The lack of consensus of control measures to prevent extended-spectrum β-lactamase producing Enterobacterales (ESBL-E) transmission in the hospital setting is of great concern. We describe the prevalence and species distribution of ESBL-E and carbapenemase producing Enterobacterales (CPE) in patients admitted in a tertiary Hospital during an active surveillance screening program for detecting ESBL-E carriers and reducing the ESBL-E transmission (R-GNOSIS Project).
MethodsFrom March-2014 to March-2016, 15,556 rectal swabs were collected from 8209 patients admitted in two medical (Gastroenterology, Pneumology) and two surgical (Neurosurgery, Urology) wards. Swabs were seeded onto ChromoID-ESBL and -CARB/OXA-48 agar plates. Growing colonies were identified by MALDI-TOF MS. ESBL and carbapenemases were phenotypically detected. Changes in species diversity (SDI) and distribution over time were analyzed.
ResultsESBL-E incidence (8.4%) tended to decrease over time (p=0.003) and CPE carrier prevalence remained unchanged during the study (2%). The contact isolation strategy targeted to reduce ESBL-E transmission was ineffective in reducing ESBL-E carriers but significant differences were observed with CPE (p=0.017). SDI did not change among ESBL-E and E. coli was predominant (78.5%) during the study. K. pneumoniae (54%) was the most frequent CPE species, followed by E. coli (19%). SDI decreased among the CPE population over time mainly due to K. pneumoniae dominance and increased E. coli prevalence in the last part of the study.
ConclusionsDuring the R-GNOSIS project, contact precautions were not effective in reducing the ESBL-E transmission but may have had a positive collateral effect on the CPE containment.
La falta de consenso en las medidas de control necesarias para prevenir la transmisión de enterobacterias productoras de β-lactamasas de espectro extendido (BLEE-E) en el entorno hospitalario es muy preocupante. En este trabajo describimos la prevalencia y la distribución de especies de BLEE-E y las enterobacterias productoras de carbapenemasas (EPC) en pacientes ingresados en un hospital terciario durante un programa de vigilancia activa para detectar portadores de BLEE-E y reducir su transmisión (Proyecto R-GNOSIS).
MétodosEntre marzo-2014 y marzo-2016 se recogieron 15.556 hisopos rectales de 8.209 pacientes ingresados en 2 servicios médicos (Gastroenterología, Neumología) y 2 quirúrgicos (Neurocirugía, Urología). Los hisopos se sembraron en las placas de agar ChromoID-ESBL y CARB/OXA-48. Las colonias crecidas fueron identificadas por MALDI-TOF MS. La producción de BLEE y carbapenemasas se confirmó fenotípicamente. Se analizaron los cambios en la diversidad de especies (SDI) y su distribución en el tiempo.
ResultadosLa incidencia de BLEE-E (8,4%) tendió a disminuir (p=0,003) y la prevalencia de portadores de CPE permaneció sin cambios durante el estudio (2%). La estrategia de aislamiento de contacto dirigida a reducir la transmisión de BLEE-E fue ineficaz, pero se observaron diferencias significativas en las EPC (p=0,017). La SDI de las BLEE-E no cambió durante el estudio y E. coli fue la especie predominante (78,5%). K. pneumoniae (54%) fue la especie de EPC más frecuente, seguida de E. coli (19%). El SDI disminuyó entre la población de EPC, principalmente debido al dominio de K. pneumoniae y al aumento de la prevalencia de E. coli en la última parte del estudio.
ConclusionesDurante el proyecto R-GNOSIS, las precauciones de contacto no fueron efectivas para reducir la transmisión de BLEE-E, pero pudo haber tenido un efecto colateral positivo en la contención de EPC.
ESBL-producing Enterobacterales (ESBL-E), currently disseminated worldwide, are considered endemic in many countries, including Spain. Last available data indicate that the overall prevalence of ESBL-E fecal carriers in Spain is approximately 6–8%, with an increasing trend in the community.1–3 ESBLs and particularly CTX-M enzymes are mainly detected in community-acquired Escherichia coli isolates and nosocomial clones of Klebsiella pneumoniae.4,5 The increasing consumption of carbapenems antibiotics for the treatment of infections caused by ESBL producers has been directly related to the rapid emergence and dissemination of carbapenemase-producing Enterobacterales (CPE).6,7 In Spain, the epidemiology of CPE has been largely associated with the expansion of hospital-adapted OXA-48 and VIM-1-producing K. pneumoniae clones.8–10 However, an increased prevalence of OXA-48-producing E. coli has also been described in our area, even associated with the ST131-E. coli clone, commonly related to CTX-M production.11,12
The risk for carbapenemases spread into the community, as happened with CTX-M enzymes, is increasing and depicts a great public health threat worldwide. As a consequence, regional guidelines have been implemented to curtail the dissemination, and declaration of CPE to Public Health authorities is now mandatory in different Autonomous Communities.13 Nevertheless, few official guidelines related to infection control measures for reducing ESBL-E carriers in general hospital wards have been published and the effectiveness of patient isolation for preventing the ESBL-E transmission in non-outbreak situations is uncertain.14
This study aimed at assessing the incidence of ESBL-E and CPE carriers in a tertiary hospital in Madrid during the R-GNOSIS project and determining the efficacy of the contact isolation (CI) with respect to standard precaution (SP) strategy in reducing the nosocomial transmission of ESBL-E isolates within the hospital. Additionally, species diversity and distribution of both CPE and ESBL-E populations were analyzed over time.
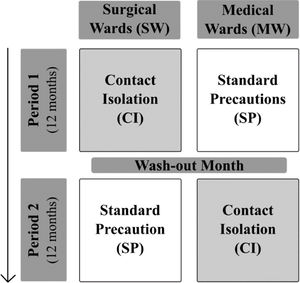
MethodsStudy designR-GNOSIS “Resistance in Gram-Negative Organisms: Studying Intervention Strategies” is a European collaborative research project funded by the European Commission under the Seventh Framework Programme (FP7) for Research and Technology (R-GNOSIS-FP7-HEALTH-F3-2011-282512) (http://www.r-gnosis.eu/). Two work packages (WP) were performed at the University Hospital Ramón y Cajal (Madrid, Spain) as a part of the R-GNOSIS project: WP5 (“Patient isolation strategies for ESBL carriers in medical and surgical hospital wards”) and WP7 (“Functional microbiology & within-host transmission dynamics of genes, plasmids and clones of MDR-GNB”). WP5 objective was to evaluate the additional effects of contact isolation (CI) compared to standard precaution (SP) strategies on the incidence density of nosocomial ESBL-producing Enterobacterales (ESBL-E) acquisition among adult patients hospitalized in two surgical (SW) and two medical wards (MW). During the first period (12 months), CI was implemented in SW (Urology and Neurology) while only SP were used in MW (Gastroenterology and Pneumology). Both strategies were switched after a wash-out period of one month (Fig. 1). CI and SP interventions are summarized in Table 1. Following the R-GNOSIS protocol, all patients were screened on admission to the ward or as soon as possible within the first 3 days. Repeated surveillance cultures were obtained from patients staying longer than 7 days on a specific day each week. Samples were also recovered at discharge from patients staying up to 3 days.15 The study was approved by the Hospital Ethical Committee (Ref. 251/13).
Intervention strategies for ESBL-producing Enterobacterales (ESBL-E) carriers during the R-GNOSIS Project.15
| Intervention strategies | |
|---|---|
| Standard Precautions (SP) | Hand hygiene (HH).Use of gloves and other barriers by healthcare workers (HCWs) during the care of all patients. |
| Contact Isolation (CI) | Standard Precautions (SP).Use of gloves and gowns by visitors during the care of patients known to be colonized/infected.Isolation of colonized/infected patients in a single room or in a shared room with patients colonized/infected with the same microorganism. |
In our Hospital, unlike the other centers, patients included in the R-GNOSIS project were also screened for CPE intestinal colonization. Following the Spanish guidelines, infection control measures and active surveillance (rectal cultures) in colonized and/or infected patients were strictly implemented after detection of each new case of carbapenemase production.13 On the other hand, WP7 was aimed at studying the emergence, persistence, resistance mechanisms and clonal relationship of MDR-GNB recovered during the WP5.
Surveillance culturesA total of 15,556 rectal swabs from 8209 patients were collected in our Hospital between 4 March 2014 and 30 March 2016 as a part of the R-GNOSIS Project; 6810 samples from 4027 patients and 8016 swabs from 3872 patients were recovered during the first (4 March 2014–28 February 2015) and second (1 April 2015–31 March 2016) intervention period, respectively. Furthermore, 730 swabs from 310 patients were also collected during the wash-out period (March 2015). In the subsequent analysis, the wash-out month was considered to be within the first period. The total rectal swabs recovered in each ward during both study periods are shown in Table 2.
Recovered rectal swabs and patients screened at the University Hospital Ramón y Cajal during the R-GNOSIS Project.
| Wards | No. rectal swabs | No. patients |
|---|---|---|
| Gastroenterology | ||
| Period 1 | 2026 | 1083 |
| Wash-out | 197 | 82 |
| Period 2 | 2225 | 999 |
| Total | 4448 | 2164 |
| Pneumology | ||
| Period 1 | 1812 | 1078 |
| Wash-out | 224 | 88 |
| Period 2 | 2218 | 1007 |
| Total | 4254 | 2173 |
| Neurosurgery | ||
| Period 1 | 762 | 421 |
| Wash-out | 87 | 35 |
| Period 2 | 972 | 456 |
| Total | 1821 | 912 |
| Urology | ||
| Period 1 | 2210 | 1445 |
| Wash-out | 222 | 105 |
| Period 2 | 2601 | 1410 |
| Total | 5033 | 2960 |
| TOTAL | 15,556 | 8209 |
Period 1=March 2014–March 2015; Period 2=April 2015–March 2016.
Rectal swabs were directly seeded on both ChromID-ESBL and -CARB/OXA-48 selective chromogenic agar plates (BioMérieux, Marcy l’Étoile, France) and incubated at 37°C for 24–48h. A single colony per color and morphology growing on each selective chromogenic agar-media was selected for microbiological studies. Bacterial identification was performed by MALDI-TOF MS (Bruker Daltonics, Bremen, Germany). ESBL production was phenotypically detected by the double-disk synergy test (DDST) and ESBL and AmpC Screen Kit (RoscoDiagnostica, Taastrup, Denmark). Production of carbapenemases was also investigated by the Modified Hodge and KPC/MBL/OXA-48 Confirm Kit (RoscoDiagnostica) tests.
Diversity analysisThe Simpson Diversity Index (SDI) was estimated16 to calculate the bacterial species diversity of both CPE and E-ESBL populations throughout the study.
Statistical analysisDifferences in accumulated incidence rates over time were assessed using Poisson regression (Stata Statistical Software: Release 11. College Station, TX: StataCorp LP). Differences in prevalence rates among different wards and periods were assessed using the Fisher's Exact test and statistical significance among SDI disparities was also estimated by Paired T-test (RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/). In all cases p-values<0.05 were considered statistically significant.
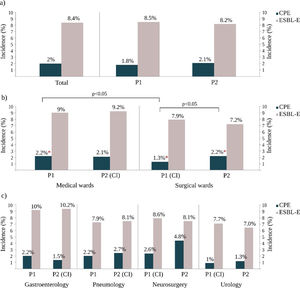
ResultsCPE and E-ESBL colonization ratesDuring the study period, 730 non-duplicated ESBL-E isolates were recovered from 687 patients and the total colonization rate was 8.4% (95% CI 7.8–9%). During the same period, 198 non-duplicated CPE were isolated from 162 patients and this result represented an overall CPE accumulated incidence rate of 2% (95% CI 1.7–2.3%). It should be noted that 15.3% (105/687) of the ESBL-E carriers were simultaneously colonized with a CPE isolate and that 55.5% (110/198) of CPE isolates were also ESBL producers.
Colonization rates and infection control measures strategiesDuring the R-GNOSIS Project, the incidence rate of ESBL-E carriers tended to decrease over time (p=0.003) and non-significant differences were detected with the implementation or withdrawal of CI (IRR=1.04, 95% CI=0.86–1.25; p=0.07). However, although the colonization incidence density with CPE was also invariable throughout the study (IRR=1.01, 95% CI=0.98–1.02; p=0.41),17 statistical differences were found depending on the intervention strategy implemented for ESBL-E carriers (p=0.017). All incidence data are shown in Table S1 and represented in Fig. 2. Significant differences were not observed in both ESBL and CPE colonization rates comparing both studied periods (Fig. 2a). At ward level, during the first intervention period and coinciding with the CI implementation for ESBL-E carriers in SW, both ESBL-E and CPE incidences were higher in MW with respect to SW (Fig. 2b), but statistical differences were only found among CPE carriers (MW=2.2%, SW=1.3%) (OR=1.7; 95% CI=1.0–2.8; p=0.03). As is shown in Fig. 2b, an increasing CPE incidence was observed in SW (Period 1=1.3%, Period 2=2.2%) (OR=0.6; 95% CI=0.4–1.0; p=0.05) in the second part of the study, coinciding with the withdrawal of CI measures in patients colonized with ESBL-E. On the contrary and despite the implementation of the CI strategy, the CPE incidence in MW remained invariable with respect to the first period (Period 1=2.1%; Period 2=2.1%). Remarkably and despite the CI measures, the ESBL-E colonization rate during this second intervention period was also significantly higher in MW (9.2%) than SW (7.2%) (OR=1.3; 95% CI=1.0–1.6; p=0.03).
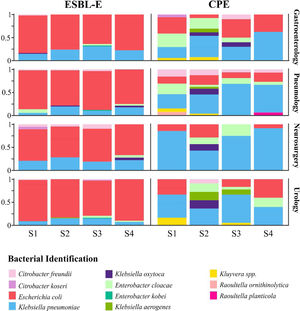
Diversity and bacterial species distributionDuring the R-GNOSIS study, a total of 198 CPE and 730 ESBL-E non-duplicated isolates were collected. As shown in a previous study,16K. pneumoniae (53.5%; 106/198) was the most frequent CPE species, followed by E. coli (19.2%; 38/198) and Enterobacter cloacae complex (11.1%; 22/198). Other CPE species such as Citrobacter freundii (4.5%; 9/198), Klebsiella oxytoca (4%; 8/198), Klebsiella aerogenes (3%; 6/198), Kluyvera spp. (3%; 6/198), Citrobacter koseri (0.5%; 1/198), Raoultella ornithinolytica (0.5%; 1/198) and Raoultella planticola (0.5%; 1/198) were sporadically detected, mainly during the first period. Conversely, the most frequent ESBL-producing microorganism was E. coli (78.5%; 573/730), followed by K. pneumoniae (17%; 124/730), while other ESBL-E species were occasionally detected (12 E. cloacae complex, 11 C. freundii, 4 K. oxytoca, 3 Enterobacter kobei, 2 C. koseri and 1 K. aerogenes). In order to analyze and compare the species distribution of both CPE and ESBL-E populations over time, four semesters within the R-GNOSIS study were defined (S1=March 2014–August 2014; S2=September 2014–March 2015; S3=April 2015–September 2015; and S4=October 2015–March 2016). CPE and E-ESBL species distribution and diversity are shown in Table S2 and Table S3, respectively. According to our previous study, the CPE species diversity decreased notably throughout the R-GNOSIS study (SDIS1=0.75, SDIS2=0.77, SDIS3=0.62 and SDIS4=0.43) (p=0.051). Changes in the species distribution were observed during the studied period, as a higher K. pneumoniae prevalence was found in the last part of the study, along with an increased E. coli population and a reduced incidence of other minority species. Conversely, species diversity among ESBL-E carriers remained virtually stable over time (SDIS1=0.29, SDIS2=0.36, SDIS3=0.41, and SDIS4=0.34) (p=0.9). Overall, changes in species distribution were not observed and ESBL-producing E. coli was the predominant species throughout all the study. Composition ratio of CPE and E-ESBL species by semesters and wards is shown in Fig. 3.
DiscussionDuring the R-GNOSIS study, the incidence of patients colonized with ESBL-E in our Hospital was 8.4%. Previous studies have reported a similar prevalence in Spain among healthy fecal carriers.2 The compromise between benefits and cost-effectiveness of CI for the containment of multidrug-resistant Enterobacterales is controversial and it has been suggested that it may be related to the local epidemiology.18 Some studies have demonstrated that transmission dynamics of ESBL and advantages of CI implementation in the hospital setting are dependent on the circulating species and clones.19 The R-GNOSIS Project was designed to evaluate the benefits of CI over SP in the prevention of ESBL-E acquisition in non-critical care wards across different European Hospitals. In this multicentre study, no association between the incidence density of ESBL-E ward-acquired and the implementation of CI was observed. Moreover, no differences in the acquisition of ESBL-producing E. coli and K. pneumoniae isolates between CI and SP strategies were found.15 In agreement with the general results obtained across all participant centers, effectiveness of CI measures in reducing the ESBL-E transmission in our Hospital was not demonstrated. Furthermore, species diversity and distribution among ESBL-E were not affected by the different intervention strategies and ESBL-E. coli (79%) predominance remained throughout all the study. This fact is consistent with the idea that ESBL-E. coli prevalence is not associated with a hospital transmission and that the reservoir of these microorganisms is more probably community associated. A recent work has confirmed that during the R-GNOSIS study, prevalence at admission of ESBL-E fecal carriers in our hospital was 7.7%, supporting the hypothesis of a community reservoir.20 Nevertheless, further research should be performed to investigate if the ESBL-K. pneumoniae population (17%) could have been acquired in our institution and could have been affected by the CI implementation, remained undetectable under a high prevalence of community ESBL-E. coli. In this respect, some studies have demonstrated a reduced transmission rate for ESBL-K. pneumoniae in the hospital setting after the proper implementation of control and prevention measures.14,21
On the other hand, in our Hospital, CPE incidence during the R-GNOSIS study was 2% and significant differences were found coinciding with the implementation or the withdrawal of the CI measures for ESBL-E carriers. It should be noted that during the last part of the study and coinciding with the withdrawal of the CI strategy, we detected an outbreak by NDM-1-K. pneumoniae producers in the Neurosurgery ward22 along with an increase of patients colonized with the OXA-48-ST11-K. pneumoniae high-risk clone.17 Although the R-GNOSIS project was not designed to evaluate the CPE transmission, our results indicate that the isolation of ESBL-E fecal carriers could have had a protective indirect effect on the acquisition of CPE. Several studies have demonstrated that prevention and infection control measures, particularly hand hygiene and CI, are a key factor preventing and containing outbreaks in both hospital and health care centers.23–27 In contrast to ESBL-E, efficiency of CI measures among CPE populations could be related to the nosocomial acquisition of these pathogens. It should be noted that according to our previous study, 56% of CPE cases were considered to be acquired during the R-GNOSIS studied period, while 27% were related to a previous admission in our hospital or in other health care centers.17 Moreover, although carbapenemase production was detected in a wide variety of Enterobacterales species, coinciding with other studies performed in Spain, the most frequent CPE was K. pneumoniae (54%).8–10 Additionally, an increased prevalence of E. coli (19%) was found in the last part of the study. These results may reflect the efficient penetration of carbapenemases among different members of the Enterobacterales order, as happened previously with the CTX-M enzymes.28–30 It should be noted that cross-species transmission of different carbapenemases, mainly OXA-48, from K. pneumoniae to E. coli was described in our Hospital during the R-GNOSIS Project.31
During the R-GNOSIS study, the main limiting factor in studying the effectiveness of the CI measures targeted to reduce the hospital transmission of ESBL-E, could be the high prevalence of community-acquired E. coli isolates. However, although further studies are required to support these findings, our work suggests that preventive isolation of colonized or infected patients with ESBL-E may also have an indirect containment effect on the nosocomial acquisition of CPE. A combination of control precautions for all patients colonized or infected with multidrug-resistant microorganisms, along with an optimal antimicrobial agent administration is needed to contain the dissemination of CPE from hospitals to the community.
FundingMH-G was supported with a contract from Instituto de Salud Carlos III of Spain (iP-FIS program, ref. IFI14/00022). The content and scientific background of this work was supported by European Commission (grants R-GNOSIS-FP7-HEALTH-F3-2011-282512) and Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0011) co-financed by European Development Regional Fund “A way to achieve Europe” (ERDF), Operative program Intelligent Growth 2014–2020.
Conflict of interestThe authors declare that they have no conflict of interest.
We thank Mrs. Mary Harper for English correction of the manuscript.