This study reviews our experience in bisphosphonate-associated jaw osteomyelitis (BJOM), focusing on the incidence, etiology, treatment, and long-term outcome.
MethodsRetrospective review of the clinical histories adult patients diagnosed with BJOM (1995–2008) in a tertiary hospital.
ResultsBJOM was found in 30 of 132 (22.7%) consecutive patients with jaw osteomyelitis. The percentage of BJOM cases increased from 8.7% (4/46) in 1995–2005 to 30.2% (26/86) in 2005–2008. Symptoms appeared in a median of 2.5 years after intravenous use, and 4.5 years after oral exposure. Viridans group streptococci were isolated in 83.3% of cases. Actinomyces spp. was found in 16 (39.0%) of 41 bone histologies. All included patients received a median of 6 months of appropiate antibiotic therapy and a surgical procedure (debridament and/or sequestrectomy). Thirteen of 27 cases (48.1%) with long-term follow-up (median 22 months, IQR 25–75 17–28) failed. Clinical failure defined as, persistent infection or relapse, was more frequent in patients receiving intravenous than oral bisphosphonates (11/16 [68.8%] vs. 2/11 [18.2%]; P < .05) and in cases with Actinomyces spp. (7/10 [70.0%] vs6/17 [35.3%]; P = .08).
ConclusionsBisphosphonate therapy is now a frequent cause of JO. BJOM is difficult to cure and relapses are common, particularly in patients exposed to intravenous bisphosphonates.
Analizar la incidencia, la etiología, el tratamiento y la evolución clínica a largo plazo de la osteomielitis maxilar (OM) asociada al tratamiento con bifosfonatos (OMAB).
MétodosEstudio retrospectivo de pacientes adultos con diagnóstico de OMAB (1995-2008) en un hospital universitario.
ResultadosFueron diagnosticadas 30OMAB de un total de 132OM. Desde el año 1995 al 2004 fueron diagnosticadas 4OMAB de 46OM (8,7%), y desde el año 2005 al 2008, 26 de 86 (30,2%). Los síntomas de osteomielitis aparecieron en una mediana de 2,5años en los pacientes que recibieron el tratamiento con bifosfonatos por vía intravenosa y una mediana de 4,5años en los pacientes que lo recibieron por vía oral. En el 83,3% se aislaron Streptococcus del grupo viridans. En 16 (39%) de 41muestras enviadas para estudio histológico se constató la presencia de Actinomyces spp. Todos los pacientes fueron sometidos a desbridamiento quirúrgico y/o secuestrectomía y recibieron una mediana de 6meses de tratamiento antibiótico. Trece de los 27casos (48,1%) con seguimiento a largo plazo (mediana 22meses, IQR25-75 17-28) presentaron fracaso terapéutico. Estos fueron más frecuentes en pacientes que recibieron bifosfonatos por vía intravenosa en comparación con los que los recibieron por vía oral (11/16 [68,8%] vs 2/11 [18,2%], p<0,05) y en los casos con Actinomyces spp. (7/10 [70,0%] vs 6/17 [35,3%], p=0,08).
ConclusionesActualmente el tratamiento con bifosfonatos es causa frecuente de OM. Las recidivas son frecuentes en las OMAB, especialmente en pacientes expuestos a los bifosfonatos por vía intravenosa.
Bisphosphonate-associated osteomyelitis of the jaw (BJOM) is a relatively common side effect of bisphosphonate therapy.1–7 BJOM is the result of mandibular bone infection secondary to bone exposure due to bisphosphonate-associated osteonecrosis.
More than 90% of the cases are associated with intravenous therapy, with an estimated risk in the range of 2% to 10%.1–7 Less commonly, BJOM is associated with prolonged oral bisphosphonate therapy, with a current risk of 0.001–0.1%.8 Bone infection is a common complication in advanced stages of this condition. Although several studies have been published on this subject, they have mainly focused on the initial clinical features and risk factors of the disease. Little information is available on the microbiology and antimicrobial susceptibility of the isolated microorganisms or the findings at long-term follow-up.2,6,7,9,10
Our clinical experience suggests that patients with BJOM are difficult to treat. Furthermore, the bone can be infected with microorganisms resistant to conventional antibiotics used for the treatment of maxillofacial infections. The purpose of this study was to review our clinical experience in patients with BJOM, focusing on the etiology, antimicrobial resistance features and long-term outcome of this condition.
Patients and methodsWe retrospectively reviewed the medical records of all consecutive adult patients with jaw osteomyelitis (JO) diagnosed at Vall d’Hebron Hospital from January 1995 to December 2008, focusing on those cases associated with bisphosphonate use. Our hospital is a 1250-bed tertiary referral center for complicated maxillofacial surgical patients. Patients were identified from the Infectious Diseases Department's JO registry. All patients diagnosed with BJOM were evaluated and followed-up by the same Infectious Disease Department staff member.
The diagnosis of bisphosphonate jaw osteonecrosis was established according to the report of a task force of the American Society for Bone and Mineral Research,11 which includes current or previous treatment with bisphosphonate, exposed bone in the maxillofacial region persisting for >8 weeks, and no history of radiation therapy to the jaws.
The diagnosis of JO was established according to published criteria12: (a) presence of a compatible clinical picture with purulence, fistula, or recurrent abscess; (b) consistent imaging findings on plain radiographs, and/or computed tomography, and/or increased uptake on triphasic technetium bone scan; and (c) a histological picture consistent with osteomyelitis, and/or a positive leukocyte bone scan. Microorganisms were isolated in samples of excised bone obtained by surgery, percutaneous bone biopsy samples, or pus aspiration from adjacent tissues. Only Streptococcus viridans isolated of bone and pus aspiration samples were considered evaluable. Samples were cultured as previously described.12 Streptococci intermediately resistant to penicillin (0.2–2mg/L) were considered resistant. Appropriate antibiotics were given according to culture findings, antibiotic allergy history, and susceptibility studies.
We collected demographic data; underlying medical illnesses, particularly osteoporosis and neoplastic disease; clinical features; type and duration of bisphosphonate therapy and previous antibiotic therapy used for BJOM; radiological and histological findings; bacteriologic cultures; treatment and outcome. To assess the effectiveness of antibiotic and surgical therapy, only patients followed-up for at least one year to detect relapses after completion of antibiotic therapy were considered. Clinical failure was established on persistent clinical signs of infection (purulence) or relapse. Relapse was defined as recurrence of clinical signs of osteomyelitis (purulence, new fistula or abscess) and/or recurrence of pain, plus a new positive leukocyte bone scan or new bone sequestrum on imaging study, plus isolation of a microorganism in bone or pus aspiration samples.
All cases were classified according to the AAOMS staging system for BJOM,22 as stage II (evidence of infection) or stage III (evidence of infection and at least one of the following criteria: pathologic fracture, extra-oral fistula, or osteolysis extending to the inferior border). None of the cases were classified in stage I, since this stage applies to patients with bone exposure without signs of infection.
Quantitative variables are reported as the median and interquartile range25–75 (IQR). The chi-square test (or Fisher's exact test when appropriate) was used to compare the distribution of categorical variables. Differences were considered significant at a P-value of <0.05. Statistical analyses were performed with Microsoft SPSS-PC+, version 15.0 (SPSS, Chicago, IL, USA).
ResultsDuring the study period, 30 of 132 (22.7%) consecutive cases of JO were found to associate with bisphosphonate use. Twenty-five (83.3%) were women, and median age was 69 years (IQR 57.5–75.0 years). The percentage of BJOM cases increased from 8.7% (4/46) in the 1995–2005 period,12 to 30.2% (26/86) during 2005–2008. Twenty-eight patients (93.3%) had mandibular involvement (one had bilateral lesions) and two had maxillary involvement. There was a history of diabetes mellitus in 3, smoking in 3 and alcoholism (>100g/day) in one. The 30 patients suffered a total of 44 episodes of JO.
Bisphosphonate therapyBisphosphonates were administered intravenously for metastatic neoplastic disease in 19 (63.3%) patients (breast cancer in 13, multiple myeloma in 3, prostate carcinoma in 2, and renal carcinoma in 1); zoledronate was used in 18 and pamidronate in 1 patient. In the remaining 11 patients, bisphosphonates were administered orally for osteoporosis (alendronate: 8 and risedronate: 3). The median duration of intravenous bisphosphonate therapy was 2.5 years (IQR 1.5–4 years), whereas the median of oral exposure was 4.5 years (IQR 4.5–5.0 years). One patient who additionally received an antiangiogenic agent (sorafenib) for underlying neoplastic disease developed BJOM after only 6 months of intravenous bisphosphonate therapy.
Clinical featuresClinical features of JO were present for a median of 4.5 months (IQR 2–10.5 months). The most common symptoms were pain (100%) and intraoral exposed necrotic bone was observed in 30 cases, followed by external swelling in 22, sinus formation in 6 and chin paresthesia in 6. In 12 patients (40%), the symptoms appeared after a recent (<1 month) tooth extraction, with non-healing of the extraction site.
All patients had prior exposure to one or more cycles of antibiotics, for short periods (less than 3 weeks), for relapsing maxillofacial dental infection; twenty-one had received a beta-lactam, 14 clindamycin, and 5 other antibiotics (levofloxacin, azithromycin) before the microbiological diagnosis of BJOM was established.
Radiological findingsMaxillary computed tomography was performed in all cases and findings were consistent with osteomyelitis in all patients. Bone sequestrum formation was observed in 25 (83%) patients, and 3 had pathologic fractures. According to the AAOMS, 18 (60%) patients were classified as having stage II and 12 patients as having stage III BJOM. Leukocyte bone scanning showed increased uptake in all 18 patients in whom it was performed.
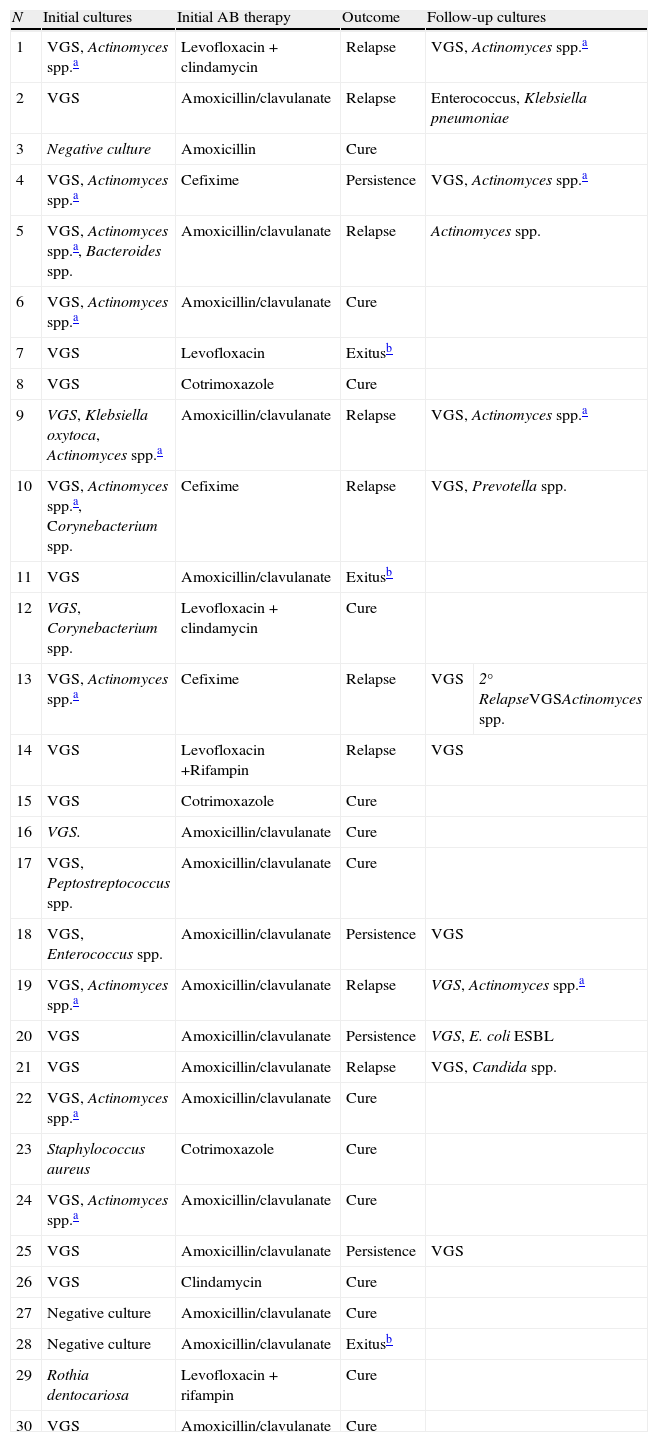
MicrobiologyAmong the 30 cases studied, the infection was monomicrobial in 14 (47%) patients and polymicrobial in 13 (Table 1). Viridans group streptococci (VGS) were the most commonly isolated agents (83.3%). On bone histological study, Gram-positive bacilli suggestive of Actinomyces spp. were observed in 10/30 cases. Overall, initial episode plus further biopsies of clinical failures, in 16/41 (39%) episodes, the histological findings were suggestive of Actinomyces spp. infection (Table 1). All three cases of culture negative BJOM had clinical and radiological signs compatible with JO and a histological picture suggestive of osteomyelitis. All 3 patients had received previous antibiotic therapy, which may have negativized the culture results.
Etiological agents, initial antibiotic therapy, and outcome from 30 patients with BJOM.
| N | Initial cultures | Initial AB therapy | Outcome | Follow-up cultures | |
| 1 | VGS, Actinomyces spp.a | Levofloxacin+clindamycin | Relapse | VGS, Actinomyces spp.a | |
| 2 | VGS | Amoxicillin/clavulanate | Relapse | Enterococcus, Klebsiella pneumoniae | |
| 3 | Negative culture | Amoxicillin | Cure | ||
| 4 | VGS, Actinomyces spp.a | Cefixime | Persistence | VGS, Actinomyces spp.a | |
| 5 | VGS, Actinomyces spp.a, Bacteroides spp. | Amoxicillin/clavulanate | Relapse | Actinomyces spp. | |
| 6 | VGS, Actinomyces spp.a | Amoxicillin/clavulanate | Cure | ||
| 7 | VGS | Levofloxacin | Exitusb | ||
| 8 | VGS | Cotrimoxazole | Cure | ||
| 9 | VGS, Klebsiella oxytoca, Actinomyces spp.a | Amoxicillin/clavulanate | Relapse | VGS, Actinomyces spp.a | |
| 10 | VGS, Actinomyces spp.a, Corynebacterium spp. | Cefixime | Relapse | VGS, Prevotella spp. | |
| 11 | VGS | Amoxicillin/clavulanate | Exitusb | ||
| 12 | VGS, Corynebacterium spp. | Levofloxacin+clindamycin | Cure | ||
| 13 | VGS, Actinomyces spp.a | Cefixime | Relapse | VGS | 2° RelapseVGSActinomyces spp. |
| 14 | VGS | Levofloxacin +Rifampin | Relapse | VGS | |
| 15 | VGS | Cotrimoxazole | Cure | ||
| 16 | VGS. | Amoxicillin/clavulanate | Cure | ||
| 17 | VGS, Peptostreptococcus spp. | Amoxicillin/clavulanate | Cure | ||
| 18 | VGS, Enterococcus spp. | Amoxicillin/clavulanate | Persistence | VGS | |
| 19 | VGS, Actinomyces spp.a | Amoxicillin/clavulanate | Relapse | VGS, Actinomyces spp.a | |
| 20 | VGS | Amoxicillin/clavulanate | Persistence | VGS, E. coli ESBL | |
| 21 | VGS | Amoxicillin/clavulanate | Relapse | VGS, Candida spp. | |
| 22 | VGS, Actinomyces spp.a | Amoxicillin/clavulanate | Cure | ||
| 23 | Staphylococcus aureus | Cotrimoxazole | Cure | ||
| 24 | VGS, Actinomyces spp.a | Amoxicillin/clavulanate | Cure | ||
| 25 | VGS | Amoxicillin/clavulanate | Persistence | VGS | |
| 26 | VGS | Clindamycin | Cure | ||
| 27 | Negative culture | Amoxicillin/clavulanate | Cure | ||
| 28 | Negative culture | Amoxicillin/clavulanate | Exitusb | ||
| 29 | Rothia dentocariosa | Levofloxacin+rifampin | Cure | ||
| 30 | VGS | Amoxicillin/clavulanate | Cure | ||
BJOM: bisphosphonate-related jaw osteomyelitis, AB: antibiotic, VGS: Viridans group streptococci, ESBL: extended-spectrum beta lactamases.
Antimicrobial susceptibilities of VGS are shown in Table 2. Only 35% of the isolates were susceptible to erythromycin studied and 52.9% to clindamycin. All nonsusceptible clindamycin streptococcal strains were nonsusceptible to erythromycin, which suggests an erm-encoding MLS resistance. All patients infected with nonsusceptible VGS strains except one had been previously exposed to the same antibiotic.
Antimicrobial susceptibilities of viridans group streptococci.
| Antibiotic | N studied | N susceptible (%) |
| Penicillina | 34 | 29 (85.3%) |
| Clindamycin | 34 | 18 (52.9%) |
| Erythromycin | 34 | 12 (35.3%) |
| Rifampin | 34 | 34 (100%) |
| Trimethoprim-sulfamethoxazole | 34 | 28 (82.4%) |
aStreptococci intermediately resistant to penicillin (0.2–2mg/L) were considered resistant.
Once BJOM was diagnosed, bisphosphonates were stopped in all patients.
All patients had been previously treated conservatively with antibiotic therapy and clorhexidine rinses.
Definitive antibiotic therapy was adjusted to surgical culture results. It was administered orally in all patients except one, who additionally had an extended-spectrum beta-lactamase Escherichia coli infection, and was treated with 8 weeks intravenous ertapenem therapy. The initial antibiotic therapy administered in the 30 cases is shown in Table 1.
Patients with Actinomyces spp. infection were treated orally for a median of 6 months (IQR 3–6 months) with a beta-lactam antibiotic (amoxicillin or amoxicillin–clavulanate).
The length of antibiotic therapy ranged from 2 to 13 months, median 6 months. The duration of antibiotic therapy was similar for stage II and III BJOM episodes.
Hyperbaric oxygen therapy was applied in one patient who presented a BJOM relapse, with unsuccessful results.
Surgical therapyAll 30 patients underwent at least one surgical procedure. Surgery ranged from debridement to marginal resections. Sequestrectomy was performed initially in 19/25 (76%) patients with bone sequestra. Twelve patients (5 in which a sequestrum had not been removed initially, 2 in which a sequestrum developed during the follow-up and in 5 in which a new sequestrum was observed during the follow up) additionally required sequestrectomy; thus, sequestrectomy was performed in 27/30 (90%) patients and overall, in 31/44 (70.5%) episodes. Primary surgical closure of bone exposure was carried out in 4 cases (all treated during the last study year) and with no relapses, and secondary closure was performed in a second surgical procedure in 5 cases.
Follow-upAmong the 30 patients studied, 3 died due to the underlying neoplastic disease before completion of antibiotic therapy, and 27 had a long-term follow-up, median 22 months (IQR 17–28). Clinical failure occurred in 13 (48.1%). Four had persistent infection and 9 experienced BJOM relapse (1 relapse in 8 patients and 2 relapses in 1), requiring further antibiotic therapy and surgery (mean, 2.24 surgical procedures). The median time to relapse was 4.5 months (IQR 2–9 months). Patients without relapse were followed-up for a median of 26 months (IQR 19.7–44.7 months) after stopping antibiotic therapy. Among the 13 clinical failures, the same microorganism (VGS cases had the same antibiotic susceptibility pattern) was isolated in the second culture in 8 (61.6%) patients, and 5 were considered reinfections. In 6/13 (46.2%) clinical failures, Actinomyces spp. was observed on the second bone histological study.
Clinical failure (persistent infection or relapse) was more common in patients exposed to intravenous bisphosphonates (11/16, 68.8%) than in those with oral phosphonate therapy (2/11, 18.2%) (P=0.05). Clinical failure rates were 54.5% (6/11) in stage III disease and 43.8% (7/16) in stage II (P=NS). Of the 25 patients who initially had a bone sequestrum, clinical failure occurred in 3/6 cases (50%) in which bone sequestra were not removed and in 5/19 (26.3%) in which sequestra were removed (P=NS). Clinical failure was documented in 7/10 cases (70%) with Actinomyces spp. microorganisms on the first histological study versus 6/17 (35.3%) without these findings (P=0.08). Clinical failure had no relation with the duration of the antimicrobial therapy.
DiscussionThe results of our study show that bisphosphonate therapy is now a common cause of JO. In our earlier experience (1993–2005), 8% of JO cases were associated with this therapy,12 whereas our current data (2005–2008) show that one-third of cases are related to this treatment. Furthermore, this drug-related complication is difficult to cure: the failure rate is 48.1%, a percentage considerably higher then the 5% observed in our general series of JO.12
This drug-related side effect has been particularly associated with intravenous administration, with a risk as high as 10%.1–7 Nowadays, use of oral nitrogen-containing bisphosphonates accounts for 5–10% of cases of BJOM. Higher periods of drug exposure are needed in oral therapy. In our study, the median time of oral exposure before the development of BJOM was 4.5 years, a value similar to previously reported data (4.4 years),8 and the median time of intravenous exposure was 2.5 years.
Although the potency, dose and length of bisphosphonate exposure are the most important risk factors for developing BJOM,2–6 it has been suggested that other factors, such as traumatism, poor dental hygiene, smoking, chemotherapeutic drugs, and corticosteroids, might have a role in the development of the disease.3,11
In vitro and animal studies have shown that bisphosphonates also inhibit angiogenesis.14 In one patient of our series who was under treatment with an antiangiogenic agent, clinical symptoms of BJOM appeared after only 6 months of intravenous bisphosphonate exposure. A higher than usual incidence (18.3%) of jaw osteonecrosis has recently been reported in metastatic prostate cancer treated with bisphosphonates and docetaxel, prednisone and 2 antiangiogenic agents such bevacizumab and thalidomide, which suggests that antiangiogenic therapy may enhanced the effects of bisphosphonates on avascularization.15 In this situation, close follow-up may enable earlier detection of jaw osteonecrosis and facilitate a prompt intervention.
Viridans group streptococci were the most commonly isolated microorganisms with a percentage 75.6% that was similar to the 83.3% of our previous global series of JO.12 The high incidence of clindamycin-resistant strains (half the cases) and the resistance to penicillin in some cases (15%), which we also observed in other types of JO,12 are likely related to previous therapy with these antibiotics. These findings argue against empirical antibiotic therapy and suggest that antimicrobial susceptibility tests are needed.
Actinomyces-like organisms were observed on histological study in 33% (10/30) of the patients, a percentage quite higher that the 7.3% (3/46%) in our global series of JO12; however, the microorganism could not be isolated on culture. These findings are consistent with recent observations of a nearly universal presence of this pathogen on histological study, with few cases of isolation on culture.1,9,16–20 Isolation of this pathogen is likely difficult, particularly in patients who have previously received antibiotic therapy.16 Given the low yield of culture, molecular procedures such as polymerase chain reaction (PCR) testing of decalcified bone specimens may prove to be a useful alternative for identification of Actinomyces spp.17 This can have therapeutic implications, since high doses of antimicrobials and a prolonged course (6 months or longer) are required to cure the disease.16,18 The higher incidence of Actinomyces spp. found in cases of JO associated with bisphosphonate therapy could be due to a higher anaerobic environment secondary to the avascular jaw ostenecrosis caused by bisphosphonates which may predispose to the growth of this microorganism.
Even though bisphosphonates were stopped once BJOM had been diagnosed, specific antibiotics were administered according to susceptibility testing, and aggressive surgery with sequestrectomy was performed in most cases, BJOM relapsed in half our patients, particularly those with advanced disease (stage III) and those in whom bone sequestra were not removed. Management of this disease is difficult. Some authors have advocated an aggressive surgical approach with extensive bone resection and sequestrectomy,2,3,13,21,22 whereas others have proposed a more conservative approach particularly for stages I and II.3–6,9,23,24 With the currently available data, it is difficult to establish the ideal surgical approach. Although our results are not conclusive, our data showing clinical failure in 50% of patients without bone sequestrum removal versus 26% with removal, and data from a recent German multicenter study in 78 patients, reporting response in only 38% of the conservative group versus 86% in the radical bone resection group, favor a more aggressive attitude, at least in advanced stages of the disease. In a recent study the extent of radiographic appearance, surgical therapy vs conservative treatment, extensive surgical treatment and number of debridements were associated with lower recurrence rates.25 Because of our high failure rate, the surgical team has attempted to close the bone exposure in the most recently treated patients. Although, our experience in this line is limited, the long-term follow-up in four cases treated with this approach has indicated success. Attempts to close the exposed area or cover it with a soft vinyl splint may be useful to avoid super-infection,1 which occurred in 38.5% of our clinical failures.
The role of hyperbaric oxygen and ozone therapy for this condition, which in one small study seemed to show some benefit,26 should be established in prospective studies.
Our study has some limitations. As in all retrospective studies, there is a potential for flaws because of bias and statistical imprecision. The lack of data on bisphosphonate use in our hospital and the fact that we do not have data on how many cases were referred from other centers hinders an analysis of the true incidence of this drug side effect. Some variables such as dental care and the administration of antineoplasic antiangiogenic agents were not systematically recorded; however, data on type and length of bisphosphonate therapy were collected in all cases by the same infectious disease staff members. Furthermore, Actinomyces spp. was not routinely investigated by prolonged incubation in microbiological cultures or by PCR analysis; hence the incidence of this microorganism may be underestimated.
ConclusionIn conclusion, bisphosphonate therapy is now a common cause of JO. Even with specific antimicrobial therapy and surgery, the disease is difficult to cure and relapses are common, especially in patients exposed to intravenous bisphosphonates, those with stage III disease, and cases involving actinomycosis. Further studies focusing on the degree o aggressiveness required in surgery and the role of Actinomyces spp. in this condition are needed.
Conflict of interestAuthors have no conflict of interest to declare.
We thank Celine Cavallo for English language editing.