Introduction
The advent of highly active antiretroviral therapy (HAART) has significantly reduced the morbidity and mortality of HIV-1 infection, even in those patients affected by aids-defining conditions1,2. This benefit is obtained due to an increase in the absolute number of circulating naive CD4 T lymphocytes, a concomitant reduction in the number of T lymphocytes with activation markers, and restoration of the response to memory antigens3. Nevertheless, despite the clinical efficacy of HAART4,5, this treatment by itself is unable to eradicate the infection, even if it were administered for more than 60 years6,7. This limitation is mainly because therapy cannot eliminate latent HIV-1 in the form of integrated ≤ proviral DNA, in addition to the existence of low levels of viral replication, which makes possible even cell-to-cell infection8,9. Furthermore, HAART is incapable of restoring the immune-specific response to HIV10,11, and, in fact, leads to a fall in the specific CTL response due to the lack of antigenic exposure12. Recent reports have shown that the helper proliferative response to HIV p24 Ag presented by some HAART patients does not reflect an improvement in the immune phenotype or function of CD4 or CD8 cells, but is secondary to the small increases in viremia typically observed in patients taking HAART13. This would explain the rapid "rebound" of viral load after suspending HAART, in a question of days or weeks, even after several years of effective therapy14,15. This rebound occurs even if HAART is initiated in very early-stage HIV-infected patients, in whom the immune system is theoretically still well preserved (circulating CD4+ T lymphocytes > 500 cells/mL; viral load in plasma (PVL): 5,000-10,000 copies/ml). Similarly, these viral dynamics occur even when immune restoration is practically complete in terms of the homeostasis of T lymphocytes and their subpopulations, and in terms of the capacity for response to polyclonal stimuli and memory antigens with HAART14,16.
These findings reinforce the need for suitable long-term treatment. Resistance, adverse effects in the medium-long term, and cost are important limitations for lifelong adherence to this therapy17. These concerns mean that new therapeutic strategies must be evaluated. The two possibilities being investigated at present are simplification of therapy18 and the combination of HAART with immune therapy to restore and/or boost such immune responses with the primary objective of controlling viral replication in the absence of HAART10. The idea is that HAART-free periods could be longer if we used pre-HAART withdrawal strategies aimed at stimulating the immune system to partially control viral replication after withdrawal10.
Pathogenic basis for the design of immune-mediated strategies
The main question to be answered is whether the immune system can contain viral replication without HAART, even if only for limited periods. This hypothesis arises from the following facts:
1. Although in most infected patients replication leads to the progressive destruction of the immune system and evolves inevitably towards aids, a small number of immunologically "privileged" individuals, or "Long Term Non-Progressors" (LTNP), have a potent and sustained response of anti-HIV-1 CTL, Th cells, and neutralizing HIV-1 antibodies. This is associated with a control of viral replication and the presence of very low or undetectable viral concentrations in plasma in the absence of HAART19.
2. The anti-HIV-1 cytotoxic response (CTL) is detected in all cases studied during the acute phase of the infection, and it is believed to reduce the peak of PVL which characterizes it to the stabilization level, or "setpoint", of PVL, which is established at the end of the acute phase. Direct data on the critical role of the CTL response in the control of viral replication have been obtained both in the infection model with macaques devoid of CD8+ T lymphocytes20,21, and in the immunodeficient murine model22.
3. There is clear evidence that a specific helper T response against HIV is crucial in obtaining an optimal specific CTL response which can control viral replication both in human10,23 and in animal models24. This concept is consistent with other recently reported data on chronic viral infections in murine models25.
4. Studies in primate and murine models show that high levels of neutralizing antibodies can block infection regardless of the route of exposure to the virus26.
Despite the importance of the immune response in infection, it cannot contain viral replication. Alterations of the immune system may allow us to explain this inability or dysfunction. Even though CD4+ and CD8+ cells capable of secreting interferon gamma (IFN-gamma) can be found, in most HIV-infected patients, the proliferative CD4 responses are normally absent11,23,27, and the CD8+ cells are defective with regard to their cytolytic activity28-30. One explanation of these functional deficits of CD4 and CD8 responses would be that the antigen-presenting functions of the dendritic cells could be deteriorated in these patients, and this could contribute to the functional defects observed in the Th1 and CTL cellular responses31,32. The absence of a correct proliferation and expansion of the CD4+ responses may in turn influence the lack of cytolytic activity of the CD8+ cells27,33. In animal models, there is a clear deficit in the secretion of cytokines by CD4+ cells which starts when PVL peaks in primary infection34. Lastly, the selective infection of HIV-specific CD4+ cells in infected patients would explain why these responses are quickly lost in HIV infection35.
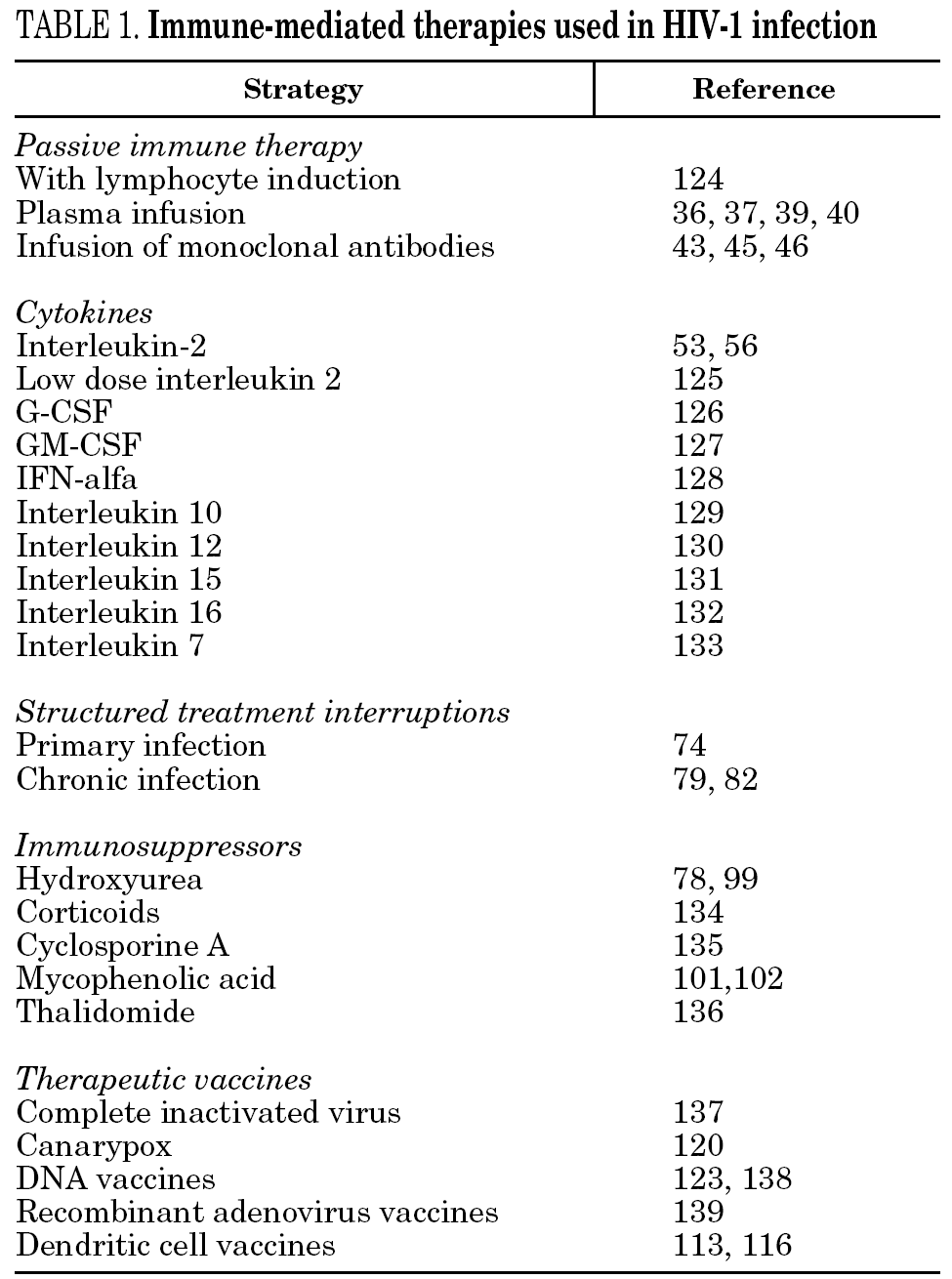
Apparently, therefore, cellular and immune responses, and the relationship between them, are vital for a correct functioning of the immune system. The defects would be more from alterations of these responses than from viral escape. Different types of immune-mediated therapies have been examined to solve these problems, including passive immune therapy, cytokines, structured treatment interruptions, immunosuppressors and therapeutic vaccines (table 1). This review will try to summarize the different approaches.
Passive immune therapy
Two types of passive immune therapy have been investigated in HIV-infected patients. The first type is based on infusion, of both CD4+ and CD8+ cells, and the second on plasma or neutralizing antibody infusion.
Passive immune therapy by cell infusion
Several studies on the infusion of specific CTL cells have been performed, although the results to date are not very promising36-38. Brodie et al39,40 investigated the functional activity of HIV-specific CTL cells and the capacity of these effector cells to migrate in vivo to areas of infection. Briefly, these authors expanded HIV-specific autologous CTL gag clones in vivo and injected them into HIV patients. The transferred cells retained their lytic activity in vivo, accumulate in territories close to where HIV-infected cells are found in the lymph nodes and reduce transitorily the circulating levels of HIV-infected CD4+ cells. Apart from expanding and infusing CTL, other groups have tried to infuse expanded CD4+ cells in vitro by a strategy which allows only virus-free cells to be conserved41. After infusion, a moderate improvement is observed in the CD4+ lymphocyte figure with a reduction in the CCR5 co-receptor, which implies a relative reduction in the infective capacity of these cells. In summary, passive therapies based on the transfer of cells are still very experimental, and have provided us with a better knowledge of the immune pathogenesis of the disease, although with no immediate clinical application.
Passive immune therapy by infusion of plasma or neutralizing antibodies
Plasma passive immune therapy (PIT) and monoclonal neutralizing antibodies appeared many years ago (although they are no longer used) for use in daily clinical practice. PIT as therapy for aids patients was investigated during the first half of the 1990s. It was proposed and initiated by Abraham Karpas, a virologist from the University of Cambridge (UK), who published his first results in 1988. This technique involved the intravenous infusion in advanced aids patients of plasma from asymptomatic HIV+ patients. The first study used a monthly infusion of 500 ml of plasma for 3 months in 10 advanced patients (7 with aids and 3 with ARC "aids-related complex", according to the classification criteria from this period). The plasma was previously inactivated with propiolactone to eliminate the infectivity of the donor virus. The results showed that PIT produced negative p24 antigenemia values and increased neutralizing activity with no adverse effects42. Shortly afterwards (1990), Karpas demonstrated the efficacy of the PIT protocol by observing reductions in circulating viral load43. In this second study, Karpas also showed that the monthly plasma infusion increased and maintained a high level of neutralizing activity in serum. The hypothesis which resulted from these studies was that asymptomatic HIV-infected patients had serum containing high titers of viral neutralizing activity, and that this activity disappeared as patients progressed to aids. Karpas concluded that this neutralizing activity was correlated with non-progression to aids and therefore, passive transfer of these antibodies in the plasma of asymptomatic patients would help recipients to control and slow down the progression of their immunodeficiency44. Given that there was no treatment for the infection at this time, these studies aroused great interest in PIT as a possible therapy, with the result that PIT study groups soon began to be formed. Double-blind and placebo-controlled randomized clinical trials were started with a large number of cases led by two independent groups, one in California45 and the other in Paris46. The California trial45 studied 220 aids patients with CD4+ T cell counts of between 50 and 200 cells/microL, which were randomized into three groups to receive over 12 months a monthly infusion of (i) 500 ml of hyperimmune plasma; (ii) half the dose of plasma (250 ml of plasma diluted to 500 ml with 5% human seroalbumen), and (iii) 500 ml of 5% seroalbumen, as a placebo. Mortality fell and the number of CD4 cells grew compared with the placebo group, and with the group which received half the plasma dose. The French trial included 86 aids patients who were randomized to two groups to receive over 12 months a fortnightly infusion of: (i) 300 ml of hyperimmune plasma, and (ii) 300 ml of plasma from healthy donors (non-immune) as placebo. The group treated showed a smaller incidence of aids-defining events (p = 0.009), a smaller accumulation of these events (3 times smaller), and a lower mortality rate (p = 0.009). The conclusion of both studies was clear, in the sense that the infusion of plasma from non-advanced patients to advanced patients had no adverse effects and was clinically useful in "curbing" progression of the disease. Later, systematic and controlled PIT clinical trials were practically abandoned, given the efficacy of the antiretroviral agents which appeared.
There has been renewed interest in passive immune therapy, but with specific monoclonal antibodies, based on studies of the macaque model, which show that passive transfer of antibodies prevents infection by oral, vaginal or intravenous inoculation of the virus47-50. This current interest in antibodies as a potential therapy or prophylaxis is beginning to be seen in human clinical care. Phase I trials have begun in infected patients to evaluate the pharmacokinetics and safety of human monoclonal antibodies (known as 2F5 and 2G12), which were taken some years back from two non-progressors. Unlike other human monoclonal antibodies, these can inhibit in vitro infection by R5 strains and X4 strains51. A recent study has shown that the administration of a neutralizing antibody called TNX-355 produces some antiviral efficacy (a fall of 0.5-1 log10) and an increase in the number of CD4+ lymphocytes. This effect persisted in patients for up to four weeks after infusion of the antibody52. If the efficacy of these monoclonal antibodies is confirmed in humans, in vitro and experimental animal models, they may soon be clinically useful, although for now they are only a promising possibility.
Cytokines
Several studies and clinical trials have used cytokines (table 1), all with the aim of restoring the cytokine imbalance caused by HIV infection, and suitably controlling infection using neutralizing antibodies, especially that caused by specific CTL cells. The best options for human medicine are IL-2, IL-12, IL-15, growth hormone and GM-CSF.
IL-2 infusion with different strategies, doses and routes leads to a clear increase in the CD4+ lymphocyte count53,54. The most widely recommended dose at present is 4.5 M IU/q 12 h x 5 days. In general, an induction phase is with 6 X 5-day cycles every 8 weeks, followed by a maintenance phase with a number of variable cycles if there is a new fall in the CD4+ count. Toxicity is dose-dependent with a frequency of grade 3-4 adverse effects in < 10% of cases. The most common local adverse effects are nodules and blisters at the injection site. The most common systemic adverse events are pseudoflu syndrome (90% of patients), skin lesions (60%), gastrointestinal disorders, edema, disorders of the central nervous, respiratory and endocrine systems. Other uncommon effects (< 10%) include cytopenia, electrolytic alterations, and cardiovascular disorders (arrhythmias, congestive cardiac insufficiency, ischemic cardiopathy, hypotension). It is the most widely studied and clinically advanced drug used in immune therapy. Nevertheless, after years of research, it remains unclear whether the increase in CD4+ T cells affects clinical progression positively, although there are studies in progress which try to answer these questions.
Apart from increasing total CD4+ lymphocytes, IL-2 has been used with at least three other objectives:
1. The first is as a cytosine which tries to restore the T cell repertoire by increasing the total CD4+ lymphocyte count. Progression of HIV infection is known to be associated with a more rapid loss of naïve cells than of memory cells. Immune control of viral infections depends on the immunocompetent cells having a wide repertoire, and HIV infection leads to the loss of important parts of this repertoire. Administration of IL-2 is associated with polyclonal increases both of naive cells and of memory cells in HIV-infected patients, but analysis of repertoire has shown that defects are not corrected by the administration of IL-255.
2. Therefore, the combination of IL-2 and other immune-mediated therapies has been proposed to restore the dysfunction of the helper response (perhaps due to a lack of sufficient endogenous IL-2) in HIV-infected patients. Nevertheless, several pilot clinical trials have failed to show the usefulness of IL-2, at least when it is combined with structured treatment interruptions56,57, or a canarypox vaccine (ALVAC-HIV vCP 1433)58.
3. Some years ago, an attempt was made to eliminate the virus from the reservoirs by stimulating the IL-2 of quiescent HIV-infected cells which, when stimulated, produce viruses, which would be inactivated by HAART. In one clinical trial, patients who received HAART and IL-2 showed a lower quantity of detectable infectious viruses than the control group, which only received HAART59. Nevertheless, on withdrawing therapy in both groups, the viral rebound displayed similar dynamics, which would suggest that IL-2 had little effect on the viral reservoir60.
Other cytokines have been proposed in human medicine (table 1). The most important are IL-12 and IL-15, which lead to an increase in the specific CTL response in vitro61-63. Both are produced mainly in activated antigen-presenting cells and are thought to promote the development of TH-1 type cellular responses. This type of response is essential for stimulating CTL responses. Other effects of these cytokines are the increase in lytic activity by the natural killer cells and the increase in the HIV-specific proliferative capacity62-64. Petrovas et al65 recently reported that IL-15, administered twice a week for four weeks to SIV-infected cynomulgus macaques, increased the proliferation and expansion of CD8+ cells without affecting viral replication.
The growth hormone (GH) has been suggested in clinical practice for HIV-infected patients to promote the T cell response and generate a lymphopoietic effect and induce effects in peripheral T cells. Therefore, it seems to be an appropriate immunomodulating agent in states of immunodeficiency. In HIV+ patients treated with GH a marked increase in thymic mass has also been observed. This increase was accompanied by a significant increase in the number of naïve CD4 T cells, an increase in the HIV-specific CD4 and CD8 responses, and induction in the differentiation of T cells from functional memory effector cells66,67. These results suggest that GH produces an increase in thymopoiesis and therefore that it has important effects on the human immune system, including reversion of thymic atrophy in HIV-1-infected adults.
Finally, GM-CSF has been combined with treatment interruptions, and a slight improvement in virological control and a smaller fall in CD4+ lymphocytes after definitive interruption of treatment have been observed. Nevertheless, the results are currently unacceptable in terms of toxicity, given that more than 80% of patients had local and general reactions68.
Structured treatment interruption (STI)
Since the description of the anecdotal cases presented by Franco Lori and Douglas Nixon69,70, the concept of antiretroviral therapy interruption as a therapeutic strategy has been investigated with interest by several groups. Initially, this strategy was considered as "autovaccination" with an attenuated autologous virus, in which the attenuation came from the gradual reintroduction of antiretroviral therapy. With time, other objectives of STI, which were not important initially (e.g. savings in medication, reduction of secondary effects, etc.), have come to the fore and are currently among the most widely investigated strategies71. Nevertheless, it must be stressed that one of the most important concerns in the application of STI in HIV+ patients receiving treatment is the risk of selecting resistance72. This review will examine neither the latter type of interruptions nor the so-called "therapy vacations" used in patients experiencing therapeutic failure73, rather we shall concentrate on STI as an immune-mediated strategy.
Several studies show that STI in patients who started antiretroviral therapy during the acute phase of HIV infection allow viral replication to be controlled transitorily, and stress the intrinsic potential of the immune system to adequately control the disease74. However, these data have not been confirmed by other groups56, and it has been observed that the virological efficacy of STI started during acute infection is lost over time, therefore more research is necessary into whether starting therapy (with or without STI) during the acute phase is beneficial for long-term patients75.
Re-exposure to viral antigens boosts and stimulates virus-specific immune responses, although only 20% of chronic patients who use this strategy manage to effectively control viral replication in the short-medium term76-82. It is important to understand why there is a lack of control of viral replication despite the induction of CTL and helper responses in chronic HIV-infected patients. These conclusions serve to design other immune-mediated strategies which allow more effective control of viral replication for a longer period.
1. First, during the interruption, we can observe very high peaks of viral load in some patients and, given that the CD4+ cells with an HIV-specific response are more infected after viral rebound35,83, clonal elimination of these cells could occur, which may explain the lack of response84,85. Plana et al recently studied a group of 40 patients with intermittent therapy. In these patients, the helper response was shown to be induced weakly during the interruption cycles, and it is lost during definitive interruption of therapy86. This would explain the inability of the CTL response to control viral replication87-89. Contrary to the helper response, the CTL response is induced considerably (both in magnitude and in amplitude) after definitive interruption of antiretroviral therapy77,78, but it is incapable of controlling viral replication. Some authors report that CTLs induced after discontinuation of therapy would not be functional (they would be in a pre-terminal stage and would produce fewer perforins), and they attribute the inability of these strategies to stimulate an efficacious CTL response to the loss of a specific T helper response90. Strategies aimed at avoiding clonal detection of T cells with the capacity of an HIV-specific response caused by STI, could be predicted to improve the control of viral replication by inducing a functional CTL specific response.
2. Second, many authors have contributed data from sequencing and cloning of the env gene. They suggest that, in a viral rebound, the virus which appears may be very different from that observed in other rebounds or that which appears at baseline91-93, and that the specific immunity recovered is no more than an increase in the existing immunity77,94, probably due to the fact that they are repeatedly exposed to the same antigen95. This mobilization of viruses from different reservoirs, and the inability to create a new specific response may be what prevents efficacious control of viral replication. Therefore, we should use immunogens capable of inducing new efficacious HIV-specific responses, both functionally, since this would prevent the clonal disappearance of helper T cells and improve the CTL response, and in amplitude, so that they could control replication of ancestral viruses present in memory cells, which would finish by replicating once HAART was interrupted.
Immunosuppressors
Parallel to the fall in the CD4 count, HIV infection is characterized by an intense and sustained state of immune activation manifested by a high number of T and B lymphocytes, natural-killer cells, and a marked release of pro-inflammatory cytokines such as IL-7 and TNF-α10. A constant element of this process is the high count of activated T-CD8 clones which express surface receptors, DR+/CD38+, a phenomenon which is today considered as a true marker of disease progression96,97. This sustained activation process can lead to an exhaustion of the immune system, similar to an increase in cellular infectivity, thus allowing dissemination. This phenomenon of immune activation lays the foundations for the use of immunosuppressors such as corticoids, hydroxyurea (HU), mycophenolate mophetil (MPM), thalidomide and cyclosporine A, as adjuvants to antiretrovirals.
The results obtained on the control of viremia in macaques, treated since acute infection by SIV, using HU as an adjuvant and treatment interruption cycles, lead us to consider this drug as a clinically useful immunuosuppressor in the future98. We can, therefore, formulate the hypothesis that HU inhibits activation of T lymphocytes during interruption cycles, thus preventing infection in the target cells and the production of high peaks of viral replication without destroying the specific immune response. Although it is well known and clinically proven that HU inhibits the ribonucleotide-reductase enzyme98,99, it also induces a cytostatic effect by halting the cellular cycle at the beginning of the S phase, and a reduction in cellular activity. It was this property that led García et al78 to evaluate the usefulness of the drug in patients who were programmed to undergo intermittent interruptions of HAART. Five interruption cycles of 2 weeks' duration were scheduled, but continuing with HU only for the last two cycles of interruption. This schedule made it possible to evaluate the effect of HU on viral dynamics between interruption cycles with and without the drug. Thus, if HU manages to reduce the initial phase of viral rebound, which starts from the reservoirs, resting lymphocytes, macrophages, and dendritic cells, where it has been shown that HU is excellent as monotherapy100, the effect of HU was observed even when this ceased to be administered. Secondly, HU can slow down later phases of viral replication from activated T lymphocytes, mainly due to its cytostatic effects. In this case, the control of viral replication is only obtained by maintaining HU when HAART is withdrawn. There were no differences in viral rebound after three interruption cycles, although when HU was maintained after interruption, the viral load was on average 1 log lower than that obtained during previous interruption cycles and lower than in the control group, treated with HAART only. This phenomenon shows the cytostatic activity of HU and its potential use against the intracellular viral reservoir. From a clinical viewpoint, the use of HU significantly increased the number of patients who achieved sustained viral replication (8/9 patients with viral load < 5,000 copies RNA/mL) for 48 weeks after 5 HAART interruption cycles, regardless of baseline viral load (4.6 log RNA HIV).
Other groups have studied the capacity of other immunosuppressors such as mycophenolic acid as an adjuvant to HAART. Chapuis et al101 studied in vitro and in vivo the mechanisms by which mycophenolic acid (MPA) and its sterilic derivative mycophenolate mophetil (MPM) suppressed infection by HIV. MPA selectively inhibits the synthesis of guanosine nucleotides by competitively inhibiting the dehydrogenase inosin-monophosphate enzyme. Given that there are no alternative enzymatic pathways for the synthesis of guanosine nucleotides in lymphocytes, MPA produces a profound cytostatic effect by depletion of this substrate. Furthermore, in vitro results show that MPA inhibits the proliferation of activated T cells, especially in those with low or intermediate expression of the CD4 receptor, by leading them to apoptosis even in the presence of IL-2. These data were confirmed in a clinical trial involving patients treated with abacavir and amprenavir, who were randomized to receive or not receive MMF101. In the MMF group, a reduction in the actively dividing CD4 and CD8 "pool" (Ki67+) was observed. Furthermore, the authors suggest that MMF can have an effect on the "pool" of latently infected CD4 cells, as they observed that, in patients treated with MMF, the ability to isolate viruses from the total population of T-CD4 was reduced. Even though MMF does not affect resting cells and therefore does not affect their number, once they have been activated in the presence of MMF, apoptosis and cell death are induced101. Other authors have investigated the role of MMF on viral load in plasma and in lymph tissue during and after intermittent interruptions of HAART102. Patients treated for at least one year with an abacavir-containing regimen were randomly assigned to receive or not receive MMF with HAART for four months before the interruption cycles. In those treated with MMF, the "pool" of dividing T-CD4 cells was reduced. The same occurred with the set-point of viral load after interrupting HAART. In this trial, lymphoproliferation was used to evaluate the capacity of serum in patients to reduce the response of a T cell line in vitro, using sequential samples at different time points after the dose of MMF. The changes obtained in the dynamics of viral load, especially those observed in the set-point of PVL after HAART interruption, were only observed in those patients who reduced lymphocyte proliferation to below 40% in the T cell line (CEM) for more than four hours after administration of MMF. In another context, MMF was used as part of a rescue schedule when added as an isolated drug to a HAART rescue regimen containing abacavir in patients with multiple resistance to antiretrovirals103. A significant reduction in viral load (> 0.5 log) was observed in those patients who increased their quotient: Carbovir (the antiviral active metabolite of abacavir) and deoxyguanosine triphosphate, owing to inhibition of the dehydrogenase ionosine monophosphate enzyme and subsequent depletion of guanosine nucleotides.
New therapeutic alternatives were proposed after the publication of the results of a pilot study in patients with acute HIV infection treated with short-term cyclosporine A (CyA) and HAART104. This trial involved reducing the high level of cellular activation which thus generated massive viral replication. This high level viral replication led to clonal exhaustion of HIV-specific CD4 lymphocytes. Despite the fact that CyA interferes with the synthesis of the viral gag proteins, its main effect is by inhibition of proliferation and differentiation of T cells. The CD4 count of patients treated with CyA was restored both in percentage terms and in absolute numbers, by maintaining the number of secreting CD4-HIV-?IFN. These data suggest that the use of CyA or any other drug with immunosuppressor properties during primary infection could reduce the number of active CD4 cells which sustain massive viral replication, and prevent the hijacking of these clones in lymph tissue, where antigens are presented and HIV infection is perpetuated. This process may have an impact on the clone of the resting T cells which shelter viruses with replicative capacity. Nevertheless, we do not know whether establishing a new immunological set-point to slow down the rate of progression offers a clinical benefit in long-term infection.
As several studies have shown, there are many doubts about the use of immunosuppressors as immune-mediated therapy in HIV infection. Knowing which patients are most suited, which drug to use, how long therapy should last and the ideal time to start therapy are questions which must be answered by clinical trials with a large number of patients and long-term follow-up. There are also concerns over long-term safety, such as the influence on the development of opportunistic infections or lymphoproliferative diseases, which limit extensive use of this type of drugs in the long term. Nevertheless, pharmacological strategies which interfere with the HIV life cycle by acting at the level of viral target cells, rather than by inhibiting viral enzymes, are still attractive. This has the advantage of avoiding the development of genomic mutations against antiviral drugs. The use of immunosuppressor drugs as an adjuvant to HAART must be evaluated with caution until we have more information on long-term efficacy and safety.
Therapeutic vaccines
Immune recovery of the HIV response has also been tried using therapeutic vaccination. In general, the capacity of the vaccines used to increase the CTL-specific response has been very limited and study results have been discouraging, as immunogenicity has not been demonstrated and there has been no clear impact on viral load105-109.
The Remune vaccine has received most attention. This is a vaccine of an inactivated complete vaccine in which the envelope protein has been removed during the process of inactivation which is carried out for synthesis. This vaccine stems from a virus originally obtained in Zaire and contains a type-A envelope and type-G gag. It has been administered to more than 3,000 people with an antiviral-controlled virus. The results showed that it was capable of inducing gag-specific helper responses which are sometimes very potent. Nevertheless, these studies did not observe a capacity for immunological control of viral replication110-112. The study which best demonstrates the capacity of a therapeutic vaccine to efficaciously increase specific immunity for the control of viral replication used dendritic cell (DC) vaccine in the SIV animal infection model (SIVmac251). In this study, four immunizations with cells pulsed by the same virus were made every two weeks for a period of eight weeks. In most of the inoculated animals (7/10), there was a significant reduction in viral load in plasma after the third immunization. This was sustained for the 34 weeks of the study. There was a 50 and 1,000-fold reduction in the SIV DNA and SIV RNA loads in peripheral blood, respectively. The analysis of lymph tissue revealed a correlation between the reduction in SIV DNA and RNA levels and the increase in the SIV-specific T cell response. The fall in viral load and subsequent increase in CD4 count was accompanied by an increase in circulating antibody levels113. Very similar results were obtained by two independent groups in a murine model with preventive vaccination114,115. Despite these incredible results, a clinical trial involving 12 patients with chronic infection receiving antiretroviral therapy from early stages of the infection, using a dendritic cell vaccine pulsed with heat-inactivated autologous viruses has offered much more moderate results116. In this study, in a first treatment interruption 18 months before receiving the first dose of vaccine, three plasmaphereses were performed in which 1800 cc of plasma was extracted. The median viral load of patients during plasmapheresis was 27,000 copies/ml. The virus was then inactivated by heat and concentrated using ultracentrifuge in 1 cc, all under conditions of good clinical practice (GCP). A schedule of five subcutaneous doses was administered every six weeks. The first dose was a control with non-pulsed DC. Every pulsed vaccine contained 2 x 106 DC, pulsed with five virions per cell during the first immunization and three virions/DC in the remaining vaccinations. In general, the results showed that a vaccine did not cause important adverse events, as in only 3 of the 60 doses administered (5%) was there an adverse reaction (only 1 mild local reaction and 2 episodes of flu symptoms 24 hours after the dose). This vaccine was able to control viral replication partially and transitorily, and was associated with a transitory, but not significant increase in the lymphoproliferative response to HIV P24 Ag, and with the changes in the CTL-specific response for peripheral HIV and in the CTL cells of lymph tissue. In lymph tissue, there was also a trend towards greater control of viral replication associated with an increase in CD4 and CTL cells in this tissue116. Furthermore, there was no significant increase in the neutralizing activity of the serum of these patients. Despite these moderate results, we must remember that the dose of antigen used in the human trial was 1,000 times lower that that used in macaques, therefore, new trials with a greater dose of antigen are necessary. If these results are confirmed, they would suggest that the defect in immunological control of HIV could be due to alterations in the induction phase of the immune response, which is consistent with recent studies on the induction of the immune response in the absence of a helper response117,118, and with data which suggest that antigen-presenting functions are altered in HIV-infected patients. This could contribute to functional defects in HIV-specific CTL and helper responses31,32,119.
Other vaccine trials have examined ALVAC, whose vector is a recombinant canarypox. Kinloch et al120 recently presented long-awaited results from the QUEST study. This international study was carried out on patients who started treatment during the acute phase. After a mean of two years of virological control, 79 were randomized to receive immunization with ALVAC VcP1452, ALVAC plus Remune, or placebo. After 24 weeks' immunization, HAART was interrupted. There was no difference between the groups in terms of viral rebound dynamics or in viral load figures.
Another therapeutic vaccination study has recently been presented by Cooper et al using patients with primary infection121. After a mean of four years of HAART, 35 patients with controlled viral replication were randomized to be vaccinated with a fowlpox vector free of HIV sequences, a vector containing gag/pol sequences, or a vector containing gag/pol sequences and a gene which encodes human interferon gamma. Surprisingly, there were few differences between the groups in terms of presence of CDL cells measured by ELISPOT or in cytolytic responses after vaccination and before interruption of treatment. Treatment was not interrupted in 10 patients. There were no differences in the control of viral replication between the placebo group and the group vaccinated with gag/pol. However, patients immunized with gag/pol and interferon gamma had better control of viral replication, with a mean viral load of 0.8 log10 less than the other two groups. The absence of immune responses in the two vaccinated groups is disappointing and the response in the interferon group is surprising.
Other vaccines with the potential to be used in therapy are those based on DNA which includes the proteins env-tat-nef. These have been tried as a preventive vaccine with promising results by inducing a primary response with DNA and a booster with the Ankara virus122. The vaccine tried as therapy presents the whole HIV genome minus the integrase gene and has shown promising results after intradermic administration in monkey models with STI123.
Conclusions
We have a limited knowledge of the immunological control of HIV viral replication, the causes of cellular and humoral immune deterioration, and a lack of clear immunological methods to correlate with an efficacious immune control of HIV in vivo. The efficacy of immune therapy and therapeutic vaccines has been modest in the best of cases. We must redouble our efforts to understand better the mechanisms of protection, virological control and immune deterioration. Without this knowledge, an efficacious therapeutic vaccine is a long way off. Nevertheless, given the toxicity and long-term efficacy problems with current drugs, this remains a priority line of investigation.
Acknowledgments
Some of the studies and data have received support from: Ministry of Health, Abbott Laboratories, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merck Sharp and Dohme and Roche (FIPSE) 01/36259, SAF 01/2591, Red Temática Cooperativa de Grupos de Investigación en Sida (RIS) del Fondo de Investigación Sanitaria (FIS), marató de TV3, Objectif recherche vaccin sida (ORVACS). We are grateful to Dr. Teresa Gallart, Dr. Emilio Fumero and Dr. Margarita Bofill for their comments and help in preparing this manuscript.