Although the incidence of tuberculosis (TB) has declined, TB drug resistance remains a major problem. The TB rate in Gipuzkoa (northern Spain) is higher than the European average. The objective of this study was to determine the antimicrobial susceptibility of 1855 Mycobacterium tuberculosis complex isolates (94.5% of confirmed cases between 2000 and 2015).
MethodsSusceptibility testing was performed using the agar proportion method and a commercial broth system (MGIT 960). In isoniazid- or rifampicin-resistant strains, we studied genetic determinants of drug resistance and genotype (MIRU-VNTR).
ResultsThe annual mean incidence of TB was 24.5 cases per 100,000 population on average, and tended to decrease. The multidrug-resistant TB rate was 0.5% (9/1855), and no extensively drug-resistant TB strains were detected. Rates of resistance to isoniazid and rifampicin were 3.9% (range, 3.4–4.3%) and 0.6% (range, 0.4–1.4%), respectively. TB resistance was more common among foreign-born individuals and those who had received previous TB treatment. Genotyping of 102 resistant strains showed predominance of the Euro-American lineage, although 4/9 multidrug-resistant strains had Eastern lineages (2 East African-Indian, and 2 East Asian [Beijing]).
ConclusionsIn Gipuzkoa, with a moderate incidence of TB, resistance was very low, mostly being detected among individuals who were born abroad or who had a history of TB treatment.
Aunque la incidencia de tuberculosis (TB) está descendiendo, la resistencia a fármacos antituberculosos continúa siendo un grave problema. Gipuzkoa, norte de España, tiene una tasa de TB superior a la media europea. El objetivo de este trabajo fue estudiar la sensibilidad a los antibióticos de 1.855 aislamientos de Mycobacterium tuberculosis complex (94,5% de los casos confirmados entre 2000 y 2015).
MétodosEl estudio de susceptibilidad se realizó mediante el método de las proporciones en agar, y mediante dilución en caldo (sistema MGIT 960). En las cepas resistentes a isoniacida o rifampicina se analizaron determinantes genéticos de resistencia y el genotipo (MIRU-VNTR).
ResultadosLa incidencia media anual de TB fue de 24,5 casos por 100.000 habitantes, con una tendencia decreciente. La tasa de multirresistencia fue del 0,5% (9/1.855), y no se detectaron cepas con resistencia extrema. Las tasas de resistencia a isoniacida y rifampicina fueron del 3,9% (rango: 3,4-4,3%) y 0,6% (rango: 0,4-1,4%), respectivamente. La TB resistente fue más frecuente entre las personas nacidas en el extranjero y entre los que recibieron tratamiento antituberculoso previo. El genotipado de 102 cepas resistentes mostró predominio del linaje Euro-Americano, aunque 4/9 cepas multirresistentes pertenecieron a linajes orientales (2 East African-Indian, y 2 East Asian [Beijing]).
ConclusionesEn Gipuzkoa, con una incidencia moderada de TB, la resistencia fue muy baja, y asociada especialmente a personas nacidas en el extranjero o que recibieron tratamiento antituberculoso previo.
Tuberculosis (TB) is a public health problem worldwide, exacerbated by a growing resistance to TB drugs. It has been estimated that in 2016, 4.1% of new cases of TB and 19% of previously treated cases were resistant to rifampicin (RR-TB) or multidrug-resistant (simultaneous resistance to rifampicin and isoniazid, MDR-TB).1 In 2015, the World Health Organisation (WHO) started the End TB Strategy, with the objective of ending the global TB epidemic by 2035. One of the cornerstones of this programme is early diagnosis of TB including universal drug-susceptibility testing (DST).2
Spain is among the countries in the European Union with the highest incidence of TB (10.8 cases per 100,000 people in 2014),3,4 and, historically, the Basque Country has been one of the most affected regions, with a higher incidence than Spain as whole.4 The objective of this study was to determine the antimicrobial susceptibility of the strains of Mycobacterium tuberculosis complex causing episodes of TB between 2000 and 2015 in Gipuzkoa, a province of the Basque Country. We also examined demographic factors and genetic determinants of the microorganisms associated with resistance, as well as the genotypes of the resistant strains.
2Materials and methods2.1Tuberculosis surveillance in GipuzkoaBetween 2000 and 2015, the mean total population of Gipuzkoa was 694,857 (range, 673,563–702,897), of whom 89,091 were under 15 years old and 32,608 under 5 years old. The mean foreign-born population was 4.8% (1.4% at the start of the study, rising to 6.8% in 2015).5
The TB registry was managed by the Gipuzkoa Epidemiology Unit, under the Programme for the Control and Surveillance of Tuberculosis in the Basque Country.6 This is an active surveillance programme that has covered Basque public and private health care since 1995. It uses the definitions of possible, probable and confirmed cases approved by the European Union Member States.7
The following demographic and epidemiological data were collected for all the patients: age, sex, country of origin, current place of residence, TB treatment for 1 month or more in the past, and date of initiation of TB treatment for the current episode. The registry also includes cases of people living in Gipuzkoa who had their disease diagnosed and microbiologically confirmed in other Spanish regions.
2.2TB drug resistance analysisThe study was conducted by the Microbiology Department of Donostia University Hospital (Gipuzkoa's TB Reference Laboratory). This laboratory receives all the isolates of M. tuberculosis from hospitals of the public health network in Gipuzkoa, which receive more than 95% of cases of TB in this province, and the few isolates obtained in private hospitals.
To analyse M. tuberculosis susceptibility, we considered the results from only one strain per patient, except in cases in which there were isolates obtained at least 1 year apart, or when the different isolates showed a distinct susceptibility pattern. To assess susceptibility to first-line drugs (isoniazid, rifampicin, streptomycin and ethambutol), we used the proportion method8 and, from 2009 onwards, complemented this with a commercial broth method, the BACTEC MGIT 960 system (BD; Becton, Dickinson and Company, Sparks, MD, USA). Briefly, the antibiogram was obtained with the proportion method using Middlebrook 7H11 culture medium plates, with antibiotic test discs (BD BBL; Becton, Dickinson and Company, Sparks, MD, USA) embedded in the culture medium onto which we inoculated two dilutions (10−2 and 10−4) of a suspension of a pure culture of the microorganism (McFarland 0.5–1). The final concentrations of the drugs in the medium were as follows: isoniazid, 0.2μg/mL and 1μg/mL; rifampin, 1μg/mL; streptomycin, 2μg/mL and 10μg/mL; and ethambutol, 5μg/mL. The plates were incubated for 21 days at 35°C in a CO2-enriched atmosphere (5% CO2).
The growth of colonies in the presence of an antibiotic, greater than 1% respect the inoculum control was considered to indicate resistance to the antibiotic. The BACTEC MGIT 960 system for testing susceptibility was used following the manufacturer's instructions, including pyrazinamide as well as the four aforementioned drugs. The final concentrations of the drugs in the medium were: isoniazid, 0.1μg/mL; rifampicin, 1μg/mL; streptomycin, 1μg/mL; ethambutol, 5μg/mL; and pyrazinamide, 100μg/mL. The TB H37Rv strain, that is susceptible to all the antibiotics tested, was used as a reference. Susceptibility of MDR-TB strains to second-line drugs (kanamycin, amikacin, capreomycin, ofloxacin, ethionamide and cycloserine) was assessed in the National Microbiology Centre (Madrid, Spain).
In the case of isoniazid-resistant or rifampincin-resistant strains, we investigated possible genetic determinants of this drug resistance. For this purpose, we used the GenoType MTBDRplus VER 2.0 line probe assay system (Hain Lifescience GmbH, Nehren, Germany), according to the manufacturer's instructions. This system analysed mutations in the rpoB gene (associated with resistance to rifampicin) and in the katG gene and the promoter region of the inhA gene (associated with resistance to isoniazid).
2.3M. tuberculosis complex species identification and genotypingFrom 2009 onwards, systematic genetic testing was carried out to identify the M. tuberculosis complex species, using the GenoType MTBC line probe assay system (Hain Lifescience GmbH, Nehren, Germany), according to the manufacturer's instructions. Identification was also carried out on selected isolated strains during the first period, either in our laboratory or in the National Microbiology Centre. We analysed the genotypes of the resistant strains using 24-loci mycobacterial interspersed repetitive-unit-variable-number tandem repeat (MIRU-VNTR) typing.9 Genotypic lineages (clades or families) were obtained from the MIRU-VNTRplus web application.10
2.4Statistical analysisThe incidence of TB was calculated based on the number of TB cases recorded in Gipuzkoa divided by the number of people living in this region according to the official census records 2000–2015.5 We analysed the relationship of isoniazid resistance and multi-resistance with the demographic and epidemiological data collected for the cases, using the chi-squared test. The variables which obtained a p<0.05 in the univariate analysis were entered into a multivariate analysis with a logistic regression model (enter method), and we calculated adjusted odds ratios (ORs) and corresponding 95% confidence intervals to identify the variables that predicted resistance. We examined changes in the rate of resistance during the period with a Chi-square test for linear trend. All the statistical analysis was performed using IBM SPSS, Statistics for Windows, Version 21.0.
3Results3.1Tuberculosis surveillance in GipuzkoaA total of 2724 cases of TB were registered between 2000 and 2015, of which 1697 were in men. Overall, 762 cases were classified as possible or probable (28%) and 1962 were microbiologically confirmed (72%). The average annual incidence of TB during the study period was 24.5 cases per 100,000 people, with the highest incidence being registered in 2000 (36.1 cases per 100,000), and a progressive decline to 2015 (13.7 cases per 100,000). The median age of patients was 41 years old, with a range of 0–99. During the study period, there were 66 cases in under-15-year-olds, 27 of these being in children under 5 years of age (incidence rates of 4.3 and 6 cases per 100,000 people, respectively).
3.2Drug resistance analysisAntimicrobial susceptibility was tested in 94.5% (1855/1962) of the confirmed cases (1147 men, 61.8%, and 708 women, 38.2%). This testing was performed in the laboratory of Donostia University Hospital in 1710 cases (92.2%), and in other regions in the other 145 cases (strains from these laboratories not being available for additional testing). A total of 99 patients (5.3%) had received previous TB treatment and 250 (13.5%) had been born outside Spain.
Identification of M. tuberculosis complex species led to the identification of M. tuberculosis (670 strains), Mycobacterium bovis ssp. bovis (31 strains), M. bovis ssp. caprae (2 strains) and Mycobacterium africanum (2 strains). The other 1150 strains were classified as M. tuberculosis complex.
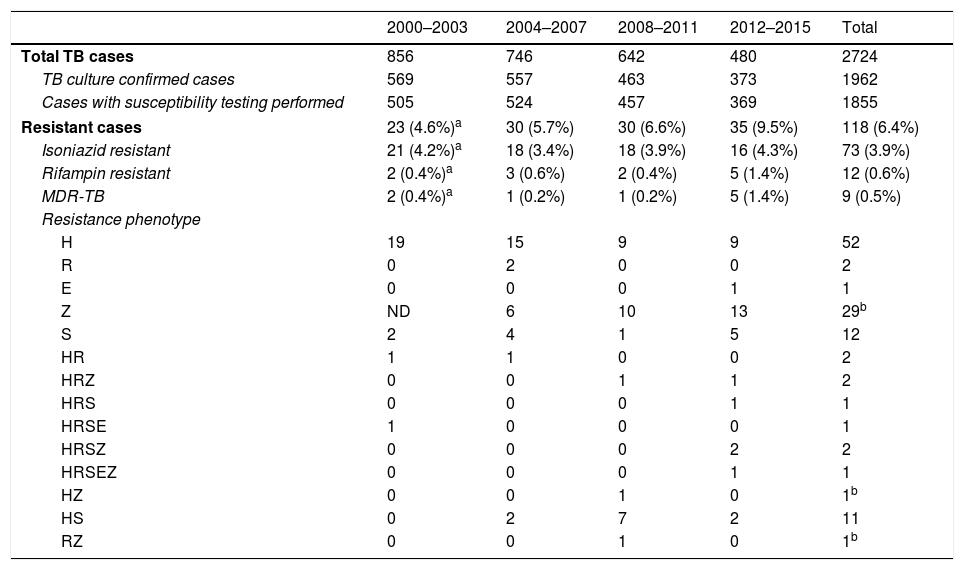
We detected resistance to at least one of the aforementioned TB drugs in 6.4% (118/1855) of cases (Table 1). In order to establish some tendencies, the 16 years the study lasts were divided in four four-year periods. The rate of resistance to isoniazid was 3.9% (73/1855), with hardly any variation over the study period. Overall, 0.6% of strains (12/1855) were RR-TB. This type of resistance increased over the last 4 years of the study, in which we detected 1.4% RR-TB strains. As in the cases of rifampicin resistance, there were more cases of MDR-TB during the 2012–2015 period, when 5 of the 9 MDR-TB cases were detected (4 in 2015 alone), but the differences were not statistically significant compared to the first period in either case. None of the MDR-TB strains were extensively drug resistant.
Antimicrobial susceptibility of 1855 strains of Mycobacterium tuberculosis complex in Gipuzkoa, north of Spain, between 2000 and 2015.
| 2000–2003 | 2004–2007 | 2008–2011 | 2012–2015 | Total | |
|---|---|---|---|---|---|
| Total TB cases | 856 | 746 | 642 | 480 | 2724 |
| TB culture confirmed cases | 569 | 557 | 463 | 373 | 1962 |
| Cases with susceptibility testing performed | 505 | 524 | 457 | 369 | 1855 |
| Resistant cases | 23 (4.6%)a | 30 (5.7%) | 30 (6.6%) | 35 (9.5%) | 118 (6.4%) |
| Isoniazid resistant | 21 (4.2%)a | 18 (3.4%) | 18 (3.9%) | 16 (4.3%) | 73 (3.9%) |
| Rifampin resistant | 2 (0.4%)a | 3 (0.6%) | 2 (0.4%) | 5 (1.4%) | 12 (0.6%) |
| MDR-TB | 2 (0.4%)a | 1 (0.2%) | 1 (0.2%) | 5 (1.4%) | 9 (0.5%) |
| Resistance phenotype | |||||
| H | 19 | 15 | 9 | 9 | 52 |
| R | 0 | 2 | 0 | 0 | 2 |
| E | 0 | 0 | 0 | 1 | 1 |
| Z | ND | 6 | 10 | 13 | 29b |
| S | 2 | 4 | 1 | 5 | 12 |
| HR | 1 | 1 | 0 | 0 | 2 |
| HRZ | 0 | 0 | 1 | 1 | 2 |
| HRS | 0 | 0 | 0 | 1 | 1 |
| HRSE | 1 | 0 | 0 | 0 | 1 |
| HRSZ | 0 | 0 | 0 | 2 | 2 |
| HRSEZ | 0 | 0 | 0 | 1 | 1 |
| HZ | 0 | 0 | 1 | 0 | 1b |
| HS | 0 | 2 | 7 | 2 | 11 |
| RZ | 0 | 0 | 1 | 0 | 1b |
TB: tuberculosis; MDR-TB: multidrug-resistant tuberculosis; H: isoniazid; R: rifampin; S: streptomycin; E: ethambutol; Z: pyrazinamide; ND: not done.
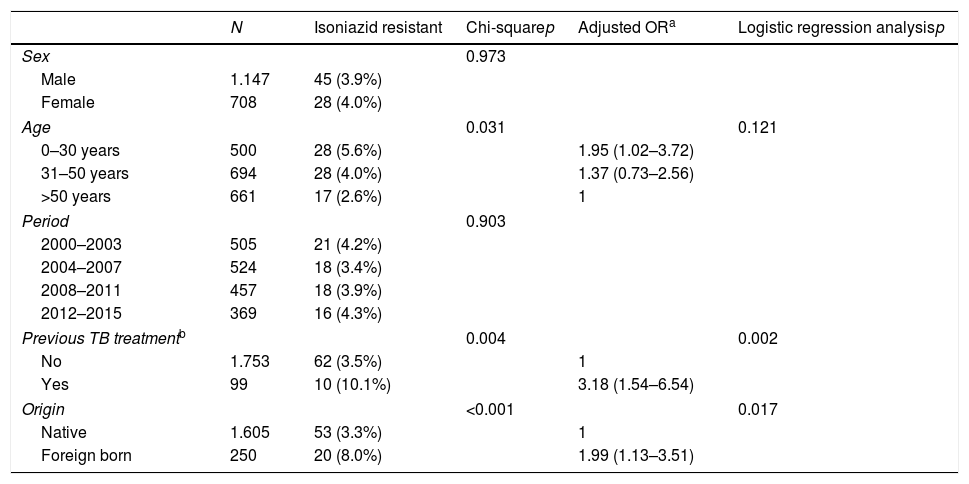
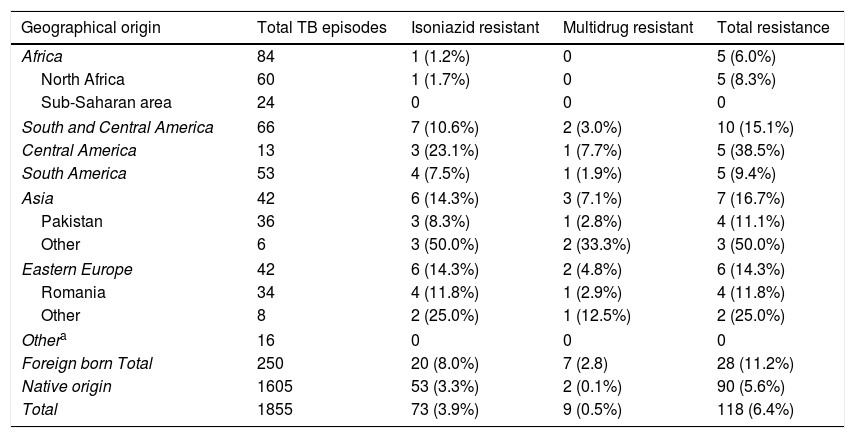
The prevalence of TB drug resistance was greater among the foreign-born population and among those who had received these drugs before the current episode of TB (Table 2). Moreover, 7 of the 9 MDR-TB cases occurred in people of foreign origin. These two factors were independently associated with both isoniazid and multi-resistance. Among foreign-born individuals and those with a history of TB treatment, the risk of isoniazid resistance was 2- and 3-fold higher, respectively, and the risk of multi-resistance was 18- and 9-fold higher, respectively, than in the general population. The highest rates of incidence in foreign-born individuals were found among those from Asia and Eastern Europe (Table 3).
Factors associated with resistance to tuberculous drugs in 1855 episodes of TB in Gipuzkoa, north of Spain, between 2000 and 2015.
| N | Isoniazid resistant | Chi-squarep | Adjusted ORa | Logistic regression analysisp | |
|---|---|---|---|---|---|
| Sex | 0.973 | ||||
| Male | 1.147 | 45 (3.9%) | |||
| Female | 708 | 28 (4.0%) | |||
| Age | 0.031 | 0.121 | |||
| 0–30 years | 500 | 28 (5.6%) | 1.95 (1.02–3.72) | ||
| 31–50 years | 694 | 28 (4.0%) | 1.37 (0.73–2.56) | ||
| >50 years | 661 | 17 (2.6%) | 1 | ||
| Period | 0.903 | ||||
| 2000–2003 | 505 | 21 (4.2%) | |||
| 2004–2007 | 524 | 18 (3.4%) | |||
| 2008–2011 | 457 | 18 (3.9%) | |||
| 2012–2015 | 369 | 16 (4.3%) | |||
| Previous TB treatmentb | 0.004 | 0.002 | |||
| No | 1.753 | 62 (3.5%) | 1 | ||
| Yes | 99 | 10 (10.1%) | 3.18 (1.54–6.54) | ||
| Origin | <0.001 | 0.017 | |||
| Native | 1.605 | 53 (3.3%) | 1 | ||
| Foreign born | 250 | 20 (8.0%) | 1.99 (1.13–3.51) | ||
| N | Multidrug resistant | Chi-squarep | Adjusted ORa | Logistic regression analysisp | |
|---|---|---|---|---|---|
| Sex | 0.738 | ||||
| Male | 1.147 | 5 (0.4%) | |||
| Female | 708 | 4 (0.6%) | |||
| Age | 0.171 | ||||
| 0–50 years | 1.194 | 8 (0.7%) | |||
| >50 years | 661 | 1 (0.2%) | |||
| Period | 0.199 | ||||
| 2000–2007 | 1.019 | 3 (0.3%) | |||
| 2008–2015 | 826 | 6 (0.7%) | |||
| Previous TB treatmentb | 0.007 | 0.003 | |||
| No | 1.753 | 5 (0.3%) | 1 | ||
| Yes | 99 | 3 (3.0%) | 9.29 (2.10–41.06) | ||
| Origin | <0.001 | <0.001 | |||
| Native | 1.605 | 2 (0.1%) | 1 | ||
| Foreign | 250 | 7 (2.8%) | 18.24 (3.63–91.80) | ||
Resistance to tuberculosis drugs by geographical area among the population resident in Gipuzkoa, north of Spain, between 2000 and 2015.
| Geographical origin | Total TB episodes | Isoniazid resistant | Multidrug resistant | Total resistance |
|---|---|---|---|---|
| Africa | 84 | 1 (1.2%) | 0 | 5 (6.0%) |
| North Africa | 60 | 1 (1.7%) | 0 | 5 (8.3%) |
| Sub-Saharan area | 24 | 0 | 0 | 0 |
| South and Central America | 66 | 7 (10.6%) | 2 (3.0%) | 10 (15.1%) |
| Central America | 13 | 3 (23.1%) | 1 (7.7%) | 5 (38.5%) |
| South America | 53 | 4 (7.5%) | 1 (1.9%) | 5 (9.4%) |
| Asia | 42 | 6 (14.3%) | 3 (7.1%) | 7 (16.7%) |
| Pakistan | 36 | 3 (8.3%) | 1 (2.8%) | 4 (11.1%) |
| Other | 6 | 3 (50.0%) | 2 (33.3%) | 3 (50.0%) |
| Eastern Europe | 42 | 6 (14.3%) | 2 (4.8%) | 6 (14.3%) |
| Romania | 34 | 4 (11.8%) | 1 (2.9%) | 4 (11.8%) |
| Other | 8 | 2 (25.0%) | 1 (12.5%) | 2 (25.0%) |
| Othera | 16 | 0 | 0 | 0 |
| Foreign born Total | 250 | 20 (8.0%) | 7 (2.8) | 28 (11.2%) |
| Native origin | 1605 | 53 (3.3%) | 2 (0.1%) | 90 (5.6%) |
| Total | 1855 | 73 (3.9%) | 9 (0.5%) | 118 (6.4%) |
Resistance to pyrazimamide was detected in 36 (1.9%) cases (29 of which were only resistant to this antibiotic), and the related species was M. bovis spp. bovis in 31 of them.
The genetic analysis identified mutations associated with resistance in 73.2% (41/56) of the isoniazid-resistant strains analysed. We found a mutation in the katG gene in 24 strains (22 being the S315T1 mutation) and in the promoter region of the inhA gene (the C15T mutation) in 17 strains. The strains that underwent mutation in the katG gene showed associated resistance to other antibiotics with high frequency (8 isoniazid-resistant and streptomycin-resistant strains; 3 MDR-TB-resistant strains). Strains that underwent mutation in the inhA gene were only isoniazid-resistant. Notably, we detected mutations in the rpoB gene in all (9/9) of the rifampicin-resistant strains analysed.
The genotype was assessed in 86% (102/118) of the resistant strains, revealing a predominance of the Euro-American lineage with 67 strains (67.1%), distributed amongst the following clades: 34 LAM strains, 14 Haarlem strains; 8 Cameroon strains, 7 S strains; and 4 Uganda I strains. In addition, there were six strains belonging to the East African-Indian lineage and two to the East-Asian (Beijing) lineage, all of which were isolated in foreign-born individuals, and the remaining 27 were M. bovis ssp. bovis. We identified 5 clusters (identical MIRU pattern) grouping a total of 18 strains, all belonging to the Euro-American lineage; the largest cluster included 7 strains. We did not find any epidemiological relationships between patients infected with the strains from these clusters.
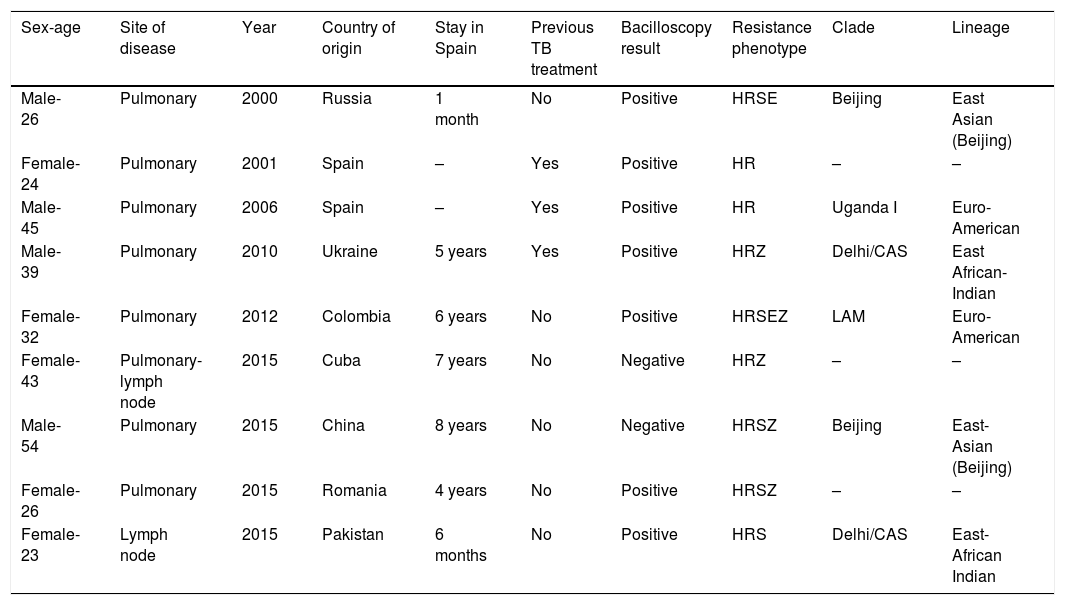
Table 4 provides details concerning the nine cases of MDR-TB. No secondary cases linked to patients with MDR-TB were reported.
Characteristics of nine multidrug-resistant tuberculosis (MDR-TB) cases detected in Gipuzkoa, north of Spain between 2000 and 2015.
| Sex-age | Site of disease | Year | Country of origin | Stay in Spain | Previous TB treatment | Bacilloscopy result | Resistance phenotype | Clade | Lineage |
|---|---|---|---|---|---|---|---|---|---|
| Male-26 | Pulmonary | 2000 | Russia | 1 month | No | Positive | HRSE | Beijing | East Asian (Beijing) |
| Female-24 | Pulmonary | 2001 | Spain | – | Yes | Positive | HR | – | – |
| Male-45 | Pulmonary | 2006 | Spain | – | Yes | Positive | HR | Uganda I | Euro-American |
| Male-39 | Pulmonary | 2010 | Ukraine | 5 years | Yes | Positive | HRZ | Delhi/CAS | East African-Indian |
| Female-32 | Pulmonary | 2012 | Colombia | 6 years | No | Positive | HRSEZ | LAM | Euro-American |
| Female-43 | Pulmonary-lymph node | 2015 | Cuba | 7 years | No | Negative | HRZ | – | – |
| Male-54 | Pulmonary | 2015 | China | 8 years | No | Negative | HRSZ | Beijing | East-Asian (Beijing) |
| Female-26 | Pulmonary | 2015 | Romania | 4 years | No | Positive | HRSZ | – | – |
| Female-23 | Lymph node | 2015 | Pakistan | 6 months | No | Positive | HRS | Delhi/CAS | East-African Indian |
H: isoniazid; R: rifampin; S: streptomycin; E: ethambutol; Z: pyrazinamide.
Gipuzkoa is a region with a moderate incidence of TB. The mean incidence between 2000 and 2015 was 24.5 cases per 100,000 people. This is higher than the values for Spain as a whole for the same period (mean: 13.5 cases per 100,000), and makes our region one of the areas with the highest incidence of TB in the European Union, where there were 765,000 cases between 2007 and 2016 (mean: 12.6 cases per 100,000).3 As observed across Europe and in the rest of the world,1,3,4 in our setting, the incidence of TB is decreasing. In Gipuzkoa, the cumulative reduction of incidence was 62% in 16 years. This being attributable to current active control programmes such as that implemented in our region over the last 20 years.
The current study analysed the susceptibility of 1855 strains causing TB over the last 16 years. The susceptibility testing included almost 95% of the strains causing TB episodes, meeting one of the objectives stated in the WHO programme for the control of the disease across the world.2
Our data show a low rate of resistance to TB drugs in Gipuzkoa. Resistance to one of the first-line drugs was found in only 6.4% of the episodes studied, MDR/RR-TB strains being exceptional, with a prevalence of 0.6%. The rates of resistance were found to be higher from 2009 onwards, although this is attributable to the fact that in that year we started to systematically assess susceptibility to pyrazinamide. The majority of the pyrazinamide-resistant strains belonged to the specie M. bovis spp. bovis, which has intrinsic resistance. Excluding pyrazinamide, the rate of resistance to first-line drugs in Gipuzkoa did not change over the study period, with a mean of 4.8%. It has been estimated that worldwide, in 2016, 4.1% of new cases and 19% of previously treated cases were MDR/RR-TB.1 In the European Union, 11% of the strains analysed in 2015 were resistant to some of the first-line drugs (not including pyrazinamide) and 4.1% corresponded to MDR-TB.11 In Spain, the subnational data collected in 2014–2015 showed that 7.4% of cases were resistant to first-line drugs and 4.7% corresponded to MDR-TB strains.4,11 In the rest of the Basque Country, 6.9% of the strains analysed between 2008 and 2015 were resistant to some of the first-line drugs and 0.3% corresponded to MDR-TB.12 Our results indicate that Gipuzkoa maintains a favourable situation regarding antibiotic resistance in tuberculosis, which is probably closely linked to both main associated factors to this study. Treatment adherence was good in Gipuzkoa. In 2015, 94% of patients with TB completed their treatment, with only 6% being lost to follow-up, moving away from the region or withdrawing from the treatment. Further, in our region, there are relatively few foreign-born individuals from areas with a high prevalence of MDR-TB, such as China, Russia or India, representing less than 2% of the overall population. In Gipuzkoa, these two factors were independently associated with isoniazid resistance and drug multi-resistance. Notably, however, the foreign-born population living in Gipuzkoa is growing, which may explain the small increase in the number of multi-resistant strains detected in Gipuzkoa at the end of the study period.
The genotyping of resistant strains reveals two scenarios. On the one hand, there are strains resistant to some drug but which are not multi-resistant, most belonging to the Euro-American lineage, endemic to Europe13–16; they are highly diverse, with only 18 strains in the present study grouped into 5 clusters with no clear epidemiological relationship. On the other hand, there are the MDR strains, belonging to non-endemic lineages and found in foreign-born individuals. The only two cases of MDR-TB in non-foreign-born patients were detected after failure of previous treatment. Results of genotyping must be carefully interpreted, as not only were the sensitive strains not analysed, but genotyping was also not carried out on all the resistant strains.
The results of this study support the policy of, in the event of a case of TB in Gipuzkoa, administering an empirical treatment with the three first-line drugs until the susceptibility report is available. The use of four drugs could be reserved for when there is a higher risk of MDR-TB, that is, for foreign-born individuals or those who have received a previous TB treatment. Even in these individuals, in our routine diagnostic procedures, this treatment regimen is partially avoided by using methods for detecting the M. tuberculosis genome in our centres, which also allows us to detect genomic changes associated with resistance.
The main limitation of this study is that, due to the small local area analysed, extrapolation of the results to even nearby zones is limited. Moreover, methodological improvements in 16 years are important and unavoidable, like the introduction of a complementary antibiotic susceptibility method in 2009, which included pyrazinamide-susceptibility. Excluding this agent, results were presumably almost unaffected, due to the high methodological concordance. Eventually, some facts related to the health as well as the habits of the patients (VIH, smoking, drinking alcohol and diabetes), which were not analysed owing to the lack of systematic data collection, could have some influence in the results.
In conclusion, in our setting, the incidence of TB continues to fall. Antibiotic resistance currently has a relatively small impact, mostly associated to individuals who were foreign born or had a history of TB treatment, and multidrug-resistant TB is unusual. Nevertheless, this favourable situation could change with the geographical spread of resistant strains, underlining the importance of an active surveillance programme.
5Compliance with ethical standardsThis study was approved by the Ethics Committee for Clinical Research of Gipuzkoa Health Area.
6FundingThis study was supported in part by grant PI 2016111036 from the Office of Health Research and Innovation, Department of Health, Government of the Basque Country, Spain, and by grants from the Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias (CIBERES CB06/06/0056), an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
7Conflict of interestThe authors declare that they have no conflict of interest.
The authors thank Professor Emilio Pérez-Trallero for his valuable support in conceiving and developing the intellectual framework of the study.