Lymphomatoid granulomatosis (LG) is a rare entity which was first reported in 1972 by Liebow et al.1 It is defined as a lymphoproliferative disorder caused by an Epstein-Barr virus-induced transformation of B-cells in a T-cell rich environment.
LG can first appear with a huge variety of non-specific symptoms. Pulmonary lesions are found in almost all patients with LG. Extrapulmonary disease without lung involvement is uncommon and a laryngeal primary kind is exceptional.
The case of a 34-year-old man, heavy smoker of 30 cigarettes a day and suffering from HIV-1 infection is reported. Five years before his admission to our hospital, he was diagnosed with HIV-1 infection, probably sexually transmitted, in context of pulmonary tuberculosis and was subsequently treated with a nine-month chemotherapy course for tuberculosis and Tenofovir-Lamivudine-Efavirenz. The tuberculosis was successfully cured with this treatment. Later, the HAART was changed to Zidovudine-Lamivudine-Nevirapine with poor disease control (30 CD4 cell count/μl and 20 copies HIV-1/ml).
In the previous two years, he was treated with Abacavir-Lamivudine-Lopinavir/ritonavir with discordant response (last test before admission: 138 CD4 cell count/μl and 20 copies HIV-1/ml). Throughout his illness, he has suffered from two episodes of community acquired pneumonia.
The patient was admitted to our centre because of a fever syndrome of unknown origin which he had suffered for several months, as well as a recent dysphonia. The endoscopic examination of the larynx found bilateral Reinke's edema. We did not find any cause to explain the fever.
Blood tests showed normochromic normocytic anemia and ESR 109mm/h. CD4 count cell was 124 cells/μl and viral load 32 copies/ml. Chest radiograph was normal. No evidence of renal disease was found. Blood and urine cultures, mycobacterium and parasites tests, cytomegalovirus PCR assay and latex-cryptococcus antigen detection were negative. Tumor markers levels were not raised. A liver biopsy revealed unspecific hepatic granulomas which were cultured, but no mycobacteria grew in Löwenstein-Jensen medium. Bone marrow aspiration found non-specific alterations in all blood cells, related to an unknown illness.
A cervical CT discovered a thickening of both vocal folds, compatible with Reinke's edema, but it extended into the subglottis and the normal sized laterocervical lymph nodes. Only laryngeal and cervical lymph nodes had values over their maximum standard in a PET/CT (SUV maximum 10.7).
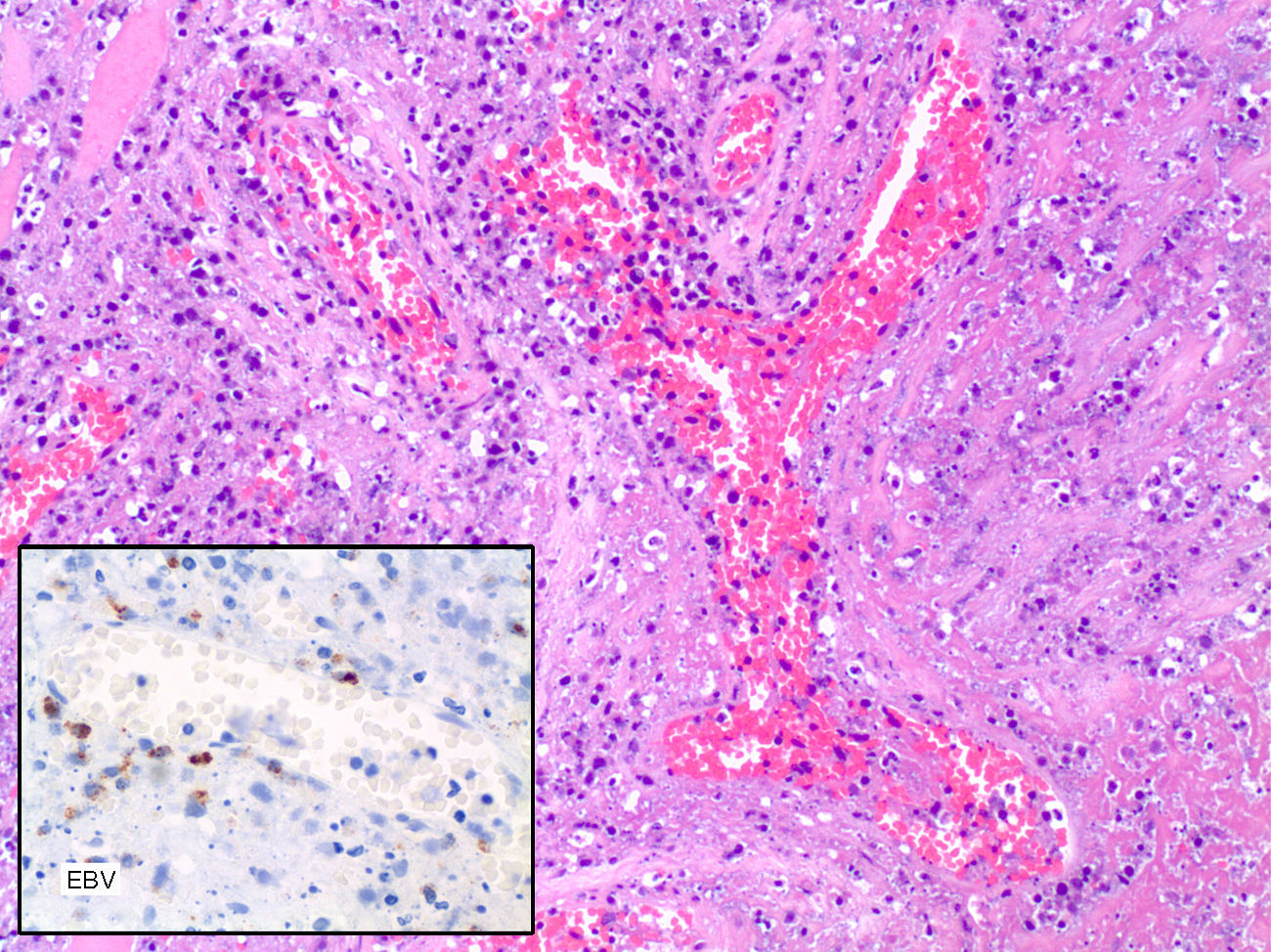
The tissue biopsy was reported as polymorph lymphoid proliferation related to post-transplant lymphoproliferative disorder. Neither T-cell nor B-cell clonality was found in the histochemical study and flow cytometry. Flow cytometry characteristics were CD1a negative, CD-3 negative, CD-5 negative, CD-10 negative, CD-20 negative, CD-30 positive in large cells, CD-68 positive in macrophages and cells surrounding vascular structures, CD-79a negative, CD-99 negative and CD-117-c-Kit negative. The EBER “in situ” hybridization was positive, pointing out the presence of EBV. Ziehl-Neelsen, P.A.S. and Congo red stains were negative.
No treatment was given after the first biopsy and the subglottic mass grew fast and aggressively: over three months, it infiltrated the para-laryngeal space and destroyed cricoid cartilage. This local progress caused recurrent episodes of massive bleeding. We started him on steroids 1mg/kg/day IV and cyclophosphamide 2mg/kg/day IV treatment and an urgent total laryngectomy was performed due to the imminent risk of fatal haemorrhage. Two weeks after surgery, a large pharyngeal fistula was developed, showing necrotic tissues. Our patient died six months after being hospitalized, due to a severe local bleeding.
The histopathological examination of larynx showed an atypical lymphoid infiltration containing large lymphoid cells surrounding vascular structures accompanied by extensive areas of necrosis, and was finally reported as lymphomatoid granulomatosis grade III according to the proportion of EBV positive B cells.
The immunohistochemical analysis showed positivity for EBV-LMP-1 and negativity for CD20. The Ki67 labelling index was high. The PCR analysis showed polyclonal expansion of T-cells with the TCR and IgH gene, and positivity for of EBV with histosonda EBER (Fig. 1).
A literature search with no date limits in PubMed using the keywords “lymphomatoid granulomatosis” and “larynx” found only two reported cases where LG affects the larynx, but in both of them, laryngeal disease was associated with pulmonary involvement.2,3 The larynx has its own lymphatic structure called larynx-associated lymphoid tissue (LALT). In the subglottis, LALT is replaced throughout life by a diffuse infiltration of strong intensity consisting predominantly of CD3-positive T lymphocytes with scattered CD20-positive B cells,4 therefore LG could be originated in subglottic tissues.
LG pathogenesis is unclear, but it has been linked to EBV and immunodeficiency. As regards the close to 100% EBV association with LG and the presumed wide expression of EBV latent encoded proteins, it has been strongly inferred that EBV is not just an innocent bystander in the pathogenesis of LG.5 When dealing with immunodeficiency, it has been proven that most patients with LG have defects in cytotoxic T cell function. That would explain how LG is less rare in many immunodeficiency states.6 In our case, CD4 count cell was 124 cell/μl due to a good treatment compliance with discordant response.
Many studies have analyzed the connection between LG and AIDS. HIV infection is associated with an increased risk of lymphomas by 60-165 fold even in the combined antiretroviral therapy era. Excellent outcomes with infusion therapy and concurrent rituximab have been reported in the treatment of some of them.7
We report that laryngeal LG can mimic Reinke's edema in its early stages. In our own experience, Reinke's edema should not be treated as a casual finding in patients with fever of unknown origin or immunodeficiency.
Our current case was classified as grade III of LG, which is histologically considered as diffuse large B-cell malignant lymphoma,8 but the first biopsy was reported as polymorph lymphoid proliferation related to a post-transplant lymphoproliferative disorder (PTLD), which can simulate a lower grade of LG. LG and PTLD are associated with immunodeficiency and are driven by EBV. Histopathologically, they present a morphological spectrum spanning polymorphic through monomorphic lymphoid proliferations.9 The distinctness of LG and PTLD has been emphasized by the difference in the immune response. While LG has a large population of background T-cells, PTLD is recognized by a poor T-cell enviroment.5
A better awareness of LG in recent years is at present allowing new therapeutic tools for this disease to be developed. Rituximab, a new monoclonal antibody anti-CD20 has shown promising results in some cases of LG with pulmonary involvement.10