To determine the clinical, epidemiological and prognostic factors of HIV-infected patients with influenza A H1N1 admitted to hospital.
MethodsThe study population was HIV infected patients with confirmed influenza infection admitted to hospital in a multicenter cohort. We analyzed demographic data, comorbid conditions, severe events (bronchopneumonia, respiratory insufficiency, respiratory distress, sepsis, admission to intensive care unit, death) and outcome. Data were analyzed using descriptive statistics. Proportions were compared using the χ2 test or Fisher exact test, when applicable. Quantitative variables were compared using the Student t test or Mann–Whitney test. Prognostic impact was analyzed using logistic regression.
ResultsA total of 43 patients, of whom 62.8% were male, were included from 22 hospitals. The mean age was 43.3 years (interquartile range [IQR], 38.4–48.4). HIV was diagnosed for a mean of 14.5 years (IQR, 8.4–20.3). CD4 lymphocyte was <200cells/μL in 38%; 85.7% were on antiretroviral therapy, and 66.7% virologically suppressed. Comorbid conditions were hepatitis B or C (74.4%), smoking (67.4%), chronic obstructive pulmonary disease (30.2%), asthma (14%), and obesity (8.6%). Seven patients had received seasonal influenza vaccination, and 2 the H1N1 vaccine. Cough (100%), fever (93%), gastrointestinal disorders (27.9%) or general – myalgia, general malaise – (67.4%) were the presenting symptoms. These were severe in 24 (55.8%) with 7 (16.3%) requiring intensive care. Two patients died. A lower CD4 lymphocyte count was associated with bacterial infection (P=.063) and longer hospital stay (P=.007). Early oseltamivir reduced severe cases (OR, 4.5; 1.1–18.3; P=.035).
ConclusionsHIV-infected patients admitted to hospital due to influenza A H1N1 had severe morbidity. Low CD4 lymphocytes correlated with longer hospitalization and bacterial infections. Early oseltamivir treatment reduced severe symptoms.
Determinar los factores clínicos, epidemiológicos y pronósticos de pacientes infectados por VIH con gripe A H1N1 ingresados en el hospital.
MétodosSe estudiaron pacientes infectados por VIH con gripe H1N1 confirmada que fueron ingresados en un hospital en una cohorte multicéntrica. Se analizaron los datos demográficos, comorbilidades, efectos adversos graves (bronconeumonía, insuficiencia respiratoria, distress, sepsis, ingreso en unidad de cuidados intensivos y muerte) y situación final. Los datos se analizaron mediante estadística descriptiva. Las proporciones se compararon mediante la prueba de X2 o la prueba exacta de Fisher si procedía. Las variables cuantitativas lo fueron mediante la prueba t o la prueba de Mann-Whitney. Para analizar el impacto pronóstico se utilizó la regresión logística.
ResultadosSe incluyeron 43 pacientes (62,8% varones) de 22 hospitales. La edad media fue de 43,3 años (rango intercuartil [IQR], 38.4 a 48.4). La infección VIH era conocida desde 14,5 años (IQR, 8.4-20.3). Los linfocitos CD4 fueron <200 células/mm en el 38%; el 85,7% estaban en tratamiento antirretroviral, y el 66,7% virológicamente suprimidos. Las comorbilidades fueron: hepatitis B o C (74,4%), tabaquismo (67,4%), enfermedad pulmonar obstructiva crónica (30,2%), asma (14%) y obesidad (8,6%). Siete pacientes habían recibido la vacuna gripal estacional y 2 la vacuna gripal H1N1. La tos (100%), fiebre (93%), alteraciones digestivas (27,9%) o síntomas generales (mialgias, malestar) (67,4%) fueron los síntomas de presentación. Veinticuatro pacientes (55.8%) presentaron un curso evolutivo grave, y 7 (16,3%) requirieron cuidados intensivos. Dos pacientes fallecieron. El número reducido de linfocitos CD4 se asoció con una sobreinfección bacteriana (p = 0,063) y estancia hospitalaria más prolongada (p = 0,007). El oseltamivir administrado precozmente redujo el número de casos graves (OR, 4,5; 1.1-18.3, p = 0,035).
ConclusionesLos pacientes infectados por VIH que ingresan por gripe A H1N1 tienen una morbilidad grave. El número bajo de linfocitos CD4 se correlaciona estancia hospitalaria más prolongada y sobreinfecciones bacterianas. El tratamiento precoz con oseltamivir redujo las manifestaciones graves.
The new strain of influenza virus (A H1N1) that emerged in Mexico in the spring of 2009 spread quickly throughout the world.1,2 Subsequent studies have defined the origin of the strain (a combined avian, porcine, and human influenza virus)3 and its associated morbidity and mortality. Severe cases were reported in groups, such as pregnant women, children, patients with morbid obesity, and with comorbid conditions (e.g. diabetes, chronic lung disease, chronic heart disease).4 Likewise, a greater risk of disease was reported in severely immunodepressed patients, such as solid organ transplant recipients.5 Although it seems logical to think that the effect on patients with diseases such as HIV infection would be similar, there are few studies that have analyzed outcomes in HIV-positive patients co-infected with this new strain of pandemic influenza. Moreover, data from immune competent patients suggest that neuraminidase inhibitors such as oseltamivir can reduce the duration of symptoms when treatment is started in the first 48h after onset. However, at the height of the pandemic, international organizations recommended this drug for immune depressed patients irrespective of the duration of symptoms.6
Although the initial epidemic has passed, we do not know whether new outbreaks will occur in the future, or during coming seasons. As a result, the World Health Organization has recommended including infection by influenza A H1N1 in the 2010 trivalent vaccine. But the number of vaccinated patients is small and, even in vaccinated patients, the response in HIV-infected may be suboptimal.
Given the need to know the behavior of AH1N1 in HIV-infected patients, we conducted a prospective multicenter clinical and epidemiological study of HIV-infected patients admitted to hospital with pandemic influenza A in order to characterize the disease and establish factors associated with severity and complications.
MethodsA multicenter prospective cohort study was proposed to all the members of GESIDA (Spanish AIDS Study Group), a scientific study group of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica) (SEIMC), that includes most physicians involved in the care of HIV-infected patients in Spain. The Ethics Committees of the participating hospitals approved the study. Inclusion criteria were: (a) age >18 years; (b) HIV infection; (c) microbiologically confirmed influenza A, and (d) signed informed consent. Patients admitted between 15 November 2009 and 30 March 2010 were invited to participate. Microbiological diagnosis of the new strain A H1N1 was confirmed using polymerase chain reaction (PCR) from a nasopharyngeal sample. Patients were admitted according to the clinical judgment of the attending physician and without prefixed clinical criteria. Treatment with oseltamivir (and specially time to) depended on the decision of the physician.
A case report form was designed for data collection. The investigators who agreed to participate were asked to collect all cases fulfilling the inclusion criteria. Data were collected weekly and stored centrally (GESIDA-SEIMC). Data collected were, patient characteristics (age, sex, date of birth, country of origin), HIV infection (e.g. date of infection, CD4 lymphocyte count nadir, CD4 lymphocyte count and viral load at admission, highly active antiretroviral therapy [HAART]), and comorbid conditions (asthma, chronic obstructive pulmonary disease, hepatitis virus co-infection, diabetes, pregnancy, cardiovascular disease, chronic renal disease, and previous transplantation). For patients co-infected with the hepatitis C virus (HCV), data were also recorded on viral genotype, degree of fibrosis, bilirubin, HCV treatment and response to it, Model for End-Stage Liver Disease (MELD) score, Child-Turcotte-Pugh score. The influenza A related data recorded were: presenting symptoms, chest X-ray on admission, baseline laboratory results, and blood gas and/or oxygen saturation values (pulse oximetry). Symptoms evolution, complications, treatment with oseltamivir, and adverse events were collected weekly. Bilateral pneumonia or affecting at least two lobules, respiratory insufficiency (oxygen saturation <90% or pO2 <60mmHg, both breathing room air), respiratory distress, sepsis, admission to intensive care, or death were considered severe events. Data were analyzed using descriptive statistics. Qualitative variables were expressed as frequencies and percentages. Quantitative variables were described using the mean, standard deviation, median, and interquartile range (IQR). Proportions were compared using the χ2 test or Fisher exact test when applicable. Quantitative variables were compared using the Student t test or Mann–Whitney test, as appropriate. The impact of prognostic variables on severity of symptoms was analyzed using logistic regression, by including only those variables that were statistically significant in the univariate analysis. Statistical significance was set at P<.05. Data were analyzed using SPSS 16.0 software.
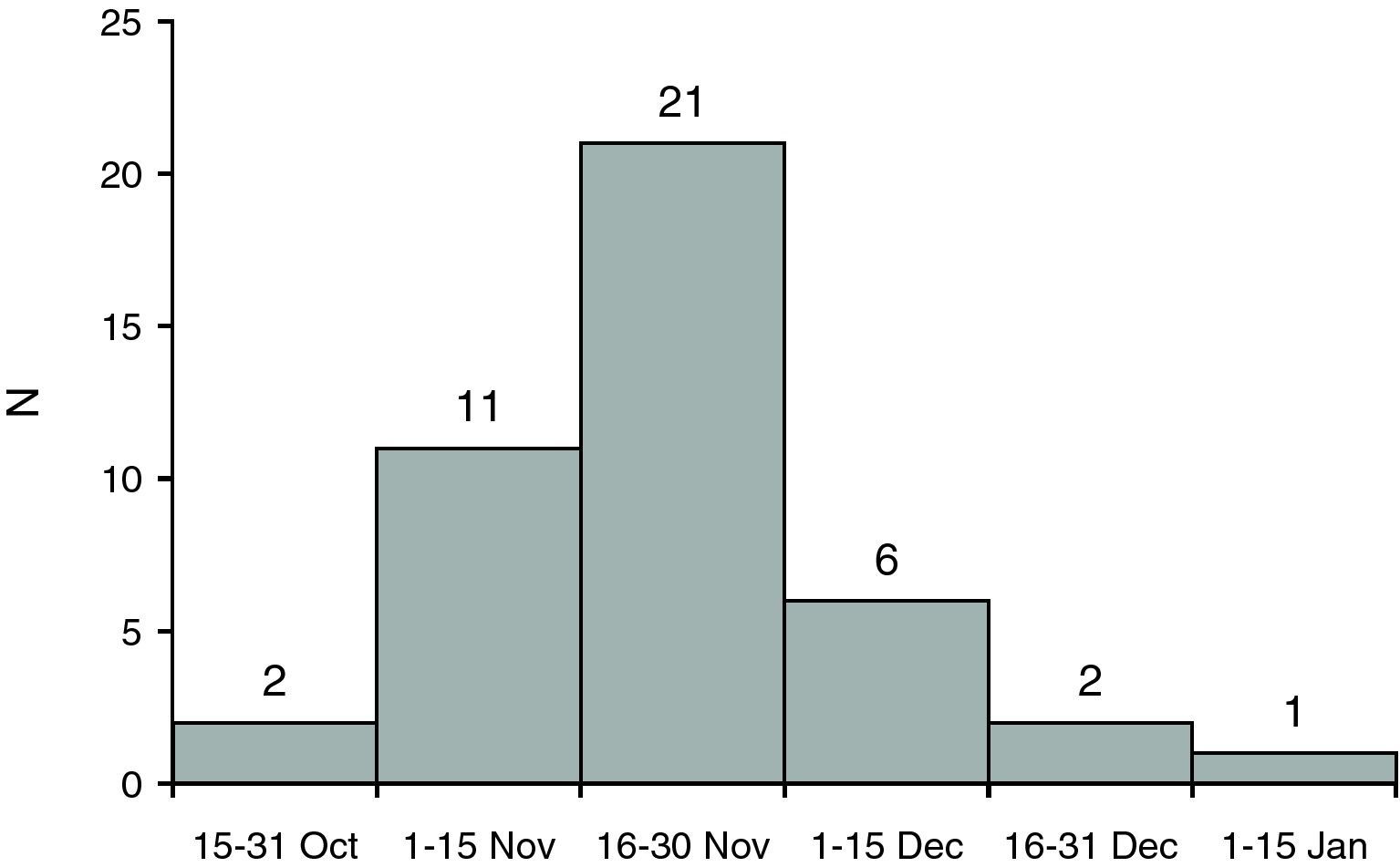
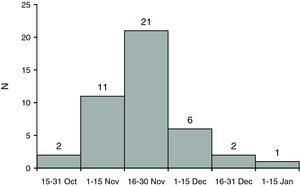
ResultsThe sample included 43 patients from 22 Spanish hospitals. Fig. 1 shows the onset of the symptoms. The mean age was 43.3 years (IQR, 38.4–48.4), and 27 patients were male (62.8%). The median time from diagnosis of HIV infection was 14.5 years (IQR, 8.4–20.3). CD4 lymphocyte count nadir was 100cells/μL. At diagnosis of influenza, 37 (85.7%) were receiving HAART (Table 1).
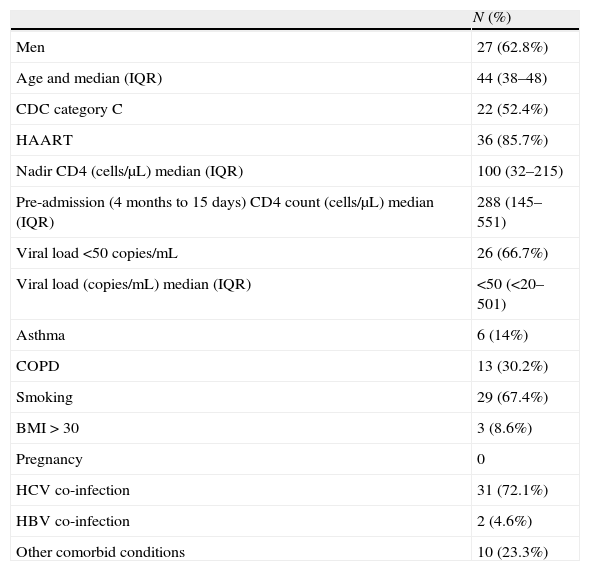
Baseline characteristics and comorbid conditions.
| N (%) | |
| Men | 27 (62.8%) |
| Age and median (IQR) | 44 (38–48) |
| CDC category C | 22 (52.4%) |
| HAART | 36 (85.7%) |
| Nadir CD4 (cells/μL) median (IQR) | 100 (32–215) |
| Pre-admission (4 months to 15 days) CD4 count (cells/μL) median (IQR) | 288 (145–551) |
| Viral load <50copies/mL | 26 (66.7%) |
| Viral load (copies/mL) median (IQR) | <50 (<20–501) |
| Asthma | 6 (14%) |
| COPD | 13 (30.2%) |
| Smoking | 29 (67.4%) |
| BMI>30 | 3 (8.6%) |
| Pregnancy | 0 |
| HCV co-infection | 31 (72.1%) |
| HBV co-infection | 2 (4.6%) |
| Other comorbid conditions | 10 (23.3%) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HAART, highly active antiretroviral therapy; CDC, Centers for Disease Control and Prevention; IQR, interquartile range.
A comorbid condition was recorded for 38 patients (88.4%) (Table 1). The most prevalent conditions were co-infection by hepatitis virus (74.4%), smoking (67%), chronic obstructive pulmonary disease (30%), asthma (14%), and obesity (8%). Only 7 (16.3%) patients had received the seasonal influenza vaccine, and 2 (4.7%) the influenza A vaccine.
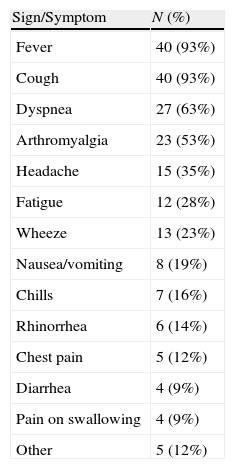
Most frequent presenting symptoms were respiratory symptoms (100%, mainly cough [93%]), fever (93%), general symptoms – myalgia, general malaise – (67.4%), and gastrointestinal symptoms (27.9%) (Table 2).
Presenting signs and symptoms.
| Sign/Symptom | N (%) |
| Fever | 40 (93%) |
| Cough | 40 (93%) |
| Dyspnea | 27 (63%) |
| Arthromyalgia | 23 (53%) |
| Headache | 15 (35%) |
| Fatigue | 12 (28%) |
| Wheeze | 13 (23%) |
| Nausea/vomiting | 8 (19%) |
| Chills | 7 (16%) |
| Rhinorrhea | 6 (14%) |
| Chest pain | 5 (12%) |
| Diarrhea | 4 (9%) |
| Pain on swallowing | 4 (9%) |
| Other | 5 (12%) |
All patients had a chest X-ray at admission. The result was normal in 21 patients (48.8%). The remaining 22 patients (51.2%) had pulmonary infiltrates, which were unilateral in 14 (32.6%) and bilateral in 8 (18.6%). In one patient, the pneumonia progressed from unilateral to bilateral. One patient had a tension pneumothorax.
Complications occurring during the admission were, invasive pneumococcal disease in 6 patients (2 pneumonia, 3 bacteremia, 1 otitis); Pneumocystis jiroveci pneumonia in 2; and disseminated Mycobacterium avium intracellulare, Staphylococcus epidermidis bacteremia, and occipital herpes zoster, each in 1 patient.
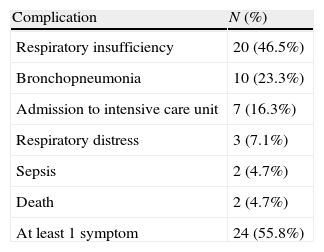
Symptoms were severe in 24 patients (55.8%) (Table 3), and 7 of them (16.3%) had to be admitted to the intensive care unit (3 required mechanical ventilation for a median of 7 days). Two patients died, one of invasive pneumococcal infection on the second day of admission, and the other of systolic dysfunction with myocarditis on the 12th day. Both had a CD4 lymphocyte count under 200cells/μL. Recovery was uneventful in 38 (88.4%) patients. Three patients had sequelae (re-admission due to nosocomial pneumonia, persistent bronchospasm, and polyneuropathy in a critical patient).
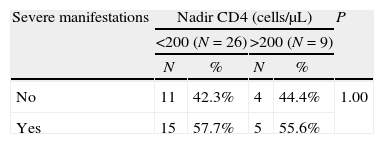
Univariate analysis revealed no differences in severity related to comorbidity, HAART or nadir CD4 lymphocyte count (Table 4). Patients co-infected with hepatitis virus had significantly fewer gastrointestinal symptoms (15.65% vs 63.6%; P=.005) and general symptoms (56.2% vs 100%; P=.008) than non-co-infected patients. Respiratory symptoms were present in 100% of patients in both groups. CD4 lymphocyte count at admission correlated with superinfection by other pathogens and with a longer hospital stay. Thus, 37.5% of patients with <200cells/μL suffered from these infections compared with 11.5% of those with >200cells/μL (P=.063) and the mean hospital stay of patients with <200cells/μL was significantly longer (13.2 days) than that of patients with >200cells/μL (8.8 days; P=.007). The proportion of patients with severe manifestations was lower in the seasonal influenza vaccinated patients than in the non-vaccinated (28.6% vs 56.25%; P=ns).
Impact of the main variables on severe manifestations.
| Severe manifestations | Nadir CD4 (cells/μL) | P | |||
| <200 (N=26) | >200 (N=9) | ||||
| N | % | N | % | ||
| No | 11 | 42.3% | 4 | 44.4% | 1.00 |
| Yes | 15 | 57.7% | 5 | 55.6% | |
| Severe manifestations | Most recent CD4 count, cells/μL | P | |||
| <200 (N=16) | >200 (N=26) | ||||
| N | % | N | % | ||
| No | 7 | 43.8% | 12 | 46.2% | 1.00 |
| Yes | 9 | 56.2% | 14 | 53.8% | |
| Severe manifestations | Seasonal influenza vaccination | P | |||
| No (N=32) | Yes (N=7) | ||||
| N | % | N | % | ||
| No | 14 | 43.8% | 5 | 71.4% | 0.235 |
| Yes | 18 | 56.2% | 2 | 28.6% | |
| Severe manifestations | Currently on HAART | P | |||
| No (N=6) | Yes (N=36) | ||||
| N | % | N | % | ||
| No | 3 | 50 | 16 | 44.4% | 1.00 |
| Yes | 3 | 50 | 20 | 56.6 | |
| Severe manifestations | Hepatitis virus coinfection | P | |||
| No (N=11) | Yes (N=32) | ||||
| N | % | N | % | ||
| No | 5 | 45.5% | 14 | 43.8% | 1.00 |
| Yes | 6 | 54.5% | 18 | 56.2% | |
| Severe manifestations | Early treatment with oseltamivir (<48h) | P | |||
| No (N=30) | Yes (N=13) | ||||
| N | % | N | % | ||
| No | 10 | 33.3% | 9 | 69.2% | 0.046 |
| Yes | 20 | 66.6% | 4 | 30.8% | |
Severe manifestations: two lobules or bilateral pneumonia, oxygen saturation <90% or po2 <60mmHg, respiratory distress, sepsis, intensive care admission or death. HAART, highly active antiretroviral therapy.
All patients were treated with oseltamivir at a dose of 75mg/12h. Time from onset of symptoms to treatment was <48h in 13 patients (32.3%) and >48h in 30 patients (69.7%). Twenty (66.6%) patients with delayed treatment suffered severe symptoms, but only 4 (30.8%) of those with <48h to treatment (P=.035). Logistic regression analysis revealed that the delayed treatment (>48h) was associated with a greater risk of severe symptoms (OR, 4.5; 95% CI, 1.1–18.3; P=.035). No oseltamivir related adverse effects were reported.
DiscussionHIV-infected patients hospitalized due to influenza A had mild to severe clinical courses, with complications and co-infection with other pathogens. Morbidity and mortality were substantial: 55.8% of patients suffered severe symptoms, and 16% had to be admitted to the intensive care unit. These findings are similar to those observed in other immunosuppressed patients, such as transplant recipients.5 Two patients died; one due to concomitant sepsis, and the other due to left ventricular systolic dysfunction with influenza AH1N1 associated myocarditis.
Most patients were on HAART, and 70% had a CD4 lymphocyte count >200/μL at admission, but the CD4 lymphocyte count nadir was low (median 100cells/μL). One noteworthy observation is that 72% of patients were co-infected with HCV, a common finding in HIV-infected cohorts in Spain. The presenting symptoms were similar to those reported in other series,4 but, paradoxically, general and gastrointestinal symptoms were more frequent in non HCV-coinfected patients. Comorbid conditions were common, but not associated with, a poorer prognosis, maybe due to the fact that there were few patients without any comorbid condition. Severely immunosuppressed patients suffer bacterial infections more frequently during the hospital stay, especially infections caused by Streptococcus pneumoniae.
A limited number of studies have analyzed the characteristics of influenza A in HIV-infected patients. A report including 56 patients seen in a hospital (but not necessarily admitted) revealed that gastrointestinal symptoms were more common in HIV-infected patients (37%) than in the non-HIV-infected (18%).7 A considerable difference in our patients was the median CD4 lymphocyte count at presentation (583cells/μL), which was notably higher. When analyzing patients admitted with influenza A only, a study that compared the characteristics and outcome of HIV-infected patients (26 patients) with HIV-negative (559 patients) did not find any differences in outcome, and the authors concluded that the influenza A was a relative benign process.8 Again, our patients were more immunodepressed (median CD4 lymphocyte count: 503cells/μL vs 288cells/μL). A study of 27 Mexican HIV-infected patients, however, reported 6 deaths and 13 opportunistic infections with a complicated course. These differences could be explained by different selection criteria in each of the series.9 In a Spanish seroincident cohort, the study revealed an influenza A incidence of 5.8 cases/1000 patients. The median CD4 lymphocyte count was 376cells/μL. Half of the patients had to be admitted to hospital, and 25% developed pneumonia. Only 1 patient had received influenza A vaccine, and only 25% of them with the seasonal influenza vaccine. Nevertheless, no patient died and 1 was admitted to intensive care unit.10
The most important finding of our study was the association between delayed treatment (>48h) with oseltamivir, and a greater risk of developing severe symptoms (OR, 4.5). This association has been reported previously in immunocompetent,11,12 and in immunodepressed patients.5,13 It has been suggested that immunodepressed patients could benefit from higher doses of oseltamivir or longer treatment.6 However, in transplant recipients with influenza A there were no differences with the dose, but there was in the early use of oseltamivir.5
In our study, only 2 patients (4.6%) had received influenza A vaccine, and only 7 (16.2%) the seasonal influenza vaccine. This is remarkable when compared with a recent study of American HIV-infected patients, of whom 79% were vaccinated against seasonal influenza.14
Available data on the interaction between seasonal influenza and HIV infection are also scarce. Early studies from the pre-HAART era suggested that seasonal influenza caused an extremely high number of cases of pneumonia and deaths in HIV-infected patients, similar to that observed in persons aged >65 years.15 Subsequent data suggested that HAART led to a reduction in the number of cases of influenza. In a retrospective cohort study comparing pre-HAART and the HAART era, seasonal influenza-related hospitalization fell from 48 to 5 admissions per 1000patients/year.16 The efficacy and safety profile of seasonal influenza vaccination in HIV-infected patients has been confirmed in 2 studies,17,18 although data on its protective efficacy is variable. An earlier study revealed poor protection rates, even after 2 doses,19 but a later clinical trial showed high rates of neutralizing antibodies production.20 Prospective studies performed during the HAART era have revealed, not only adequate production of neutralizing antibodies,21 but also clinical efficacy, with fewer cases of influenza (6.1%) in vaccinated patients when compared with non-vaccinated (21.2%; RR, 0.29).22
Our study has several limitations. It includes only patients admitted to hospital, and probably only the most severe cases, therefore it prevents us from determining the global incidence of influenza in HIV infected patients. Likewise, we cannot be sure if the admission criteria were similar in all centers; and we could not determine with any degree of certainty the coverage of the vaccine or its potential efficacy, which is a key aspect in the case of a new epidemic.
To conclude, this study shows the potential severity of the disease, and the benefit of early therapy with oseltamivir in HIV-infected patients with influenza A H1N1. We cannot be sure of the efficacy of vaccination in HIV-infected patients, but our data reinforce the indication for vaccination and, more importantly, emphasize the need for an adequate study of its role both in the prevention of influenza A, and in the prevention of severe cases. Similarly, severely immunodepressed patients are at greater risk of suffering from concomitant bacterial infections.
Conflict of interestThe authors declare no conflicts of interest.
We are grateful to the staff of GESIDA-SEIMC for coordinating the study, particularly Herminia Esteban and Esther Aznar. We thank Lucia Serrano for performing the statistical analysis, and Thomas O’Boyle for editorial assistance.
Hospital Carlos III, Eulalia Valencia Ortega; Hospital La Fe, José López Aldeguer; Hospital San Pedro, José Antonio Oteo, JR Blanco; Hospital Universitario Vall d’Hebron, Adria Curran Fabregas; Hospital Donostia, José Antonio Iribarren, María Jesús Bustinduy; Hospital del Mar, Hernando Knobel; Hospital Txagorritxu, Joseba Portu Zapirain; Hospital de Mataró, Luis Force Sanmartín; Hospital de Navarra, Julio Sola Boneta; Hospital General Universitario de Elche, Félix Gutierrez Rodero; Hospital Clínico Universitario de Valencia, Ana Ferrer Ribera; Hospital Bellvitge, Daniel Podzamzer; Hospital Marina Baixa (Villajoyosa), Concepción Amador Prous; Hospital Universitario de Guadalajara, Miguel Torralba González de Suso; Hospital Sant Pau i Santa Tecla, Rafael Ramírez Montesinos; Hospital Severo Ochoa, Miguel Cervero Jiménez; Hospital Universitario de Valme, Fernando Lozano de León; Hospital Clínico San Carlos, Vicente Estrada Pérez; Hospital Universitario de Canarias, Juan Luis Gómez Sirvent; Hospital General Yague, Carlos Dueñas Gutierrez; Hospital La Paz, Marta Mora Rillo; Hospital Universitario de Getafe, Gabriel Gaspar Alonso-Vega; Hospital Virgen de la Victoria, Jesús Santos; FUNDACIÓN SEIMC–GESIDA, Elena Barquilla.