A 43-year-old man presented with two months of fever, without other relevant symptomatology; he was admitted to the internal medicine department for further investigations. He lived in Mexico City, and his past medical history was only relevant for long-lasting poorly controlled type 2 diabetes mellitus. On admission, his laboratory evaluation revealed pancytopenia (WBC 500/μL, absolute neutrophil count [ANC] 95/μL, Hb 11.4g/dL, platelets 50,000/μL), hyperglycemia (617mg/dL; Hba1C 9.7%) and mild diabetic ketoacidosis with respiratory alkalosis (pH 7.44, HCO3− 16.8mmol/L, pCO2 25.5mmHg; urine was positive for ketones). He received treatment with insulin, crystalloids and broad-spectrum antibiotics with an appropriate response to treatment. The results of a bone marrow biopsy were consistent with acute lymphoblastic leukemia; an induction to remission regimen with daunorubicin, vincristine, and prednisone was administered.
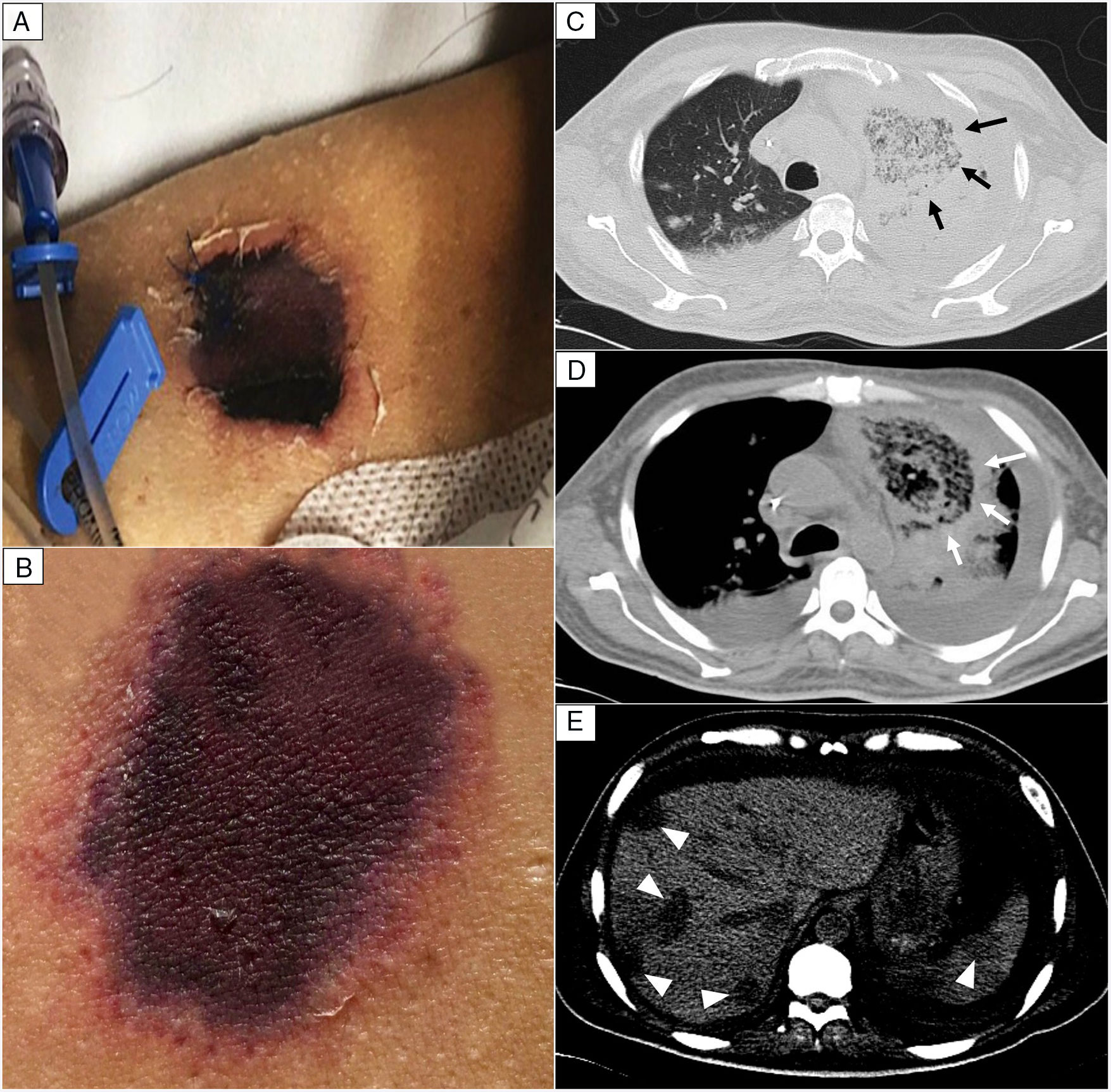
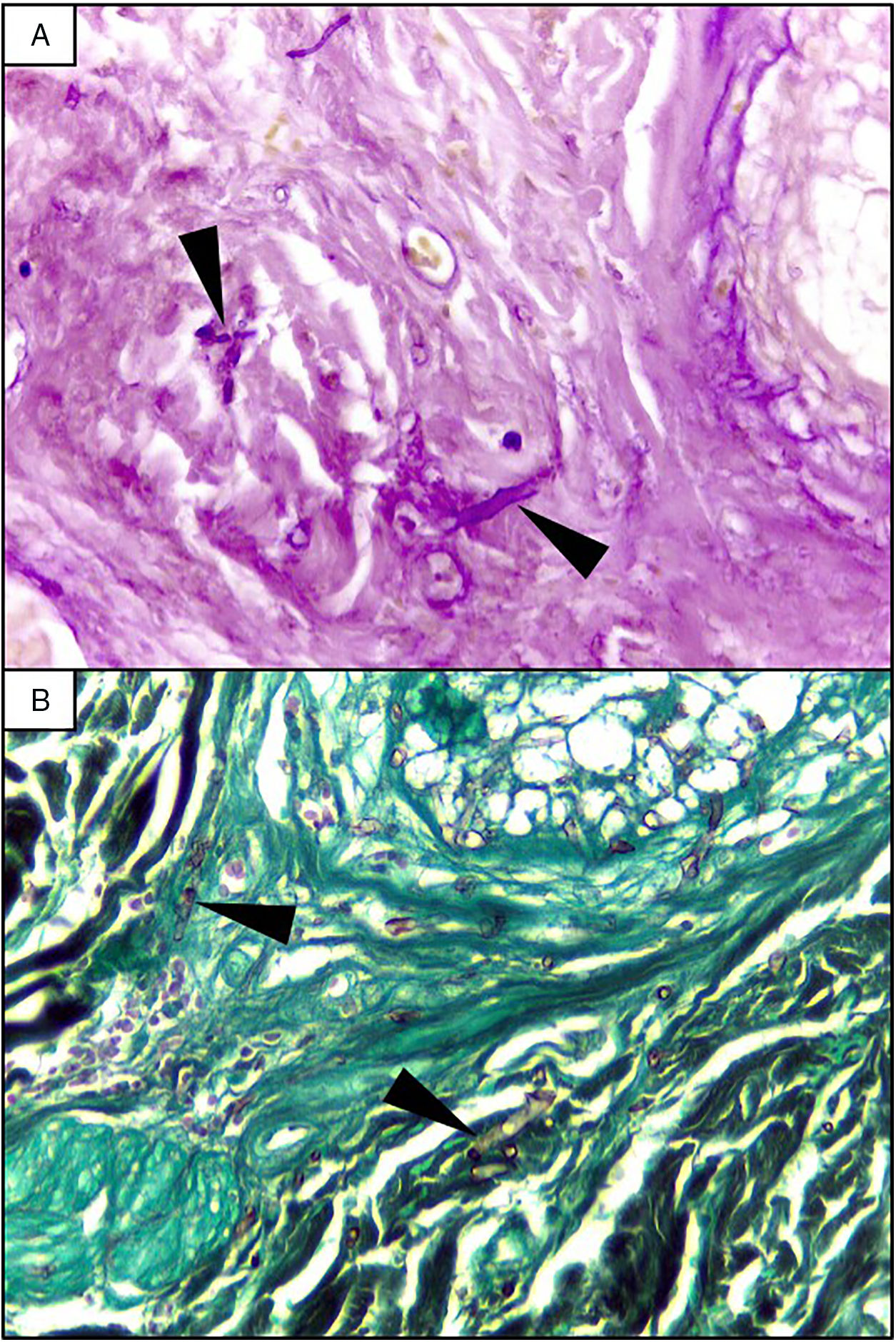
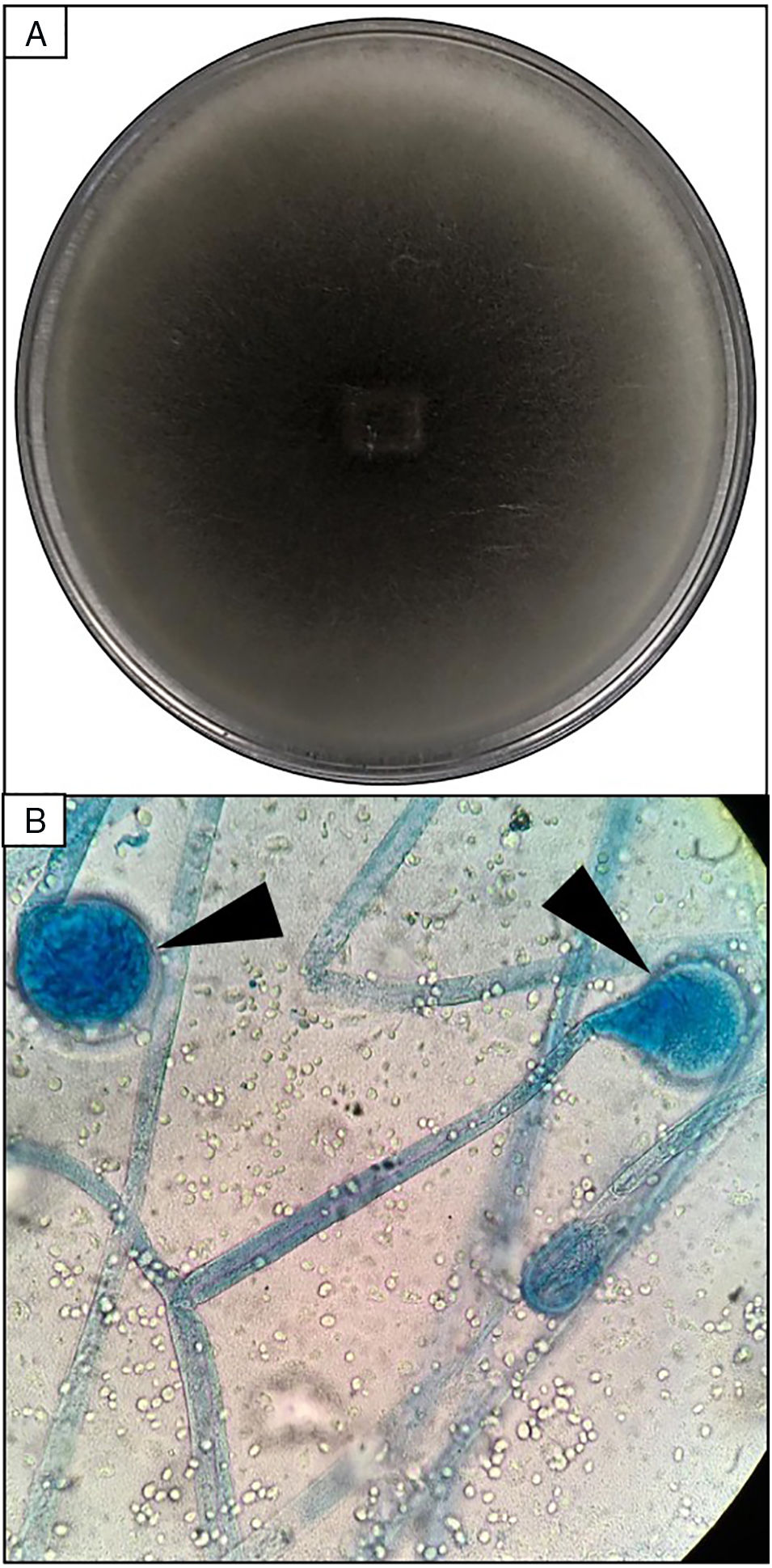
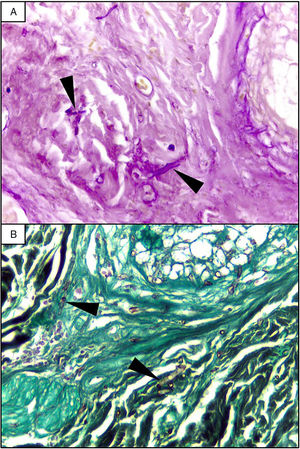
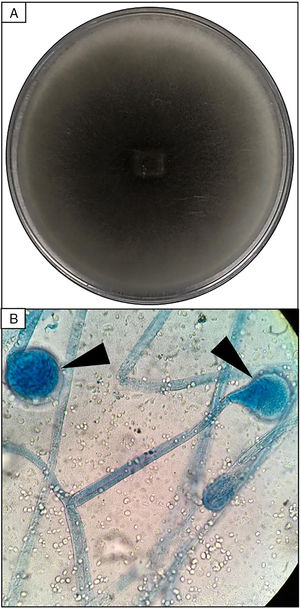
EvolutionOn day +18 (ANC: 120cells/μL), he developed fever, a necrotic ulcer on the right shoulder (Fig. 1A and B), and hypoxemia. A chest CT scan showed lung infiltrates, with a left upper lobe reversed halo sign, and abscesses in the liver and spleen (Fig. 1C and E). A skin biopsy showed broad ribbon-like aseptate hyphae (Fig. 2) consistent with Mucormycosis diagnosis; Mucor spp. were isolated from respiratory and skin cultures (Fig. 3). He was started on liposomal amphotericin B (6mg/kg daily) but given the extent of the disease, surgery was precluded. Unfortunately, the response to treatment was unfavorable, and he died as a consequence of septic shock and disease progression.
Skin lesion and radiologic findings. (A, B) Necrotic skin lesions located at the right shoulder (shown in the image) and the ventral face of the lower extremities. (C, D) Chest CT scan. The reversed halo sign (arrows), a focal round area of ground-glass attenuation surrounded by a ring of consolidation. (E) Abdomen CT scan. Multiple hypodense nodular lesions in the liver and spleen suggestive of abscesses (arrowheads).
Skin biopsy culture. (A) Culture with dark-gray cottony colonies of Mucor spp., macroscopic morphology on Sabouraud Dextrose Agar. (B) Large spherical sporangia (arrowheads) and pronounced columellae with sporangiospores, in the absence of rhizoids (microscopic morphology on Lactophenol cotton blue stain, 100×).
Mucormycosis is a deadly disease caused by fungi of the order of Mucorales (mostly Rhizopus, Mucor, and Rhizomucor); given their ubiquitous presence, humans are frequently exposed during daily activities.1,2 Because of their vasotropism, these fungi cause tissue infarctions with protean clinical manifestations; nevertheless, an underlying disruption of the immune barriers is required to produce disease (e.g., malignancy, uncontrolled diabetes mellitus).1,2 In the last decades, there has been a dramatic rise in diabetes mellitus and other immunosuppressive disorders, in consequence, clinicians may face this entity more often; nowadays, Mucormycosis is the third most common invasive fungal infection in patients with hematological malignancies and organ transplantations.2,3 It is a highly fatal disease that implies diagnostic and therapeutic challenges; the clinical hallmark of this entity is tissue necrosis.1,2 The former results from fungi angioinvasion and subsequent thrombosis; these microorganisms invade the endothelium through specific receptors (e.g., glucose-regulated protein 78 [GRP78]) that recognize antigens of the spore coating protein family (CotH) on the fungal surface. Additionally, patient-related factors may facilitate this process, such as epithelial cell damage (e.g., diabetes, chemotherapy) or the upregulation of some of their receptors and ligands (GRP78/CotH) in the context of hyperglycemia, high iron levels, or acidosis (as observed in patients with decompensated diabetes).3 Primary host responses against these microorganisms include mononuclear and polymorphonuclear cells; thus, neutropenic patients and those with dysfunctional phagocytes are at higher risk.4 In this case, several of these factors were present (i.e., neutropenia, chemotherapy, acutely decompensated diabetes), making him a perfect host for these fungi and their spread.
The most common disease forms are pulmonary and rhino-orbital-cerebral infections (usually in poorly controlled diabetic patients), but the clinical spectrum includes gastrointestinal, cutaneous and disseminated disease.2,4 The pulmonary disease is the clinical presentation that has been most commonly related to hematogenous spread.2 A reversed halo sign on CT scan (a focal ground-glass opacity surrounded by a ring of consolidation) in an immunocompromised host is highly suggestive of pulmonary mucormycosis (present in up to 19% of patients). Although nonspecific (it has been reported in patients with other infectious [e.g., aspergillosis] and non-infectious etiologies [systemic inflammatory and neoplastic diseases]), it may assist the clinician in selecting an antifungal therapy active against Mucorales.5 A metastatic skin lesion is an important hallmark in early diagnosis, although is not pathognomonic and the differential diagnosis of a necrotic ulcer includes other fungal or bacterial infections (e.g., aspergillosis, scedosporiosis, Pseudomonas aeruginosa); occlusive vasculitis; drug reactions; and infiltrative diseases.6 Mucormycosis diagnosis is based on the growth of the mold in specific cultures, or through the histopathologic analysis (evidence of broad, non-septate hyphae, with right-angle branching). The mainstay of therapy consists of aggressive surgical debridement, control of the underlying condition (e.g., glycemic control, steroids discontinuation, reduction of immunosuppressive therapy), and prompt initiation of antifungal therapy.4 Amphotericin B has become the standard therapy; notwithstanding, current guidelines recommend isavuconazole as an alternative first line treatment form mucormycosis (United States of America Food and Drug Administration), and for refractory cases or who cannot tolerate amphotericin B (European Medicines Agency).7 The disseminated form had a poor prognosis despite best therapy, with mortality rates close to 100%.4
Patient consentA written informed consent was obtained from the patient's family.
Andrea Rangel-Cordero: laboratory technician, participated in obtaining fungal culture photographs.