We describe the pertussis epidemic, based only on confirmed whooping cough cases. We have analyzed data on the diagnosis, epidemiology and vaccine history in order to understand the factors that might explain the trends of the disease.
MethodsA descriptive study of the confirmed pertussis cases reported during 2011 in the Vallès region (population 1,283,000). Laboratory criteria for confirmed pertussis cases include isolation of Bordetella pertussis from a clinical specimen or detection of B. pertussis by PCR in nasopharyngeal swabs.
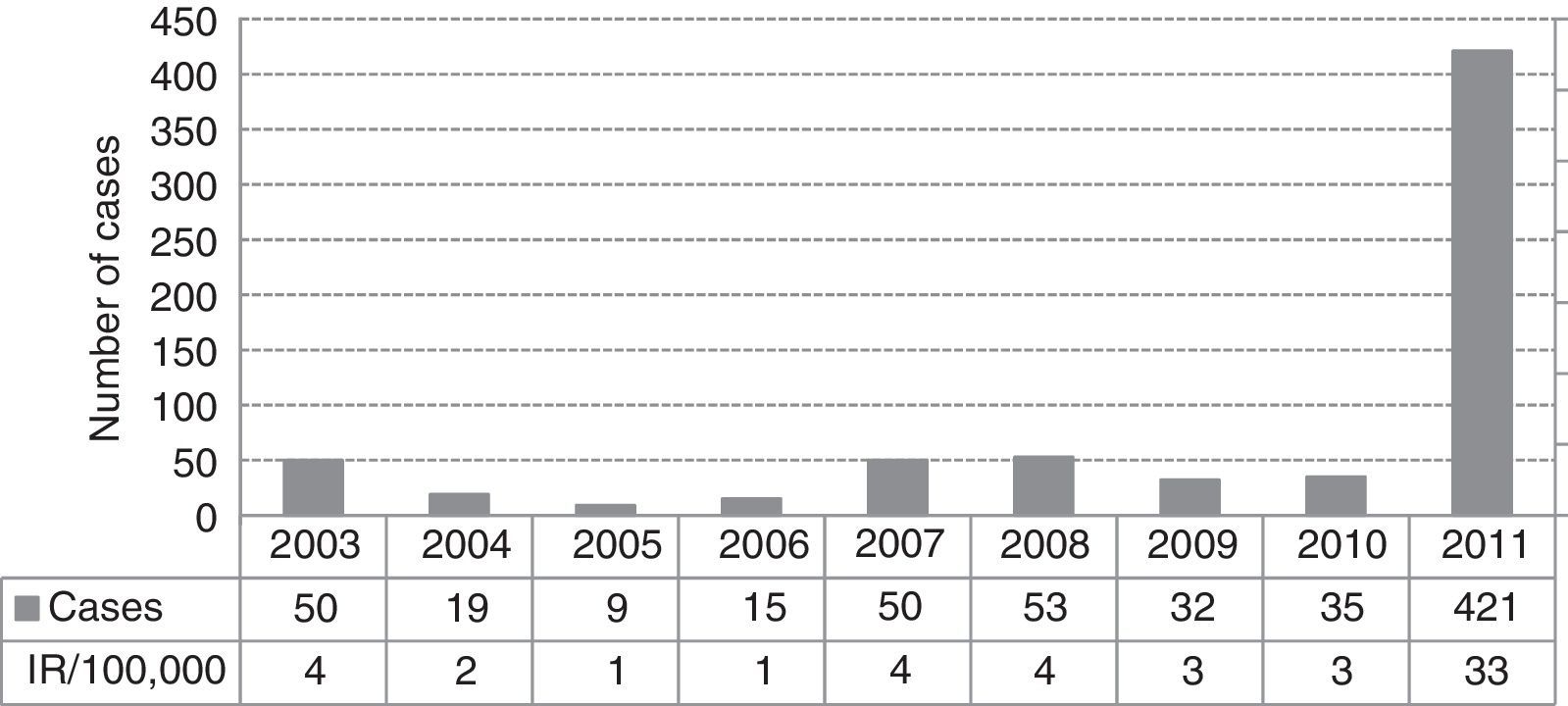
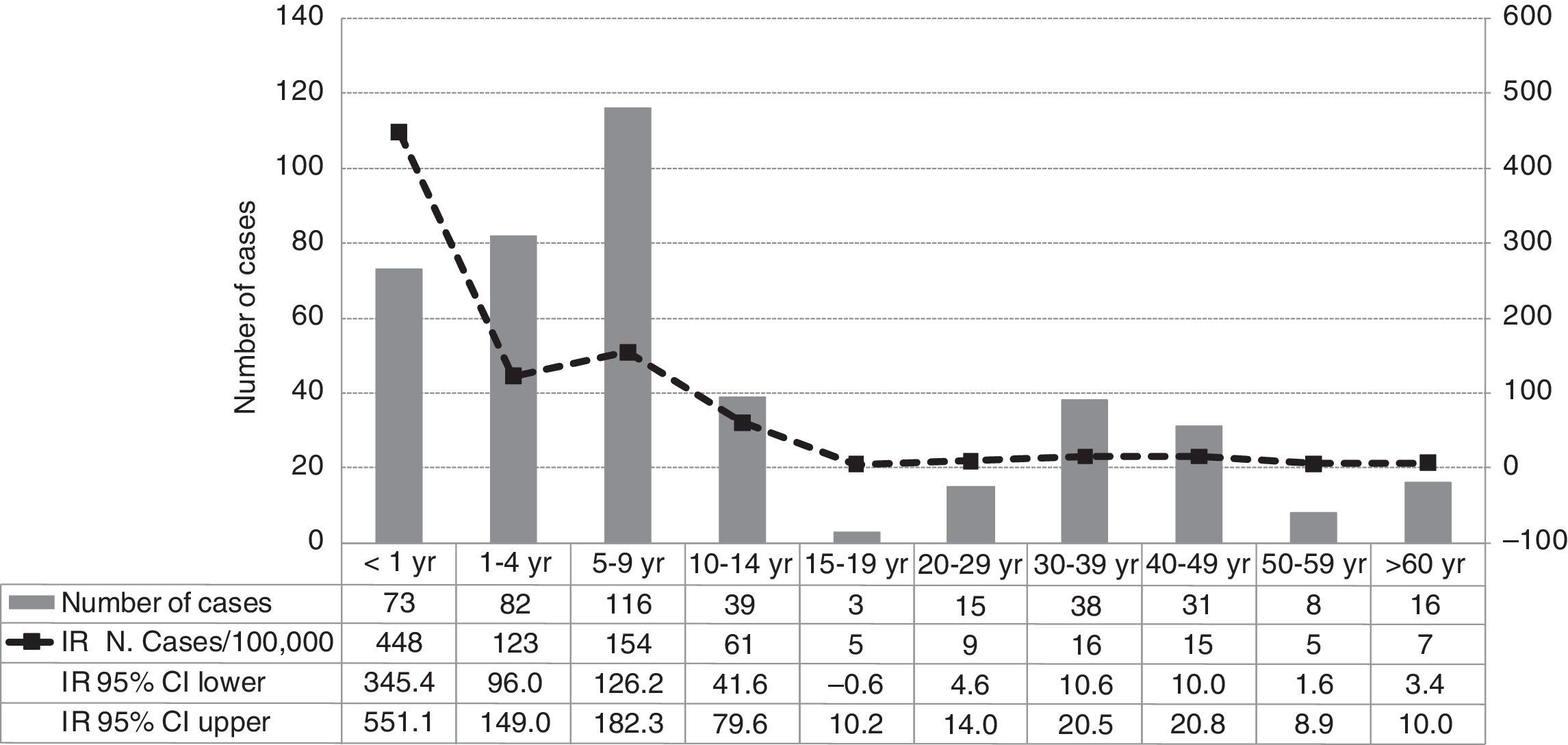
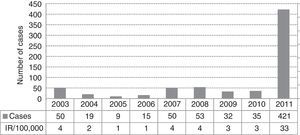
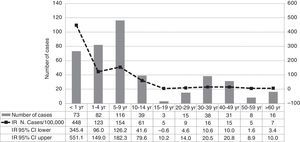
ResultsA total of 421 pertussis confirmed cases were reported, which was the highest incidence reported in the last decade (33 cases/100,000 people/year in 2011). The highest incidence rate was among infants less than 1 year old (448/100,000), followed by children 5–9 years old (154/100,000). Pertussis cases aged 2 months–1 year were 90% vaccinated following the current DTaP schedule for their age group in Catalonia, and cases of 5–9 years were 87% fully vaccinated with 5 doses of DTaP vaccine. There were no deaths, although 8% of cases were hospitalized. Pertussis was more severe in infants, 30% required hospitalization despite having received the vaccine doses corresponding to their age. Children of 5–9 years were most often identified as primary cases in households or school clusters.
ConclusionDespite high levels of vaccination coverage, pertussis circulation cannot be controlled at all. The results question the efficacy of the present immunization programmes.
Se describe la epidemia de tos ferina en el año 2011 solo en casos confirmados. Se analizan datos sobre diagnóstico, epidemiología y antecedentes vacunales que podrían explicar las tendencias de la enfermedad.
MétodosEstudio descriptivo de los casos confirmados de tos ferina notificados durante 2011 en la región del Vallès (población 1.283.000habitantes). Los criterios de laboratorio para confirmación de un caso incluyen el aislamiento de Bordetella pertussis mediante cultivo en una muestra clínica o detección de B.pertussis por PCR en muestras nasofaríngeas.
ResultadosFueron declarados 421 casos confirmados, siendo la incidencia más alta de los últimos 10años (33casos por 100.000personas/año en 2011). La mayor tasa de incidencia fue en niños <1año de edad (448/100.000), seguido de los de 5-9años (154/100.000). Los casos entre 2meses y 1año de edad estaban el 90% vacunados con DTaP según el calendario vacunal vigente en Cataluña para esta edad, entre 5-9años el 87% estaban completamente vacunados con 5dosis de DTaP. No hubo defunciones, pero el 8% de los casos fueron hospitalizados. La enfermedad fue más grave en <1año, y el 30% fueron hospitalizados a pesar de estar bien vacunados para su edad. Los casos de 5-9años fueron más frecuentemente identificados como casos primarios en los hogares o grupos escolares.
ConclusiónA pesar de los altos niveles de cobertura vacunal, la circulación de la tos ferina no se puede controlar del todo. Los resultados ponen en duda la eficacia de los programas de inmunización actuales.
Classical pertussis is initially mild but develops into severe coughing fits characterized by an inspiratory whoop and frequently followed by vomiting. The cough gradually subsides over a period of weeks to months. Complications are more frequent among infants and include apnea, pneumonia, seizures and death.1 In the past, pertussis was an important cause of childhood morbidity and mortality.2 Fortunately, the burden of pertussis morbidity and mortality has been reduced over the last several decades. Although it seems evident that the vaccine does not provide sufficient protection, the vaccine has changed the epidemiology of pertussis and in the present vaccine era the disease is usually mild in immunized children and adults. In the last years a resurgence of pertussis has been documented in different countries all over the world with high coverage of DTaP vaccine (diphtheria, tetanus and acellular pertussis),3–8 including Catalonia, where the DTaP coverage in children is over 95%.9
Paediatric a-cellular pertussis vaccine (aP) has been used in Catalonia since 2002, replacing the whole-cell vaccines (wP) that were used before. The B. pertussis vaccine contains 3 antigens and consists of a five-shot series referred to as DTaP, recommended for children at ages two, four, six and eighteen months, and at four to six years old. Despite high vaccine coverage, pertussis is the most frequent vaccine-preventable childhood disease reported in Catalonia. The incidence has been increasing in recent years including fully vaccinated cases.10 Since 2003 the diagnostic methods available are B. pertussis culture and PCR for hospitals and PCR for primary healthcare centres.
We describe the pertussis epidemic based only on confirmed whooping cough cases reported during 2011 in the Vallès region (population 1,283,000), in the Barcelona northern metropolitan area. We have analyzed the available data on diagnostic, clinical, epidemiology and vaccine status in order to understand the factors that might explain the trends of the disease.
MethodsWe conducted a descriptive study of the confirmed cases of pertussis from the Vallès region reported in 2011. Pertussis is a statutory notifiable disease in Spain. Cases from the Vallès region are reported to the Epidemiological Surveillance Unit of Vallès (ESUVV). A clinical or probable case was a person with an unexplained cough lasting at least 2 weeks with one of the following symptoms: paroxysms of coughing, inspiratory ‘whoop’, post-tussive vomiting or apnea. A confirmed case definition was the standard for the statutory reporting in Catalonia: it was a person who met the clinical definition and had laboratory confirmed pertussis by isolation of B. pertussis from a clinical specimen or detection of IS481 and IS1002 B. pertussis genes by real time polymerase chain reaction (PCR) in either nasopharyngeal swabs or nasopharyngeal aspirates. A confirmed case was also a person who met the clinical definition and was epidemiologically linked directly to a case confirmed by either culture or PCR. Cultures and serological test were not routinely made during this epidemic however swabs for PCR were available for all primary healthcare centres and hospitals. Until 2008 PCR testing was performed only by one laboratory, the Sant Joan de Déu Hospital Molecular Microbiology Department, but since 2009 the PCR test was more easily available because it was routinely provided by another laboratory (CATLAB).
A cluster was defined as two or more epidemiologically linked cases (same households, classroom, etc.). When clusters of pertussis were detected attempts were made to find the probable source of the infection, which is the primary case. The primary case was defined as the first individual in the household or school with a cough and met the clinical definition of pertussis.
Socio-demographic data, clinical and vaccine history, diagnostic tests, and epidemiological information were recorded by the ESUVV from all the cases with a standardized questionnaire. All reported cases were asked about other cases among household and family members. Parents or caregivers were interviewed when the cases were children. The information on vaccination was obtained from the computerized register of primary healthcare medical records: the number of DTP doses received until the symptoms onset and the time when the doses were administrated. Cases which had received all the vaccination doses corresponding to their age were classified as well vaccinated. The Incidence rate (IR) by age group with 95% confidence interval was calculated. Census data from 2003 to 2011 were the source for the Vallès population. The diagnosis delay was the time between the onset of cough and diagnostic confirmation by PCR or culture. One-way analysis of variance (ANOVA) was used to determine whether there were any significant differences between the diagnosis delay means of different age groups.
Follow-up was undertaken on cases with a cough lasting less than 14 days or if they had been hospitalized. Household contacts and classmates of confirmed cases with cough were requested to visit the medical centre.
Data were entered into Health Department-maintained databases and analyzed by SPSS v18. A descriptive study was performed with demographic data (sex and age), vaccination status, diagnostic confirmation, diagnostic delay, hospitalization and clusters of all the confirmed cases reported to ESUVV during 2011.
ResultsIn 2011 were reported 515 clinical cases of pertussis in the Vallès region, a total of 421 cases (82%) met the definition of a confirmed case and were included in the study. The IR of reported and confirmed pertussis was 33 cases per 100,000 in 2011, that is the largest number of confirmed cases reported in the last ten years and an 11-fold increase from 2010 (Fig. 1). Females accounted for 57% of cases. Cases aged 5–9 years were the most frequently reported (28%), followed by those aged 1–4 years (20%) and <1 year (17%). Rates of pertussis were highest in children (Fig. 2) especially in infants aged <1 year with an IR of 448 per 100,000 (95% CI, 345.4–551.1) followed by children aged 5–9 years (IR of 154 per 100,000; 95% CI, 126.2–182.3) and 1–4 years (IR of 123 per 100,000; 95% CI, 96–149). The lowest IR was in adolescents aged 15–19 years.
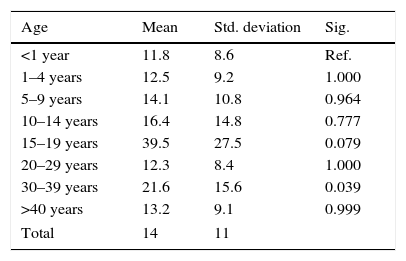
Pertussis was laboratory-confirmed in 83% of cases: 344 cases by PCR alone (82%), 8 by both PCR and culture (2%) and 69 through epidemiological linkage (16%). The diagnosis delay mean was 14 days, the shortest delay occurred in infants aged <1 year (11.8 days) and in adults aged 30–39 years was significantly highest (p=0.03) (Table 1). The results of one-way ANOVA also show that the diagnosis delays mean differed significantly between children and adults (p=0.004): was higher in cases aged 15–39 years (19.7 days; 95% CI, 14.7–24.6) than in children cases <15 years (13.3 days; 95% CI, 12.1–14.6).
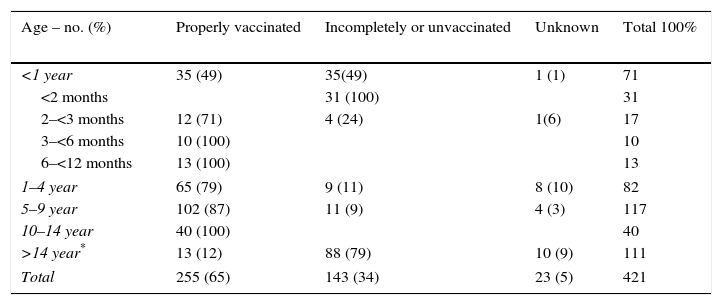
The vaccination status was known for 96% of the cases under 14 years old and 78% of them were fully vaccinated with DTaP in accordance with the Catalan schedule for DTaP. Infants aged <1 year were 49% well vaccinated according to DTaP schedule for their age group (Table 2) but 31 cases were <2 months old too young to have any doses of vaccine. The cases aged 1–4 years 79% had received 4 DTaP vaccine doses and the cases aged 5–9 years 87.2% had received 5 doses. All cases of 10 to 14 years were fully vaccinated.
Vaccination status by age group distribution.
| Age – no. (%) | Properly vaccinated | Incompletely or unvaccinated | Unknown | Total 100% |
|---|---|---|---|---|
| <1 year | 35 (49) | 35(49) | 1 (1) | 71 |
| <2 months | 31 (100) | 31 | ||
| 2–<3 months | 12 (71) | 4 (24) | 1(6) | 17 |
| 3–<6 months | 10 (100) | 10 | ||
| 6–<12 months | 13 (100) | 13 | ||
| 1–4 year | 65 (79) | 9 (11) | 8 (10) | 82 |
| 5–9 year | 102 (87) | 11 (9) | 4 (3) | 117 |
| 10–14 year | 40 (100) | 40 | ||
| >14 year* | 13 (12) | 88 (79) | 10 (9) | 111 |
| Total | 255 (65) | 143 (34) | 23 (5) | 421 |
Around 8% of cases required hospitalization for 14 days on average. Most cases (30 of 33 hospitalized cases) were infants aged <1 year. Among these 30 infants, 21 (70%) were unvaccinated because they were too young. Nine cases aged 2 months–1 year required hospitalization despite having received some DTaP doses according to the DTaP schedule. Hospitalization were required because the symptoms intensity such apnea and need of supplemental oxygen. There were not cases with serious complications such as pneumonia, seizures or encephalopathy.
We detected 70 family or school clusters of pertussis. In 59 clusters (84%) the primary case was identified. The most frequent primary cases were children aged 5–9 years (29%), followed by adults aged 30–39 years (22%) and children aged 1–4 years (19%). All primary cases aged <10 years (49% of primary cases) were fully vaccinated, except one that was too young to be vaccinated.
DiscussionA number of reasons have been given for the resurgence of pertussis in vaccinated children, although it seems to be not so much related to genetic changes in B. pertussis11,12 as to lessened potency of pertussis vaccines—it has been shown that the efficacy of DTP vaccines was greater than DTaP vaccines13—and to the waning of vaccine-induced immunity, which appears to decrease more quickly than previously thought.14 Other factors that may influence the detection of cases are greater awareness of pertussis and the availability of better laboratory tests.15 In this study on whooping cough in the Vallès region it should be borne in mind that although in the last three years the PCR test was more easily available, the PCR technique has been used since 2003 in the healthcare centres of the Vallès region. Thus the large increase in pertussis incidence in 2011 cannot be attributed only to the availability of PCR. Probably the true incidence of pertussis was even greater because, in order to have more objective conclusions, the study focused only on confirmed cases by laboratory or epidemiological relationship, discarding reported illness based only on clinical criteria of pertussis. The high incidence in 2011 cannot be attributed to PCR sensitivity or other Bordetella species because the IS481 and IS1002 sequential PCR assay was used by both laboratories in the study. This method discriminates B. pertussis from other clinically relevant Bordetella species,16 its use is recommended by ECDC since PCR has proved to be more sensitive and faster than culture and suitable for children and infants during the early stage of the disease.
The incidence of pertussis in 2011 in the Vallès region was the highest reported in the last 10 years, and coincides with the fact that the majority of the cases <15 years were fully vaccinated with DTaP according with Catalan vaccination schedule. Adolescents aged among 15–19 years accounted for only 1% of cases, the age group with the lowest IR. It is very likely that these adolescents received one or several doses of DTwP vaccine—more effective than DTaP—when they were children, which would partly explain the lower IR. The highest IR was in children aged 5–9 years, nearly 90% of them with 5 DTaP doses and in children aged 1–4 years, 80% of them had received 3–4 doses of DTaP vaccine. In addition, pertussis occurred not only in fully vaccinated children who had just received their fifth DTaP dose, but also in many immunized infants, including nine infants aged <1 year with at least one vaccine dose who needed to be hospitalized due to serious illness. All of these findings raise questions regarding the efficacy of the DTaP vaccine. Similar ecological data have been observed in other pertussis outbreaks, suggesting that the durability of the DTaP vaccine is less than expected,4,5,14,15,17–20 in contrast to that observed in outbreaks ten years ago.6,14,21–23
In clusters with the probable source of infection was identified, 29% of primary cases were fully vaccinated children. The role of other small children as the source of infection has been documented in recent outbreaks.24 Other studies provide evidence that adolescents and adults at home but also nonhousehold close contacts are important sources in transmitting the disease to more vulnerable children25 and for this reason in many countries vaccination with Tdap is recommended in adolescents and adults to reduce the burden of infant pertussis.26 This recommendation has not been made in Catalonia although in our study 49% of primary cases identified as the possible source of the infection could be targeted for Tdap.
It is very likely that incidence is higher in children also because paediatricians tend to make pertussis diagnosis more often than general practitioners, especially if the patients are infants who suffer more severe disease. Thus an observer bias could occur in regard to the age distribution of pertussis, although it does not influence the vaccine background of the cases detected and information about the DTaP doses received by cases. Underdiagnosis and underreporting could occur due to mild or atypical forms of pertussis. Pertussis in adolescents and adults may be overlooked because symptoms are often atypical, patients may have less severe paroyxmal symptoms, frequently they present only prolonged coughing but without other typical findings such as whooping or vomiting. Adults generally do not present for medical attention until they have been coughing for a prolonged time and their PCR are often negative. In addition, clinicians may not consider pertussis in the differential diagnosis of respiratory illnesses in adults resulting in diagnosis failure or delay. The low IR and the higher diagnosis delay in adults found in our study would be explained by these facts. Detection and reporting of pertussis can also be influenced by the clinical definition of cases, but it should be more specific and/or more sensitive, taking into account the signs and symptoms difference by age.27 In fact one recent study also showed the incidence of pertussis in the elderly and that the morbidity and mortality associated with this age group should not be underrated.28 Pertussis in adults and in elderly people not only results in direct morbidity in these age groups, but also poses a transmission risk to susceptible non-immune infants who are often too young to be vaccinated, as many senior citizens are carers of infants. Pertussis should be considered in the differential diagnosis of cough illness on patients of all ages, even when they are fully vaccinated. Early recognition and confirmation of pertussis are essential to limit the spread of the disease. Despite atypical presentations, when carefully questioned, most adolescents and adults with pertussis report paroxysmal symptoms.27 The availability of diagnosis tests at health centres has improved the sensitivity, since PCR is widely used, and it is especially sensitive when used less than 3 weeks after cough onset.27 Nevertheless, if laboratory diagnosis has not been made during the first 3 weeks of cough or if PCR is made later, regardless of test results physicians should treat clinically suspected pertussis with antimicrobials and report cases to the health department.29
In summary, infants aged <1 year have the highest reported incidence of pertussis. Immunity from the vaccine is not yet complete in the first year of life and childhood pertussis DTaP vaccine has not created the herd immunity that might protect incompletely vaccinated infants. Despite childhood vaccination, the resurgence of pertussis observed in infants as yet too young to have completed their three-dose vaccination schedule, should raise awareness of the need for new immunization strategies to reduce the window of vulnerability to pertussis in infants. Whooping cough can occur in children despite their being well vaccinated with five DTaP doses, suggesting that the DTaP vaccine does not confer complete immunity against pertussis. Incomplete vaccination, imperfect vaccine effectiveness, waning immunity and delays in diagnosis and treatment together can explain the pertussis epidemic.15 Although all these facts should be taken into account to control pertussis, the current vaccine schedule may be insufficient to prevent a pertussis epidemic, in fact in recently published data the resurgence of pertussis appear attributable to waning immunity from DTaP vaccines.19,20 While the current vaccine does not improve a multipronged approach will be needed5,30,31 particularly strategies aimed at improving direct protection for newborns and infants (adult and adolescent vaccination, mothers and household contacts after birth or maternal vaccination during pregnancy), along with an acceleration of the vaccine primary series for infants under 6–8 weeks old32 or the neonatal priming with anti-pertussis vaccines should be considered26,33,34 as well as research into more durable and effective vaccines.
Conflict of interestNo conflict of interest.
The authors want to thank all health professionals from primary healthcare centres, hospitals and microbiological services who reported cases or helped with the management of the cases and the implementation of control measures for the close contacts.