The continuous increase in our knowledge of HIV medicine and antiretroviral treatment has led us to draft specific consensus documents focused on topics other than antiretroviral therapy, such as treatment of opportunistic diseases, pre- and post-exposure prophylaxis, metabolic abnormalities, treatment of HBV or HCV coinfection, treatment of patients coinfected with tuberculosis, osteoporosis, kidney disorders, and cardiovascular risk. Accordingly, the AIDS Study Group (GeSIDA) of the Spanish Society of Infectious Diseases and Clinical Microbiology has promoted the drafting of this consensus document on the control and monitoring of adult patients infected with HIV. The document provides recommendations on the initial evaluation and subsequent monitoring of HIV-infected patients that will prove useful for all professionals involved in the management of this infection.
La complejidad creciente de los conocimientos relativos a la infección por el VIH y al tratamiento antirretroviral (TAR) ha condicionado la elaboración de documentos de consenso específicos que abordan aspectos diferentes al TAR. Son ejemplos, la profilaxis y el tratamiento de las infecciones oportunistas, la profilaxis post y pre-exposición, las alteraciones metabólicas y el riesgo cardiovascular, la osteoporosis, los trastornos renales, la co-infección por el VHB y el VHC o el manejo de este en pacientes con tuberculosis, entre otros. En esta línea, la Junta Directiva de GeSIDA ha promovido la elaboración de un documento de consenso sobre el control y la monitorización de los adultos infectados por el VIH. El objetivo de este documento es proporcionar una serie de recomendaciones sobre la evaluación inicial y la posterior monitorización clínica de los pacientes infectados por el VIH, que pueda servir como referencia para todos los profesionales implicados en el cuidado de estos pacientes.
Herein, we summarize the recommendations of the AIDS Study Group (GeSIDA) on the control and monitoring of adult patients infected with HIV.1 These recommendations complement those on antiretroviral therapy (ART) in adults with HIV infection, updated annually by GESIDA and the National AIDS Plan.2
Clinical evaluationRecommendations:
- -
The clinical evaluation of the HIV-infected patient should include a full history (with sociodemographic, occupational, personal, and familial data) (A-II) and an exhaustive, detailed physical examination (A-II).
- -
A series of additional steps are recommended, as follows:
- •
Inform the patient about HIV infection, make recommendations for preventing transmission of HIV and other STIs, and promote a healthy lifestyle (A-III).
- •
Start contact tracing where possible (B-III).
- •
Offer psychological support to those patients who need it (B-III).
- •
Prepare patients for initiation of ART (A-III).
- -
Successive check-ups should include questions about health problems, adherence and tolerability to ART, risk of other STIs, and substance abuse. A physical examination should be performed depending on the patient's symptoms, comorbid conditions, and the risk of developing opportunistic infections or immune reconstitution inflammatory syndrome (A-II).
Recommendations:
Table 1 shows the additional tests recommended at the initial evaluation and follow-up visits.
- -
HIV-1/2 serology testing should be performed in all cases where HIV infection has not been confirmed and PVL is undetectable (A-I).
- -
The initial laboratory evaluation should include a complete blood count, general plasma biochemistry, serology, and specific tests for HIV infection (A-II).
- -
The HLA-B*5701 allele should be determined in all patients before starting an ART regimen that contains ABC (A-I). ABC should not be prescribed if the result of the HLA-B*5701 test is positive (A-I).
Additional tests in the initial work-up and follow-up of patients with HIV-1 infection.
| Test/activity/examination | Initial evaluation | Follow-up |
|---|---|---|
| Serology for HIV-1/2 if the infection has not been previously confirmed | √ | |
| Complete blood count | √ | Every 3–6 months |
| Basic coagulation tests | √ | If clinically indicated |
| Plasma biochemistry including liver, kidney (with eGFR), and metabolic profile | √ | Every 3–6 months |
| Bone mineral profile (Ca, P, and vitamin D) | √ | If clinically indicated |
| Calculation of cardiovascular riska | √ | Every 2 years in men >40 years and in women >50 yearsb |
| Basic urine and sediment analysis | ||
| Biochemistry in a spot urine sample: proteinuria and creatinine-to-protein ratioc | √ | Annually |
| Protein profile | √ | |
| G6PDd | Optional | |
| Serology for HAV (IgG), HBV (HBsAg, HBcAc, HBsAc), HCV, and syphilise | √ | In the case of negative results, repeat testing annually if risk factors persist. Confirm HAV and HBV response after vaccination |
| Serology for toxoplasma (IgG) and CMV (IgG) | √ | If clinically indicated |
| Serology for Schistosoma, Trypanosoma cruzi,f and Strongyloides stercoralisf | Optional | If clinically indicated (see Table 5) |
| HIV-1 viral load | √ | At 4 weeks after starting ART and every 3–6 months thereafterg |
| Determination of CD4+ T lymphocytes | √ | Every 3–6 monthsg |
| Determination of CD8+ T lymphocytes and the CD4+/CD8+ ratio | Optional | Optional (every 3–6 monthsg) |
| Resistance genotyping study | √ | In cases of virological failure |
| HLA B*5701 | √ | |
| Viral tropismh | Optional | In cases where MVC is to be used and after virological failure with a CCR5 antagonist |
| HCV-RNAi,j | √ | If clinically indicated |
| Genotyping for HCVi,j | √ | If suspicion of relapse/reinfection by HCV |
| HBV-DNAk | √ | Every 3–6 months if coinfection by HBV |
| Alpha fetoprotein | Optional | If clinically indicated |
| PPD or IGRAl | √ | If the iniital result is negative, consider retesting if the patient come in contact with patients with active TB. Repeat after exposure to a patient with infectious TB, and every 2-3 years in all patients with an initial negative test |
| Chest radiographm | √ | If clinically indicated |
| Liver ultrasoundj | √ | Every 6 months if liver cirrhosis |
| Liver elastographyj | √ | At least annually if clinically indicated |
| Esophagogastroscopyj | √ | Every 2–3 years if there are no varices and every 1–2 years in cases of grade I varices |
| Cervical cytology screeningn | √ | If normal, repeat annually |
| Anal cytology screeningo | Optional | If clinically indicated |
| Electrocardiogramp | √ | |
| Bone densitometryq | Optional | If clinically indicated |
| FRAX (www.shef.ac.uk/FRAX)r | Optional | If clinically indicated |
| Screening or STIs | √ | If clinically indicated |
Note: These recommendations are for guidance and can be modified at the discretion of the attending clinician.
√: Perform.
Preferably based on the ACC/AHA ASCVD equation (www.cvriskcalculator.com), or, where this is not possible, on other equations such as that of Framingham adapted to the HIV-infected population (www.chip.dk/Tools) or the Spanish population (Score: www.heartscore.org; Regicor: www.imim.cat).
Annually in cases of previous cardiovascular disease, family history, or increased cardiovascular risk (>10% in the next 10 years).
If the patient is prescribed TDF, perform 1–3 months after starting treatment and subsequently every 6 months. If the patient has diabetes mellitus or arterial hypertension, determine microalbuminuria and the creatinine–albumin ratio in a spot urine sample.
Initiate prophylaxis with dapsone or sulfonamide if the patient is from Africa, Asia, or the Mediterranean area.
Initially, treponemal and nontreponemal tests; subsequently, only nontreponemal test if treponemal test is positive.
Schistosoma species, Trypanosoma cruzi, and Strongyloides stercoralis in patients from areas with a high prevalence of infestation, especially if this is suspected (e.g. in the case of eosinophilia) (see Table 5).
Consider less frequent determination of PVL and CD4+ T lymphocytes (every 6–12 months) in clinically stable patients with repeatedly suppressed plasma viral load and a CD4+ T lymphocyte count repeatedly >300cells/μL (at the physician's discretion).
Consult AEEH/SEIMC guidelines on management of hepatitis C (http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2017-ManejoHepatitisC-AEEHySEIMC.pdf) (see Table 3).
Sensitivity is diminished in severe immunosuppression. The specificity of PPD is diminished in vaccinated patients (BCG); therefore, use IGRA.
Especially in patients from populations with a high prevalence of tuberculosis, patients fulfilling the criteria for chronic bronchitis, and smokers.
Perform initially in all patients with risk practices (homo/bisexual men and women who engage in receptive anal intercourse) and in those with anal or perianal lesions secondary to HPV infection. If the cytology result is abnormal, perform high-resolution anoscopy and biopsy (evidence of benefit unknown, advocated by some experts).
Especially in patients with cardiovascular risk factors and/or patients who are going to initiate therapy with drugs that may affect cardiac conduction.
Identify risk factors for altered bone mineral density. Please see the GESIDA consensus statement on osteoporosis in HIV infection dated May 2016 (http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2016-osteoporosis.pdf).
Follow the recommendations of the GESIDA consensus statement on osteoporosis in HIV infection dated May 2016 (http://gesida-seimc.org/wp-content/uploads/2017/02/gesida-guiasclinicas-2016-osteoporosis.pdf).
Evaluate the risk of STI and screen for STI following the recommendations of the consensus statement on sexually transmitted infections in children, adolescents, and adults of GESIDA/PNS/GEITS/SEIP, 2017 (http://gesida-seimc.org/wp-content/uploads/2017/05/gesida-guiasclinicas-ITS-201703.pdf) (see Table 4).
Recommendations:
- -
The absolute number and percentage of CD4+ lymphocytes should be determined before starting ART. Once ART has been initiated, these parameters should be assessed at 3 months (A-III) and every 3–6 months thereafter in order to monitor the immune response (A-I).
- -
If the patient does not follow a course of ART, then the CD4+ lymphocyte count should be monitored every 3–6 months (A-III).
- -
At the physician's discretion, the interval between check-ups can be longer in clinically stable patients with suppressed viral load and a CD4+ lymphocyte count >300cells/mm3 (C-II).
Recommendations:
- -
PVL should be determined before initiating ART and at 4 weeks after initiation. Once virological suppression (<50copies/mL of HIV-RNA) has been achieved, PVL should be monitored every 3–6 months (A-II).
- -
The objective of virological suppression is a PVL <50copies/mL (A-II).
Recommendations:
- -
Perform an HIV resistance genotyping study, including the reverse transcriptase and protease genes, in all patients before initiation of ART. However, it is only necessary to wait for the result in cases where an NNRTI is to be used (A-II).
- -
The integrase gene should only be studied if there is a high degree of suspicion of transmission of resistance to this family (C-III).
- -
Perform a genotyping study to determine HIV-1 resistance in all patients who experience virological failure. The study should include the integrase gene if the regimen includes an INI (A-I).
- -
HIV-1 tropism should be determined in cases where a CCR5 receptor antagonist is to be prescribed or in cases where the regimen containing this drug has failed (A-I).
- -
In patients with a viral load <500copies/ml, resistance in plasma can be investigated using plasma concentration methods (B-II) or a proviral DNA genotyping study (C-III).
- -
Minority variants should be interpreted on an individual basis for each drug according to their genetic barrier (C-III).
- -
The results should be interpreted taking into account all previous genotyping studies the patient has undergone (B-II).
Recommendations:
- -
Determination of the plasma concentration of antiretroviral drugs is not indicated in daily clinical practice, but is reserved for clinical studies/trials or special clinical situations (BII).
Recommendations:
All HIV-infected patients should undergo a basic kidney work-up (plasma creatinine, estimated glomerular filtration rate [CKD-EPI formula], serum phosphate, urine protein-to-creatinine ratio, glycosuria, urine sediment) to test for kidney disease at the baseline visit after diagnosis of HIV infection. The work-up should be performed systematically during subsequent follow-up visits (A-II).
Patients with no risk factors for kidney disease should undergo a basic kidney work-up once per year. Patients with risk factors for kidney disease should undergo an extended kidney work-up at least every 6 months (C-III).
Evaluation of bone mineral densityRecommendations:
- -
DXA should be performed in HIV-infected patients fulfilling any of the following conditions (B-III).
- a.
Presence of major risk factors for fracture (long-term therapy with corticosteroids, history of fragility fracture, high risk of falling).
- b.
Postmenopausal women or men with confirmed hypogonadism.
- c.
Men aged ≥50 years.
- d.
Cases where the FRAX algorithm indicates a >3% risk of hip fracture and/or >10% risk of major osteoporotic fracture in the following 10 years.
- a.
In patients who do not have osteoporosis, DXA should be repeated following the sequence set out below (B-III):
- -
If the BMD value is normal or slightly reduced (t-score at any site ≤−1.5 SD): repeat after 10 years.
- -
In cases of moderate osteopenia (t-score between −1.50 and −1.99 SD) at 5 years.
- -
In cases of advanced osteopenia (t-score between −2.00 and −2.49 SD): repeat every 1–2 years.
- -
The risk of fracture should be evaluated using the FRAX algorithm in patients with osteoporosis, especially if specific treatment is being considered (B-II).
- -
The lipid profile and glycemia should be evaluated with other cardiovascular risk factors at least once per year in all HIV-infected patients (B-II).
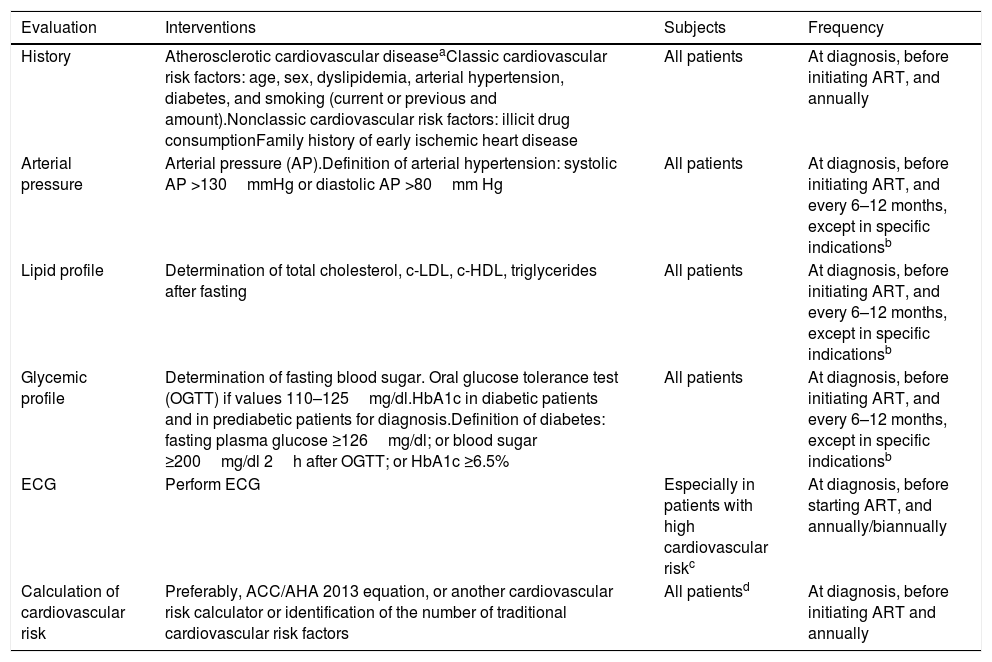
Recommendations: (Table 2)
- -
Classic cardiovascular risk factors and a family history of premature cardiovascular disease should be evaluated (AII).
- -
Nonclassic risk factors should also be assessed (illicit drug use and composition of ART regimen) (AIII).
- -
ACC/AHA guidelines recommend evaluating 10-year cardiovascular risk in the following in persons aged 40–79 years (BII).
- -
Evaluations should be repeated annually (BIII).
Evaluation of cardiovascular risk in patients with HIV infection.
| Evaluation | Interventions | Subjects | Frequency |
|---|---|---|---|
| History | Atherosclerotic cardiovascular diseaseaClassic cardiovascular risk factors: age, sex, dyslipidemia, arterial hypertension, diabetes, and smoking (current or previous and amount).Nonclassic cardiovascular risk factors: illicit drug consumptionFamily history of early ischemic heart disease | All patients | At diagnosis, before initiating ART, and annually |
| Arterial pressure | Arterial pressure (AP).Definition of arterial hypertension: systolic AP >130mmHg or diastolic AP >80mm Hg | All patients | At diagnosis, before initiating ART, and every 6–12 months, except in specific indicationsb |
| Lipid profile | Determination of total cholesterol, c-LDL, c-HDL, triglycerides after fasting | All patients | At diagnosis, before initiating ART, and every 6–12 months, except in specific indicationsb |
| Glycemic profile | Determination of fasting blood sugar. Oral glucose tolerance test (OGTT) if values 110–125mg/dl.HbA1c in diabetic patients and in prediabetic patients for diagnosis.Definition of diabetes: fasting plasma glucose ≥126mg/dl; or blood sugar ≥200mg/dl 2h after OGTT; or HbA1c ≥6.5% | All patients | At diagnosis, before initiating ART, and every 6–12 months, except in specific indicationsb |
| ECG | Perform ECG | Especially in patients with high cardiovascular riskc | At diagnosis, before starting ART, and annually/biannually |
| Calculation of cardiovascular risk | Preferably, ACC/AHA 2013 equation, or another cardiovascular risk calculator or identification of the number of traditional cardiovascular risk factors | All patientsd | At diagnosis, before initiating ART and annually |
The frequency should be higher in patients with major increases in AP, total cholesterol, LDL cholesterol, triglycerides, or glycemia who do not respond to the treatment prescribed.
The ACC/AHA equation was developed in a population aged 40–79 years. When it was evaluated in an HIV-infected population it had poorer discriminatory capacity in women, black men, and patients with a low-moderate cardiovascular risk (<10%), in whom the rates of acute myocardial infarction observed were greater than expected.
Recommendations:
- -
All HIV-infected patients should undergo screening for LTBI at diagnosis of their HIV infection (AI). Screening should be by tuberculin skin test or interferon gamma release assay (BIII).
- -
Screening for LTBI should be repeated in patients with <200 CD4+ cells/μL and an initial negative test result once this CD4+ threshold is surpassed with ART (BII).
- -
Screening for LTBI should be repeated in patients with a negative result in the baseline test and risk of exposure to TB (AII).
- -
Regular, systematic screening is not recommended in patients with a negative test result at baseline and no evidence of risk factors for TB and who are receiving ART (BIII).
Recommendations:
- -
The initial evaluation of HIV-infected patients should include testing for HCV antibodies (AI). If the result is positive, plasma viral load should be measured to determine the activity of the infection (AI). Patients with active infection should undergo viral subtyping and genotyping (AI).
- -
HCV serology testing should be repeated annually in patients with no previous history of HCV infection who engage in risk practices. HCV-RNA should be determined annually to assess the possibility of HCV reinfection in patients with an SVR who engage in risk practices (AII).
- -
Serology testing should be performed to screen for HBV (HBsAg, anti-HBs, anti-HBc, HBeAg, anti-HBe) in all HIV-infected patients (AI). HBV-DNA should be assessed in patients with positive HBsAg (AI). In the case of isolated anti-HBc positivity, the presence of occult hepatitis B should be investigated by determining HBV-DNA in serum (BII).
- -
HBV viral load should be monitored every 6 months in patients with active infection (BIII).
- -
All patients with active HBV infection should undergo serology testing for HDV. If the result is positive, HDV-RNA should be assessed (AI).
- -
All patients should undergo serology testing for HAV at the beginning of follow-up to evaluate the need for vaccination (A-I).
- -
Possible HEV infection should only be investigated in cases of clinical suspicion (A-II).
- -
Fibrosis stage should be evaluated in patients with chronic hepatitis caused by HBV and/or HCV (AI).
- -
The initial evaluation of fibrosis should be made using noninvasive methods (AI). Transient elastometry is the noninvasive technique of choice in those centers where it is available (AI).
- -
Liver stiffness should be evaluated regularly—ideally annually—in all patients with active HBV/HCV infection (AII).
Recommendations: (Table 3)
- -
HIV-infected patients with liver cirrhosis (any cause) should be evaluated at least every 6 months. The evaluation should include a clinical examination, a laboratory work-up with liver function parameters, and calculation of the Child-Pugh and MELD indices (AIII). Annual assessment of liver stiffness is also recommended (AII).
- -
HIV-infected patients with liver cirrhosis (any cause) should undergo systematic screening for hepatocellular carcinoma at diagnosis of cirrhosis (AI). Patients with chronic HBV infection and risk factors for hepatocellular carcinoma (Asian males aged >40 years, Asian women aged >50 years, Africans, and patients with a family history of hepatocellular carcinoma) should undergo screening even if they do not have cirrhosis (AIII). Screening for hepatocellular cirrhosis is by ultrasound examination every 6 months (AI).
- -
Patients with cirrhosis should undergo screening for esophageal varices using upper digestive endoscopy at diagnosis of cirrhosis (AII). Patients with liver stiffness <21kPa can be managed without endoscopy; liver stiffness should be assessed annually (AII).
Follow-up of patients with liver cirrhosis.
| Objective | Interval | Indication | Remarks | |
|---|---|---|---|---|
| Clinical and laboratory assessment including liver function | Early detection of complications. Monitoring of liver function | Every 6 months, if the patient is clinically stable. Every 3 months, if the patient has decompensated cirrhosis | All patients | Calculation of Child-Pugh and MELD at each visitIf applicable, evaluation by transplant team |
| Ultrasound | Screening for hepatocellular carcinoma | Every 6 months | All patients.Maintain regardless of whether SVR is achieved | Alpha fetoprotein is not useful as a screening method.CT/MRI if ultrasound is unclear. If CT/MRI is not conclusive, lesions should be closely monitored |
| Oral endoscopy | Screening of esophageal varices | BaselineSubsequently, depending on findings and SVR (Ref. 14) | If LS ≥21KPa | If no esophageal varices in UGE, repeat every 2 years (every 3 if SVR)Do not repeat if no esophageal varices in baseline UGE, subsequent SVR, and decrease in LS <21 kPa |
| Liver elastography | Monitoring LS. Prediction of events. Establish need for UGE | AnnuallyMaintain after SVR | All patients | Must be repeated annually if UGE not performed because LS <21kPaPerform UGE if progression to LS >21kPa |
CT/MRI, computed tomography/magnetic resonance imaging; LS, liver stiffness; SVR, sustained virological response; UGE, upper gastrointestinal endoscopy.
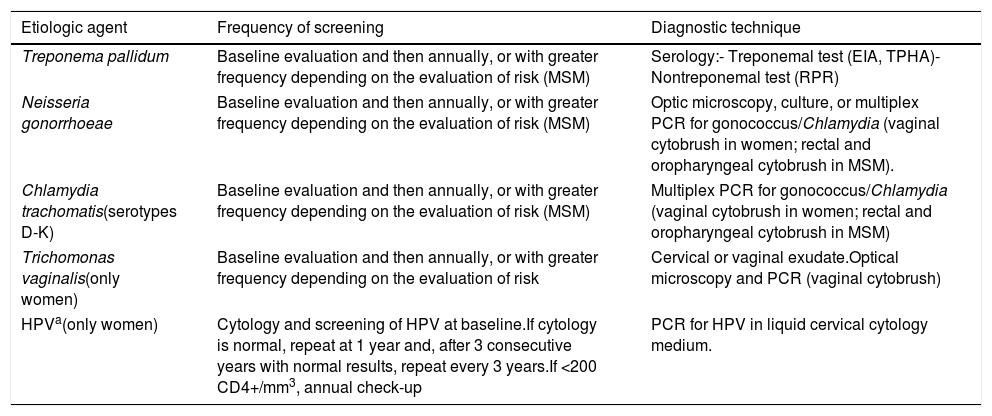
Recommendations: (Table 4)
- -
Screening for STIs is recommended at the baseline visit and annually thereafter, or more frequently depending on the individual evaluation of risk (A-II).
- -
All women should undergo cervical cytology and screening for human papillomavirus (HPV) at the baseline visit. Subsequent check-ups should be scheduled based on the initial findings (A-II).
- -
Digital rectal examination and cytology are the methods used for screening of preneoplastic lesions and/or carcinoma of the anal canal (B-II).
- -
Annual anal cytology is recommended for all men who have sex with men, women with cervical dysplasia, and patients with genital warts (B-III).
Monitoring of STI in HIV-infected patients.
| Etiologic agent | Frequency of screening | Diagnostic technique |
|---|---|---|
| Treponema pallidum | Baseline evaluation and then annually, or with greater frequency depending on the evaluation of risk (MSM) | Serology:- Treponemal test (EIA, TPHA)- Nontreponemal test (RPR) |
| Neisseria gonorrhoeae | Baseline evaluation and then annually, or with greater frequency depending on the evaluation of risk (MSM) | Optic microscopy, culture, or multiplex PCR for gonococcus/Chlamydia (vaginal cytobrush in women; rectal and oropharyngeal cytobrush in MSM). |
| Chlamydia trachomatis(serotypes D-K) | Baseline evaluation and then annually, or with greater frequency depending on the evaluation of risk (MSM) | Multiplex PCR for gonococcus/Chlamydia (vaginal cytobrush in women; rectal and oropharyngeal cytobrush in MSM) |
| Trichomonas vaginalis(only women) | Baseline evaluation and then annually, or with greater frequency depending on the evaluation of risk | Cervical or vaginal exudate.Optical microscopy and PCR (vaginal cytobrush) |
| HPVa(only women) | Cytology and screening of HPV at baseline.If cytology is normal, repeat at 1 year and, after 3 consecutive years with normal results, repeat every 3 years.If <200 CD4+/mm3, annual check-up | PCR for HPV in liquid cervical cytology medium. |
Recommendations:
- -
Patients should receive health education in order to reduce sexual risk practices and drug consumption practices (AII).
- -
Antiviral treatment should be used to prevent new infections by HBV, HCV, and HIV (AI).
- -
The use of condoms—both male and female—should be encouraged (AI).
- -
Harm reduction programs should be implemented for drug users (AI).
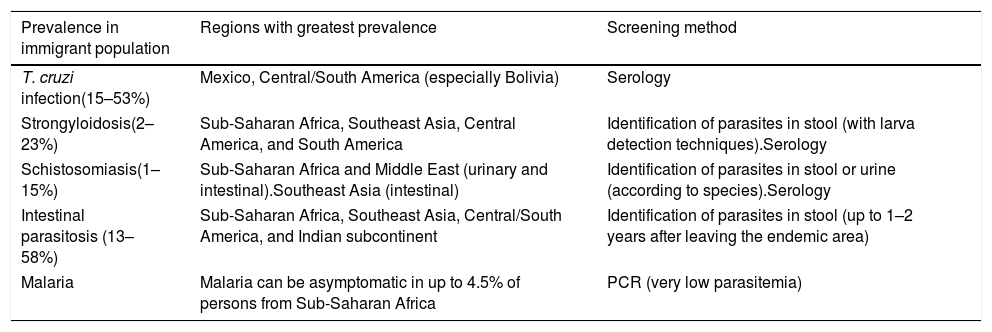
Recommendations: (Table 5)
All persons from endemic areas should undergo screening for Trypanosoma cruzi, especially in the case of compatible signs or symptoms (AII).
Geographically restricted infections that should be screened for in asymptomatic immigrants.
| Prevalence in immigrant population | Regions with greatest prevalence | Screening method |
|---|---|---|
| T. cruzi infection(15–53%) | Mexico, Central/South America (especially Bolivia) | Serology |
| Strongyloidosis(2–23%) | Sub-Saharan Africa, Southeast Asia, Central America, and South America | Identification of parasites in stool (with larva detection techniques).Serology |
| Schistosomiasis(1–15%) | Sub-Saharan Africa and Middle East (urinary and intestinal).Southeast Asia (intestinal) | Identification of parasites in stool or urine (according to species).Serology |
| Intestinal parasitosis (13–58%) | Sub-Saharan Africa, Southeast Asia, Central/South America, and Indian subcontinent | Identification of parasites in stool (up to 1–2 years after leaving the endemic area) |
| Malaria | Malaria can be asymptomatic in up to 4.5% of persons from Sub-Saharan Africa | PCR (very low parasitemia) |
Persons from endemic areas or those who have spent long periods of residence in an endemic area should undergo screening for strongyloidosis, especially if they have eosinophilia (AII).
PCR should be performed to detect malaria in high-risk persons 2 years after leaving the endemic area (BIII).
Persons from endemic areas should undergo screening for schistosomiasis, especially if they report bathing in fresh water or have eosinophilia (AIII).
Persons from tropical areas should undergo screening for parasites in stool during the 2 years following their return (AIII).
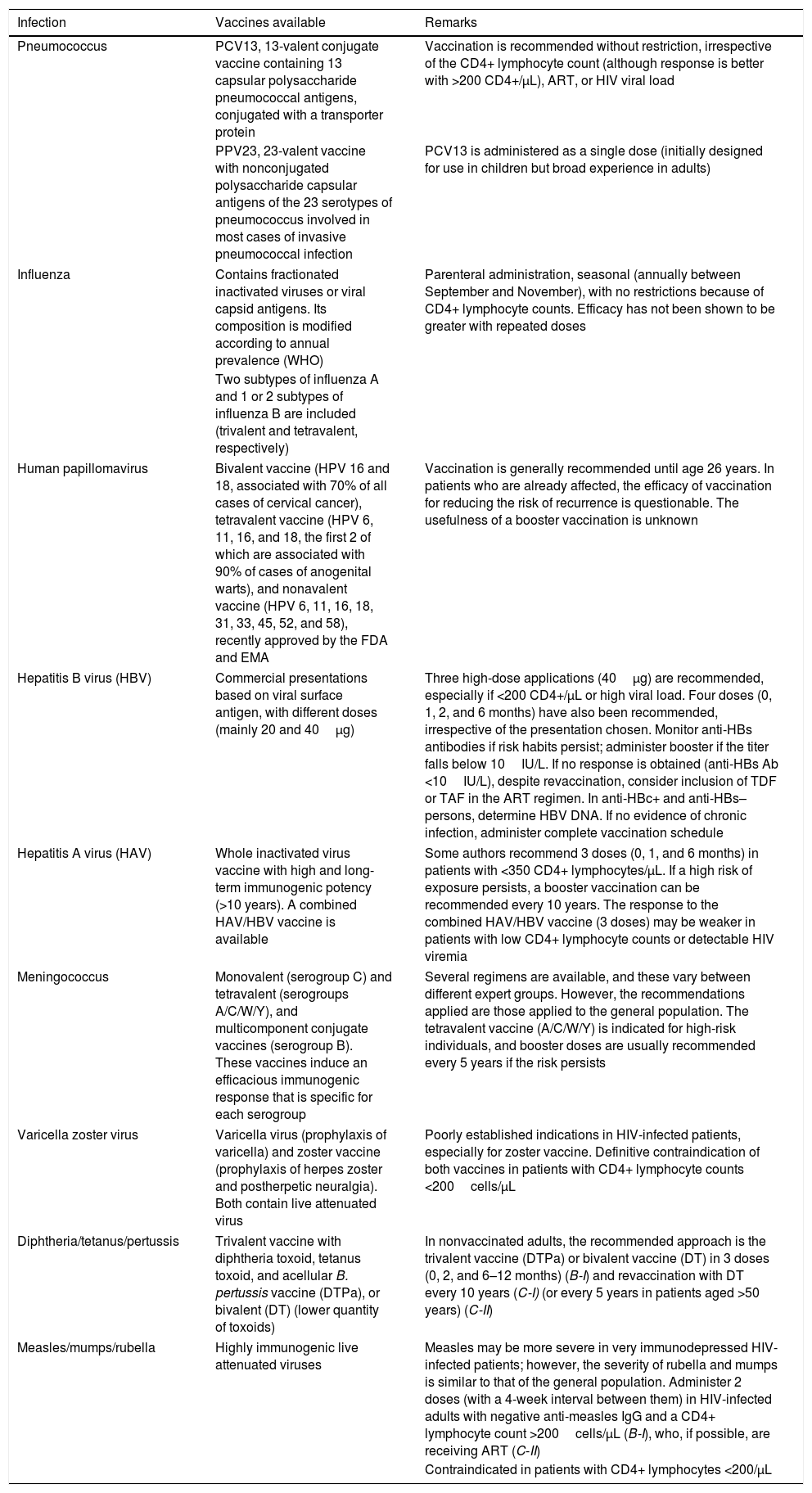
VaccinationRecommendations: (Table 6)
- -
HIV-infected adults who have not been vaccinated against pneumococcus should receive a dose of PCV13 (A-II), followed, at least 8 weeks later, by a dose of PPV23 (A-II). Patients previously vaccinated with PPV-23 can choose to be vaccinated with PCV13 at least 1 year after the last dose of PPV23 or continue being revaccinated with PPV23 every 5 years (B-III).
- -
Annual vaccination with nonreplicative (inactivated) influenza vaccine is recommended for all HIV-infected persons, including pregnant women (A-II), and persons in close contact with profoundly immunocompromised HIV-infected patients.
- -
Vaccination against HPV is recommended (3 doses; 0, 1–2, and 6 months) for all HIV-infected boys and girls aged 9–12 years (A-III) and for persons aged 13–26 years (B-III).
- -
HIV-infected patients who have not been vaccinated against HBV should be vaccinated with high doses—40μg (0, 1, and 6 months) (A-I) or 20μg (0, 1, 2, and 6 months) (B-I)—after previous initiation of ART.
- -
Postvaccination anti-HBs levels should be determined. If these are <10IU/L, then 3 new 40-μg doses should be administered at monthly intervals (B-I) or the patient should be revaccinated with the same regimen.
- -
Patients who have not been vaccinated against HAV should receive monovalent vaccine in 2 doses separated by 6–12 months (A-I). The response to anti-HAV antibodies after vaccination should be evaluated.
- -
The doses for vaccination against meningococcus in HIV-infected patients are the same as in the general population (B-I).
- -
Vaccination against VVZ (2 doses separated by 4–8 weeks) is recommended for HIV-infected children older than 12 months and adults, with negative anti-VVZ antibody titers, provided that they have CD4+ lymphocytes ≥15% or ≥200cells/μL, respectively, and are receiving effective ART (B-I).
Recommended vaccinations in HIV-infected patients.
| Infection | Vaccines available | Remarks |
|---|---|---|
| Pneumococcus | PCV13, 13-valent conjugate vaccine containing 13 capsular polysaccharide pneumococcal antigens, conjugated with a transporter protein | Vaccination is recommended without restriction, irrespective of the CD4+ lymphocyte count (although response is better with >200 CD4+/μL), ART, or HIV viral load |
| PPV23, 23-valent vaccine with nonconjugated polysaccharide capsular antigens of the 23 serotypes of pneumococcus involved in most cases of invasive pneumococcal infection | PCV13 is administered as a single dose (initially designed for use in children but broad experience in adults) | |
| Influenza | Contains fractionated inactivated viruses or viral capsid antigens. Its composition is modified according to annual prevalence (WHO) | Parenteral administration, seasonal (annually between September and November), with no restrictions because of CD4+ lymphocyte counts. Efficacy has not been shown to be greater with repeated doses |
| Two subtypes of influenza A and 1 or 2 subtypes of influenza B are included (trivalent and tetravalent, respectively) | ||
| Human papillomavirus | Bivalent vaccine (HPV 16 and 18, associated with 70% of all cases of cervical cancer), tetravalent vaccine (HPV 6, 11, 16, and 18, the first 2 of which are associated with 90% of cases of anogenital warts), and nonavalent vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58), recently approved by the FDA and EMA | Vaccination is generally recommended until age 26 years. In patients who are already affected, the efficacy of vaccination for reducing the risk of recurrence is questionable. The usefulness of a booster vaccination is unknown |
| Hepatitis B virus (HBV) | Commercial presentations based on viral surface antigen, with different doses (mainly 20 and 40μg) | Three high-dose applications (40μg) are recommended, especially if <200 CD4+/μL or high viral load. Four doses (0, 1, 2, and 6 months) have also been recommended, irrespective of the presentation chosen. Monitor anti-HBs antibodies if risk habits persist; administer booster if the titer falls below 10IU/L. If no response is obtained (anti-HBs Ab <10IU/L), despite revaccination, consider inclusion of TDF or TAF in the ART regimen. In anti-HBc+ and anti-HBs– persons, determine HBV DNA. If no evidence of chronic infection, administer complete vaccination schedule |
| Hepatitis A virus (HAV) | Whole inactivated virus vaccine with high and long-term immunogenic potency (>10 years). A combined HAV/HBV vaccine is available | Some authors recommend 3 doses (0, 1, and 6 months) in patients with <350 CD4+ lymphocytes/μL. If a high risk of exposure persists, a booster vaccination can be recommended every 10 years. The response to the combined HAV/HBV vaccine (3 doses) may be weaker in patients with low CD4+ lymphocyte counts or detectable HIV viremia |
| Meningococcus | Monovalent (serogroup C) and tetravalent (serogroups A/C/W/Y), and multicomponent conjugate vaccines (serogroup B). These vaccines induce an efficacious immunogenic response that is specific for each serogroup | Several regimens are available, and these vary between different expert groups. However, the recommendations applied are those applied to the general population. The tetravalent vaccine (A/C/W/Y) is indicated for high-risk individuals, and booster doses are usually recommended every 5 years if the risk persists |
| Varicella zoster virus | Varicella virus (prophylaxis of varicella) and zoster vaccine (prophylaxis of herpes zoster and postherpetic neuralgia). Both contain live attenuated virus | Poorly established indications in HIV-infected patients, especially for zoster vaccine. Definitive contraindication of both vaccines in patients with CD4+ lymphocyte counts <200cells/μL |
| Diphtheria/tetanus/pertussis | Trivalent vaccine with diphtheria toxoid, tetanus toxoid, and acellular B. pertussis vaccine (DTPa), or bivalent (DT) (lower quantity of toxoids) | In nonvaccinated adults, the recommended approach is the trivalent vaccine (DTPa) or bivalent vaccine (DT) in 3 doses (0, 2, and 6–12 months) (B-I) and revaccination with DT every 10 years (C-I) (or every 5 years in patients aged >50 years) (C-II) |
| Measles/mumps/rubella | Highly immunogenic live attenuated viruses | Measles may be more severe in very immunodepressed HIV-infected patients; however, the severity of rubella and mumps is similar to that of the general population. Administer 2 doses (with a 4-week interval between them) in HIV-infected adults with negative anti-measles IgG and a CD4+ lymphocyte count >200cells/μL (B-I), who, if possible, are receiving ART (C-II) |
| Contraindicated in patients with CD4+ lymphocytes <200/μL | ||
Recommendations:
- -
There is no clear evidence to recommend generalized screening for lung cancer using low-radiation chest computed tomography in patients infected by HIV. In centers where screening for lung cancer is performed in the general population, the option should be offered based on the same criteria as in asymptomatic HIV-infected patients (B-II).
- -
Screening for hepatocellular cancer in cirrhotic patients is based on 6-monthly ultrasound (A-II). Screening should be maintained in HCV-infected cirrhotic patients, even if they have reached SVR to treatment (B-II).
- -
Periodic check-ups of the oral cavity, skin, and skin adnexa are recommended (C-III).
- -
HIV-infected patients should be included in the standard tumor screening programs available to the general population (A-III).
Recommendations:
- -
In the case of HIV-infected patients with neurocognitive disorders, it is important to evaluate comorbid conditions (e.g., psychiatric diseases, drug consumption, and HCV coinfection) that may be the cause or act as triggers of the disorder (A-II).
- -
In the diagnosis of HAND, causes other than HIV infection should be ruled out, a neuropsychological examination should be performed, and interference with activities of daily living should be evaluated (A-II).
- -
Depression and anxiety disorders should be evaluated in all HIV-infected patients (A-II).
Recommendations:
- -
Adherence should be monitored and reinforced at all visits to the clinic (A-III).
- -
Adherence should be monitored by a multidisciplinary team including physicians, nursing staff, psychologists, and hospital pharmacists (A-III).
- -
Adherence and prescribed drug therapy should be reviewed systematically using a sequential, structured methodology including at least 2 different approaches (A-II).
- -
Adherence to all prescribed medication—not only ART—should be assessed every 4–6 months in polymedicated patients (≥6 drugs prescribed) and at least once per year in other patients (A-II).
- -
Annual loss to follow-up should be calculated (per 1000 patient-years), and, depending on the characteristics and facilities of the care unit, more appropriate strategies for minimizing it should be established (A-II).
- -
In order to minimize loss to follow-up and lack of adherence, we recommend the following (A-II):
- 1.
Creation of specific HIV clinics with a personalized and multidisciplinary approach.
- 2.
Adapting ART to the patient's characteristics.
- 3.
Simplification of ART regimens.
- 4.
Development of joint strategies with the patient.
- 5.
Assessment interviews in special situations.
This consensus document was drafted without grant aid or other funding, whether collective or individual, from any private institutions. The conflicts of interest not associated with this document declared by the Members of the Editorial Board are set out below.
Piedad Arazo has acted as a consultant for Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme.
María José Buzón declares that she has no conflicts of interest.
Manuel Crespo has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has held clinical research grants from Gilead Sciences and ViiV Healthcare. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and for developing training presentations for Bristol-Myers Squibb, Gilead Sciences, and ViiV Healthcare.
Adriá Curran has received grants for attending conferences from Gilead Sciences and Janssen Cilag. He has also received payment for talks and consultancy from Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and grants for running research projects and biomedical training activities from Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme.
Vicente Estrada has acted as a consultant for Gilead Sciences, ViiV Healthcare, Merck Sharp & Dohme, and Janssen Cilag and has held clinical research grants from Bristol-Myers Squibb, Merck Sharp & Dohme, Gilead Sciences, and Janssen Cilag. He has received payment for talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme.
Federico García has acted as a consultant and given talks for AbbVie, Gilead Sciences, Merck Sharp & Dohme, Roche Diagnostics, and Werfen and has received clinical research grants from Merck Sharp & Dohme and Gilead Sciences.
Arkaitz Imaz has acted as a consultant for Merck Sharp & Dohme and ViiV Healthcare and has held clinical research grants from Gilead Sciences and ViiV Healthcare. He has received payment for talks from AbbVie, Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and for training presentations from Gilead Sciences. He has also received grants to attend conferences from Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme.
José López Aldeguer has received payment for talks from Gilead Sciences.
Luis López Cortés has received research grants from and acted as a consultant and prepared training presentations for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Juan Emilio Losa has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Fernando Lozano has received payment for training presentations from Gilead Sciences, Janssen Cilag, Merck-Sharp & Dome, and ViiV Healthcare.
Mar Masiá has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and received payment for talks from Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Ana Mariño has received grants to attend conferences and scientific meetings from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, and ViiV Healthcare.
Nicolás Merchante has received payment for talks from Bristol-Myers Squibb, Gilead Sciences, and Merck Sharp & Dohme and has received grants to attend conferences from Janssen Cilag, Merck Sharp & Dohme, Gilead Sciences, and ViiV Healthcare.
Antonio Ocampo has received payment for speaking at conferences and consultancy from Fundación Galicia Sur, of which he is a member. He has also received payment for clinical trials and research grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
Rosario Palacios has acted as a consultant for Bristol-Myers Squibb and ViiV-Healthcare and received payment for training talks from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen-Cilag, Gilead Sciences, and ViiV-Healthcare.
José A. Pérez-Molina has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. He has also received research grants from Bristol-Myers Squibb, Janssen Cilag, and Merck-Sharp & Dohme and payment for talks from Bristol-Myers Squibb, Merck Sharp & Dohme, and ViiV Healthcare.
Eva Poveda has received grants to attend conferences and scientific meetings from Janssen Cilag, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. She has also received payment for talks from Janssen Cilag and Merck Sharp & Dohme and grants for developing research projects and biomedical training projects from Janssen Cilag and Gilead Sciences.
Melchor Riera has received grants for travel to scientific meetings and conferences from Gilead Sciences, Janssen Cilag, and Merck Sharp & Dohme and payment for talks from AbbVie. He has also received payment for teaching and research from AbbVie and Bristol-Myers Squibb.
Antonio Rivero has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare and has held clinical research grants from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare. He has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Merck Sharp & Dohme, and ViiV Healthcare.
Rafael Rubio has acted as a consultant for AbbVie, Bristol-Myers Squibb, Gilead Sciences, and Janssen Cilag and has received payment for talks from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen Cilag, Merck Sharp & Dohme, Roche Pharma, and ViiV Healthcare.
Miguel Santín is the principal investigator in a clinical trial on QuantiFERON®-TB Gold In-tube (QFT-GIT), specifically in the contact tracing study, for which Cellestis, Inc. (Carnegie, Australia) provided the QFT-GIT tubes.
Jesús Santos has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, and ViiV-Healthcare and received payment for training talks from Bristol-Myers Squibb, Merck Sharp & Dohme, Janssen-Cilag, Gilead Sciences, and ViiV-Healthcare.
José Sanz has participated in clinical trials sponsored by Bristol-Myers Squibb and ViiV Healthcare. He has also acted as a consultant and given paid training presentations for AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, and ViiV Healthcare.
María Jesús Téllez has received grants to attend conferences from Gilead Sciences.
Javier de la Torre has acted as a consultant for Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare and has received payment for talks from AbbVie, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline, Janssen Cilag, Merck Sharp & Dohme, and ViiV Healthcare.
The GeSIDA (AIDS Study Group) Directorates are grateful for the support and opinions of Miguel Cervero, Carlos Folguera, José Antonio Iribarren, Ramón Morillo, Miguel Ángel Rodríguez-Sagrado, and Isabel Viciana, whose efforts have improved the writing and enriched the contents of the document.
Writing Committee: Manuel Crespo,1 Fernando Lozano,2 María José Buzón,3 Adriá Curran,4 Vicente Estrada,5 Federico García,6 Arkaitz Imaz,7 Luis López Cortés,8 Juan Emilio Losa,9 Mar Masiá,10 Nicolás Merchante,2 Ana Mariño,11 Antonio Ocampo,1 José A. Pérez-Molina,12 Eva Poveda,13 Melchor Riera,14 Miguel Santín,7 Jesús Santos,15 Eulalia Valencia,16 Piedad Arazo,17 Javier de la Torre,18 José López Aldeguer,19 Rosario Palacios,15 Antonio Rivero,20 Rafael Rubio,21 José Sanz,22 María Jesús Téllez.5
1Complexo Hospitalario Universitario/IIS Galicia Sur, Vigo; 2Hospital Universitario Virgen de Valme, Sevilla; 3Vall d́Hebrón Institut de Recerca, Hospital Universitari Vall d́Hebron, Barcelona; 4Hospital Universitari Vall d’Hebrón, Barcelona; 5Hospital Clínico Universitario San Carlos, Madrid; 6Hospital Universitario San Cecilio, Instituto de Investigación Bio-sanitaria, ibsGranada; 7Hospital Universitario de Bellvitge–IDIBELL, L’Hospitalet de Llobregat, Barcelona; 8Hospital Universitario Virgen del Rocío–IBIS, Sevilla; 9Hospital Universitario Fundación Alcorcón. Alcorcón, Madrid; 10Hospital General Universitario de Elche; 11Complejo Hospitalario Universitario de Ferrol; 12Hospital Ramón y Cajal–IRYCIS, Madrid; 13Complejo Hospitalario Universitario A Coruña–INIBIC, La Coruña; 14Hospital Son Espases, Palma de Mallorca; 15Hospital Universitario Virgen de la Victoria–IBIMA, Málaga; 16Hospital Universitario La Paz. Madrid; 17Hospital Universitario Miguel Servet, Zaragoza; 18Hospital Costa del Sol, Marbella; 19Hospital Universitario La Fe–IISLaFe, Valencia; 20Hospital Universitario Reina Sofía–IMIBIC, Córdoba; 21Hospital Universitario 12 Octubre, Instituto de Investigación i+12, Universidad Complutense, Madrid; 22Hospital Universitario Príncipe de Asturias, Alcalá de Henares.