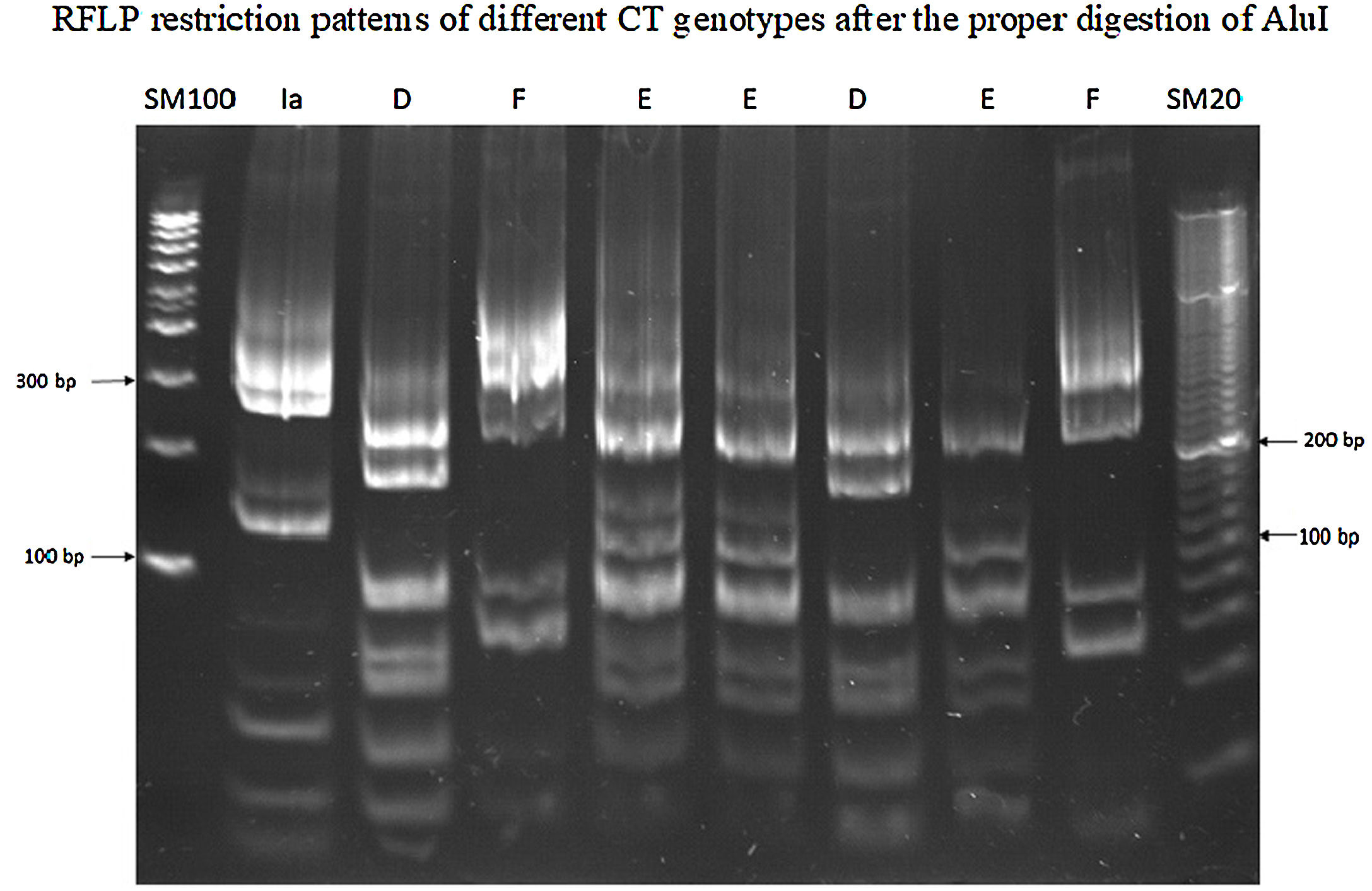
Genotypes of Chlamydia trachomatisOnly 43 (87.8%) from 49 CT-positive samples contained the ompA specific gene. Five genotypes of CT were correctly identified, 25 (51%) for F; 6 (12.2%) for D; six (12.2%) for E; three (6.1%) for L2, and two (4.1%) for Ia (Fig. 1). Table 2 shows the association between CT genotypes and seminal parameters, as well as, gynecological data of their sexual partners. No association was observed between the various abnormal sperm parameters and the identified Chlamydia genotype. In a similar manner, we did not observe a significate association between Chlamydia genotypes and Fallopian tube obstruction and endocrine disorders of sexual partners.
Identification of Chlamydia trachomatis genotypes in Mexican men whose couples female are infertile.
| Clinical data | N | Chlamydia trachomatis | RR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | E | F | I | L2 | ||||||||
| − | + | − | + | − | + | − | + | − | + | |||
| Teratozoospermia | ||||||||||||
| Yes | 38 | 33 | 5 | 32 | 6 | 16 | 22 | 37 | 1 | 35 | 3 | NS |
| No | 5 | 4 | 1 | 5 | 0 | 2 | 3 | 4 | 1 | 5 | 0 | |
| Asthenozoospermia | ||||||||||||
| Yes | 4 | 2 | 2 | 3 | 1 | 3 | 1 | 4 | 0 | 4 | 0 | NS |
| No | 39 | 35 | 4 | 34 | 5 | 15 | 24 | 37 | 2 | 36 | 3 | |
| Azoospermia | ||||||||||||
| Yes | 5 | 5 | 0 | 4 | 1 | 1 | 4 | 4 | 1 | 5 | 0 | NS |
| No | 38 | 32 | 6 | 33 | 5 | 17 | 21 | 37 | 1 | 35 | 3 | |
| Hypospermia | ||||||||||||
| Yes | 5 | 5 | 0 | 4 | 1 | 1 | 4 | 5 | 0 | 5 | 0 | NS |
| No | 38 | 32 | 6 | 33 | 5 | 17 | 21 | 36 | 2 | 33 | 5 | |
| Oligozoospermia | ||||||||||||
| Yes | 10 | 9 | 1 | 7 | 3 | 5 | 5 | 10 | 0 | 9 | 1 | NS |
| No | 33 | 28 | 5 | 30 | 3 | 13 | 20 | 31 | 2 | 31 | 2 | |
| Age of the male | ||||||||||||
| <25 | 6 | 6 | 0 | 4 | 2 | 3 | 3 | 5 | 1 | 6 | 0 | NS |
| >25 | 37 | 31 | 6 | 33 | 4 | 15 | 22 | 36 | 1 | 34 | 3 | |
| Male infertility | ||||||||||||
| Yes | 37 | 34 | 3 | 31 | 6 | 14 | 23 | 36 | 1 | 34 | 3 | NS |
| No | 6 | 3 | 3* | 6 | 0 | 4 | 2 | 5 | 1 | 6 | 0 | |
| FTO | ||||||||||||
| Yes | 7 | 7 | 0 | 5 | 2 | 3 | 4 | 7 | 0 | 7 | 0 | NS |
| No | 36 | 30 | 6 | 32 | 4 | 15 | 21 | 34 | 2 | 33 | 3 | |
| EOF | ||||||||||||
| Yes | 39 | 33 | 6 | 35 | 4 | 16 | 23 | 37 | 2 | 36 | 3 | NS |
| No | 4 | 4 | 0 | 2 | 2 | 2 | 2 | 4 | 0 | 4 | 0 | |
RR=relative risk; FTO=Fallopian tube obstruction; EOF=endocrine-ovaric factor; NS=no significant.
There are many reasons why a couple may have difficulty conceiving a child. Overall, one-third of infertility cases are caused by male reproductive issues, one-third by female reproductive issues, and one-third by both couple's issues or by unknown factors.12 For men, common causes include problems with their semen, sperm, or the testicles.12 Sexually transmitted infections (STI) are the most frequent causes of male infertility because it impairs semen.2,12C. trachomatis is the most common bacterial STI worldwide.1,2
In Mexico, there are few studies about the prevalence of Chlamydia infection in men and the few data about these are reported in men whose sexual partners are infertile.5,6 Therefore, the prevalence reported in these men is from 3.6 to 31.9%.5,6 However, in the general population, the chlamydia screening average percentage is low. A systematic review of 25 studies obtained from 2000 to 2013 by Deliesee et al.,13 indicated that the prevalence of CT infection in men is from 0.1% to 12.1%, and in women of 1.1 to 10.6%. In this study, the prevalence was less than 7.5%, which is within the range reported by Delilesee et al.,13 and by those described by our research group.5,6
The most important sequelae produced by CT in men are non-gonococcal urethritis and epididymitis.14 The seminal values in many cases are not altered, so there has been a controversy that CT causes male infertility.5,6,14 However, in this study, some seminal alterations were observed on such as morphology (6.7%), sperm number (1.5%), and seminal volume (0.9%), as well as azoospermia in 50% of these patients. These results are very similar to those recently reported by our research group that demonstrated that concordant couples showed an association between CT infection and fallopian tubal obstruction presence, suggesting an alteration in the quality of semen, particularly in men whose sexual partners present a tubal pathology.6 In this study we observed a concordance of 22.4%, similar to that obtained by López et al.6
The alterations in terms of sperm number and morphology that we observed in the present study may be related to infection of the seminiferous tubules and Sertoli cells, as was recently demonstrated by Bryan et al.15 who verified the presence of CT in testicular biopsies of men with severe and moderate infertility, and by the results of Filardo et al.16 who demonstrated the susceptibility of human Sertoli cells to experimental infection with this pathogen. The low seminal volume could be attributed to an inflammatory process of the vas deferens or to the infection of the accessory glands.17
In cases of azoospermia, could be to apparent lack of sperm production by the testes (secretory azoospermia by FSH deficiency) or by a possible obstruction of the deferent ducts (excretory or obstructive azoospermia). In this study, 6 of 12 azoospermia patients showed Chlamydia infections only two showed secretory azoospermia (data no-showed), by which we no can conclude that C. trachomatis provoke this pathology.
In Mexico, the most frequent genotypes of CT that cause infection in men are unknown. In this study, five different genotypes (F, D, E, Ia, and L2) were identified in males. Worldwide, the most prevalent genotypes are E, D, and F, according to the order of importance.7–10 The few studies of CT genotypes in Mexico have been carried out on women.4,9 In the State of Jalisco, genotype E (39.6%) was reported as one of the most frequent genotypes associated with the presence of green vaginal discharge.4 Genotype F (29.2%) was associated with pelvic inflammatory disease, and genotype D (15.6%) did not show an association with any clinical manifestation.4 However, in Mexico City, the most important genotype in women was F (54.2%), followed by genotypes E, G, K and L2 (8.7% each); and finally genotypes D and I, which were detected with a frequency of 4.2% each.9 It should be noted that in this study, genotype F (49%) was the most identified Chlamydia strain followed by genotypes D, E, L2, and Ia.9
An interesting observation was that the sexual partners (45/49) of men infected with CT showed a significant association with infertility due to ovarian endocrine disorders (p<0.001). The treatment with steroid hormones, estrogen, and/or progesterone, can influence the progression of several STIs including Chlamydia infection.18,19 Some publications inform that estrogen enhances CT entry to genital epithelial cells by means of cell membrane-associated estrogen receptors.19,20
Referring to male infertility, we observed a significantly associated especially with Chlamydia infection (p<0.001), the genotype F was the more identified. In 2006, Gomes et al.21 hypothesized that the most prevalent serovars would have the highest infectious loads because of their worldwide ecological success. However, the one exception to those Gomes's results was genotype F where men had a lower chlamydial load than women if this pathogen was the infecting genotype. Despite the above, in this study, the F genotype was merely the most common in the male population that was carefully analyzed. The seminal parameters affected by this genotype were morphology, sperm number and seminal volume, and azoospermia. However, none CT genotypes identified in this study typically produced fundamental changes in spermatic parameters.
Hosseinzadeh et al.22 demonstrated that the F genotype was more common in individuals with a low seminal volume, while the genotype E-infected individuals whose showed a low percentage in the morphology and mobility of spermatozoa, or genotype D-infected individuals whose sperm number was low. However, in this comparative study, all Chlamydia genotypes produce changes in spermatic values, and these are positively unassociated with a specific defect or alteration particular to these cells.
Another study as carried out by van Duynhoven et al.23 reported that E, F, and D genotypes were more frequent (men: 71%; women: 60%) in patients with sexually transmitted infections were. The most frequent complaints in these men were urethral discharge and dysuria, which were more frequently associated with genotypes H and J. While in women, it was lower abdominal pain that was often associated with genotypes F and G. In this study, urethral discharge, dysuria, or lower abdominal pain were not reported by patients. The differences between studies may be due to the fact that the male population that was analyzed is different.
About rare Chlamydia LGV infection correctly identified in this study is extremely interesting because the male patient not accompanied by clinical signs or typical symptoms of LGV. Several publications have accurately reported about these Chlamydia genovariants.9,24,25 At the moment we do not know if this genotype will develop LGV or if to have some DNA mutations that prevent the development of LGV. Currently, there is not extensive research about genome sequences of these strains to know what virulence factors lack. Another distinct possibility is the good immune response of the male patient, where it can precisely handle this specific pathogen due to the inability of the responsible bacterium to typically developing infectious LGV disease. However, more studies are necessary to correctly identify the molecular characteristics of the DNA of this LGV genotype.
Finally, we conclude that in this study, the F genotype is the most frequent genotype associated whose couples female are infertile.
In addition, one of the weaknesses of this study is the small number of positive samples to C. trachomatis, so it is necessary to increase the number to show if there is an association between genotypes and sperm values of sexual partners of infertile women.
Ethical approvalThe project related to this manuscript was approved by the Ethical Committee of the National lnstitute of Perinatology.
FundingThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interestAll authors declare no conflicts of interest.