Dual therapy (DT) for maintenance is nowadays a very interesting option. Several regimens have been tested, among those rilpivirine containing regimens avoid drawbacks and toxicities due to the nucleosidic backbone, while maintaining the efficacy and convenience of robust ART. Rilpivirine (RPV) in combination with dolutegravir (DTG) or darunavir (DRV) have shown to be effective, safe and well tolerated.1–4 The SWORD-1 and SWORD-21 studies had provided relevant evidence on the DTG/RPV regimen however the DRVb/RPV combination has been less investigated. DRVb/RPV combine both a high efficacy and genetic barrier with a lower pill burden, especially when DRV is used coformulated with cobicistat. Safety and effectiveness among those receiving DRV/r with those receiving DRV/c4 seems to be similar. Cobicistat, generally considered to be an equipotent inhibitor of CYP3A4, was approved to address limitations of ritonavir such as co-formulation difficulties and drug interactions secondary to its broad effects on CYP isoenzymes and drug transporters.
However, although DT has been proved effective, there is concern about whether it may be associated with a worse immune control that leads to a progressive CD4/CD8 ratio decrease. Low CD4/CD8 ratio is known to be a predictor of non-AIDS related events, non-AIDS-defining cancers and mortality in HIV-patients.
Some studies have explored the effect of DT on CD4/CD8 ratio5–8 but nowadays just few data with mixed results are available. In the case of rilpivirine in combination with dolutegravir or darunavir information is scarce and in all cases for a follow-up period no longer than 48 weeks.2,9,10
In this context, we aimed to evaluate the changes in the CD4/CD8 ratio, after 36 months of treatment, in a group of patients who changed to DT with rilpivirine containing regimens.
We conducted a retrospective, descriptive, study including all the patients from an HIV Clinic at the Arnau de Vilanova Hospital in Valencia, Spain who switched to RPV (25mg daily) and DRV cobicistat (800/150mg daily) or RPV (25mg daily) and DTG (50mg daily) between January 2014 and December 2016. Patients were previously on triple therapy and should have VL<50copies/mL for at least 6 months before switch. The last registers before switch of CD4, CD8 cell count and CD4/CD8 ratio and after 12, 24, 36 months of DT were recorded. Mean and CI 95%, were calculated for quantitative variables and frequencies for qualitative variables. The t-test for repeated measures was applied. The variables were retrieved from the medical records and analyzed with the GNU. PSPP Statistical Analysis Software. Release 1.0.1-g 818227.
The study had the approval of the ethic committee, patient's anonymity was carefully protected and all the guidelines required by the institution were strictly followed.
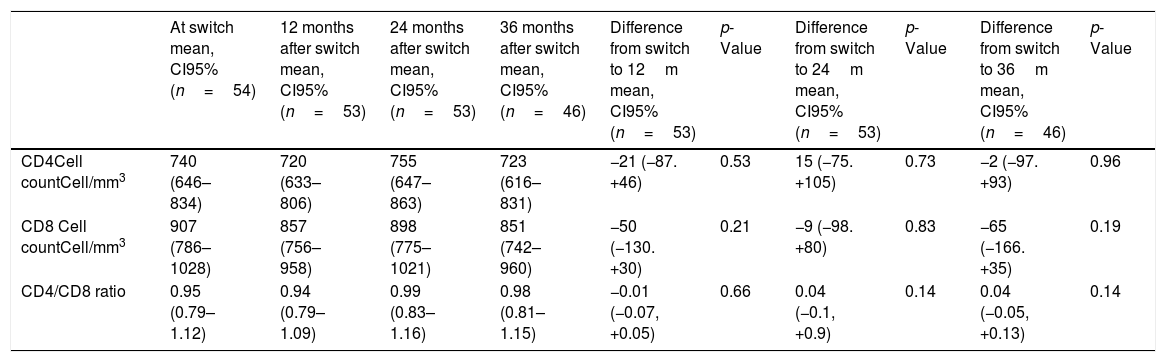
From a total of 61 patients on DT, 7 were excluded because VL was not <50copies/mL before switch. Finally, 54 patients entered the analysis, 31 of them were on RPV/DTG and 23 were on RPV/DRVc. The mean age was 51 (CI 95%: 49–53) years and 50% were men. Patients had suppressed VL for a mean of 6 (CI 95%: 5–8) years before switch and the mean of CD4 cells at switch was 740 (CI 95%: 646–834) cell/mm3. Mean time on DT was 33 months. No differences were observed among baseline characteristics between groups. During the follow-up period just one patient, in the DRVc/RPV group, had VL>50copies/mL in 2 consecutive determinations after one year of treatment, and it was due to poor adherence, finally he was lost to follow-up. No significant variations for the CD4, CD8 cell count and the CD4/CD8 ratio after 12, 24 and 36 months of treatment are found in Table 1. The results did not change after stratified analysis for type of therapy (RPV/DTG or RPV/DRVc).
Variations and their statistical significance for CD4, CD8 cell count, CD4/CD8 ratio at switch and 12, 24 and 36 months are shown in the table below. The mean with CI 95% was calculated and the t-test for repeated measures was applied.
| At switch mean, CI95% (n=54) | 12 months after switch mean, CI95% (n=53) | 24 months after switch mean, CI95% (n=53) | 36 months after switch mean, CI95% (n=46) | Difference from switch to 12m mean, CI95% (n=53) | p-Value | Difference from switch to 24m mean, CI95% (n=53) | p-Value | Difference from switch to 36m mean, CI95% (n=46) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| CD4Cell countCell/mm3 | 740 (646–834) | 720 (633–806) | 755 (647–863) | 723 (616–831) | −21 (−87. +46) | 0.53 | 15 (−75. +105) | 0.73 | −2 (−97. +93) | 0.96 |
| CD8 Cell countCell/mm3 | 907 (786–1028) | 857 (756–958) | 898 (775–1021) | 851 (742–960) | −50 (−130. +30) | 0.21 | −9 (−98. +80) | 0.83 | −65 (−166. +35) | 0.19 |
| CD4/CD8 ratio | 0.95 (0.79–1.12) | 0.94 (0.79–1.09) | 0.99 (0.83–1.16) | 0.98 (0.81–1.15) | −0.01 (−0.07, +0.05) | 0.66 | 0.04 (−0.1, +0.9) | 0.14 | 0.04 (−0.05, +0.13) | 0.14 |
Previous studies showed diverse results such as that of Mussini et al.6 which showed that patients who remained on triple regimen had a higher mean CD4/CD8 ratio increased when compared to those who switched to DT or monotherapy. In others studies, such as TivEdo study, Capetti et al.10 observed no decrease in the CD4/CD8 ratio at 48 weeks after treatment simplification with DTG/RPV. Pasquau et al.3 also reported a slight increase in the CD4/CD8 ratio at 24 weeks in patients on RPV/DRVc. In an analysis of patients switched to four different DT regimens, Monsalvo et al.8 found no decrease in the CD4/CD8 ratio at 48 weeks and no differences according to type of dual regimen.
Limitations of this study are the retrospective design and the small study population. A weakness of retrospective studies is the missed data, however in this study we included demographic and basic laboratory data (VL, lymphocytes subsets, etc.) that we use in the everyday practice and was available for all patients. Selection bias is another weakness of retrospectives studies, in order to minimize this we evaluated all patients who were on the assessed treatments in our Clinic and applied well defined inclusion criteria. The main problem with small studies is interpretation of results, in particular confidence intervals and this should also been take into account.
However, the strength of this work is that few data are currently available on CD4/CD8 ratio trend in the long term, in course of dual therapies containing new drug combinations such as RPV/DTG and specially RPV/DRVc.
Summarizing, dual therapy regimens with RPV and DTG or DRVc, in this study, have not been associated with a decrease in the CD4/CD8 ratio. More long-term studies in larger populations are needed to further assess the impact of dual therapies on the immune system.
Conflicts of interestThe authors declare that they have no conflicts of interest and that they have not received any funding for this study.