Acute otitis media is the most common respiratory tract infection in infancy and early childhood that is managed with antimicrobial agents. Ninety-three per cent of the cases diagnosed in Spain are treated with antibiotics, and Streptococcus pneumoniae and untypeable Haemophilus influenzae are the most frequently isolated pathogens. The aim of this work was to evaluate the usefulness of amoxicillin, amoxicillin/clavulanate and ceftriaxone for the empirical treatment of acute otitis media, looking at the pharmacokinetic variability and the antimicrobial susceptibility of paediatric strains of the two main pathogens responsible for AOM in Spain, Streptococcus pneumoniae and Haemophilus influenzae.
MethodsFree-drug plasma concentrations were simulated and the probability of target attainment at each minimum inhibitory concentration and the cumulative fraction of response (CFR) were determined. Microbiological susceptibility information was extracted from SAUCE 3 surveillance.
ResultsCFR with amoxicillin varied from 83% to 96% against S. pneumoniae and from 78% to 86% against H. influenzae. CFR was always >85% with amoxicillin/clavulanate. With the 3-day ceftriaxone regimen, the probability of achieving free concentrations above MIC at 72hours significantly increased compared to the single dose, with which CFR ranged from 70% to 84%.
ConclusionsHigh-dose amoxicillin (at least 80mg/kg/day) should be the first-line therapy in uncomplicated infections, whereas amoxicillin/clavulanate (40mg/kg/day) should be the choice when additional coverage for H. influenzae is desired. Administration of 3 daily doses of ceftriaxone increases bacteriological eradication probability when compared with one-day regimen, although additional clinical evaluations are necessary to establish the best target attainment with ceftriaxone.
La otitis media aguda (OMA) es la infección del tracto respiratorio más común en la infancia que es tratada con agentes antimicrobianos. El noventa y tres por ciento de los casos diagnosticados en España se tratan con antibióticos, siendo Streptococcus pneumoniae y Haemophilus influenzae no tipable los patógenos aislados más frecuentes. El objetivo de este trabajo ha sido evaluar la utilidad de amoxicilina, amoxicilina/clavulánico y ceftriaxona en el tratamiento empírico de OMA teniendo en cuenta la variabilidad farmacocinética y la sensibilidad antimicrobiana de las cepas pediátricas de los dos patógenos principales responsables de OMA en España, Streptococcus pneumoniae y Haemophilus influenzae.
MétodosSe simularon las concentraciones de fármaco libre para cada antibiótico y se calculó la probabilidad de alcanzar el objetivo terapéutico para cada valor de concentración mínima inhibitoria (CMI) y la fracción de respuesta acumulada (CFR).
ResultadosLa CFR de amoxicilina osció entre el 83% y el 96% frente a S. pneumoniae y entre el 78% y el 86% para H. influenzae. En el caso de amoxicilina/clavulánico, la CFR fue siempre >85%. Con ceftriaxona durante 3 días, la probabilidad de alcanzar concentraciones libres por encima de la CMI a las 72 horas fue significativamente superior a la probabilidad obtenida con una sola dosis, con valores de CFR que oscilaron entre el 70% y el 84%.
ConclusionesAmoxicilina a altas dosis debería ser la primera opción para el tratamiento de infecciones no complicadas, mientras que amoxicilina/clavulánico deberá utilizarse cuando se sospecha que H. influenzae puede ser responsable de la infección. La administración de ceftriaxona durante 3 días incrementa la probabilidad de erradicar la infección repecto a la administración de una única dosis, aunque son necesarios estudios clínicos para establecer el mejor objetivo terapéutico con ceftriaxona.
Acute otitis media (AOM) is the most common respiratory tract infection in infancy and early childhood that is managed with antimicrobial agents1. Ninety-three per cent of the cases diagnosed as AOM in Spain are treated with antibiotics2, and Streptococcus pneumoniae and untypeable Haemophilus influenzae are the most frequently isolated pathogens.
It is well known that antibiotics shorten the course of AOM, but many cases remit spontaneously with no complications. Thus clinicians should avoid prescribing antibiotics routinely. Initial observation seems to be suitable for many children, if follow-up can be assured, whereas antibiotic treatment may be necessary in the very young (spontaneous resolution is lower in children younger than 2 years) or in severe or prolonged cases. When antibiotic therapy is indicated, selection of the most appropriate antibiotic should be based on the patient's risk factors, physical examination, symptoms, local resistance patterns and treatment guidelines. By using pharmacokinetic (PK) and pharmacodynamic (PD) principles, an evaluation of the usefulness of treatments with antibiotics can be made in order to predict the likelihood of a successful clinical outcome3.
In patients treated with ß-lactams, bacteriological eradication can be predicted if free drug concentrations at the site of infection are above the minimum inhibitory concentrations (ƒT>MIC) of the pathogen for a time interval that exceeds 40%-50% of the dosing interval4.
In a recent study5, we evaluated the antimicrobial treatments in children with AOM in Spain taking into account the PK/PD approach. Only ceftriaxone and high-dose amoxicillin/clavulanate provided adequate efficacy indexes against S. pneumoniae and H. influenzae. Macrolides and azithromycin were not included in the study as they should not be empirically used in Spain due to the resistance of S. pneumoniae.
In that study, neither PK nor PD variability were considered. But, in fact, all organisms display a range of susceptibilities to any given antibiotic. Besides, a distribution of serum antibiotic concentration is observed in any population receiving the same antibiotic. Thus, the main objective of this work was to evaluate the usefulness of amoxicillin, amoxicillin/clavulanate and ceftriaxone for the treatment of AOM in Spain, assessing the probability of achieving the requisite PD exposure against S. pneumoniae and H. influenzae and taking into account the PK and PD variability in a simulated paediatric population.
MethodsAcquisition of microbiological dataInformation on the minimum inhibitory concentration (MIC) values of amoxicillin, amoxicillin/clavulanate and ceftriaxone from 373 paediatric strains of S. pneumoniae and 438 of H. influenzae was extracted from the SAUCE 3 surveillance and provided by the Medical Department of GlaxoSmithKline6,7. The clinical isolates of S. pneumoniae and H. influenzae were obtained from community-acquired respiratory tract infections and collected between November 2001 and October 2002. Around 49% of the S. pneumoniae and 32% of the H. influenzae isolates were from middle ear samples, whereas 32% and 67%, respectively, were collected from lower respiratory tract and 18% and 1.5% respectively, from blood. A complete description of study is provided in Reference 6.
Monte Carlo simulationThe following drug regimens were evaluated: amoxicillin and amoxicillin/clavulanate 20mg/kg, 40mg/kg, 45mg/kg and 50mg/kg every 12h (q12h) and 13mg/kg, 27mg/kg, 30mg/kg and 33mg/kg every 8h (q8h) orally; ceftriaxone 50mg/kg and 100mg/kg intramuscularly and intravenously, single dose and a 3-day regimen. A 5000 subject Monte Carlo simulation was employed to recreate steady-state free drug plasma concentration-time profiles on the basis of models and model parameter estimates reported previously in paediatrics8,9.
Amoxicillin profiles were generated using the information provided by Canafax et al8, assuming a one-compartment model. The mean population values for apparent volume of distribution (V/F), first order elimination rate constant (K) and first order absorption rate constant (Ka) were 1.44 L/kg, 0.276h−1, and 1.77h−1, respectively. The value of the unbound fraction (fu) in plasma was 0.8. The values for interindividual variability (IIV) (expressed as coefficient of variation) were 26%, 50% and 56%, respectively.
Ceftriaxone plasma concentration-time profiles were created based on a recent pharmacokinetic evaluation9. A three compartment model was used with the following pharmacokinetic parameter values (IIV expressed as coefficient of variation in parenthesis): V/F (L): 2.13 (84); apparent total plasma clearance [CL/F (L/h)]: 0.556 (27); K23 (h−1): 3.12 (78); K32 (h−1): 5.78 (37); K24 (h−1): 4.73(69); K42 (h−1): 6.13 (61), where the K's correspond to the first order rate distribution constant between the central and peripheral compartments, or absorption. When intramuscular administration was considered, bioavailability (F) and Ka (h−1) of 1 and 3.09 (183), were used.
All simulations were performed with NONMEN (Version V, Level 1.1, GloboMax LLC, Hanover, MD). Individual pharmacokinetic parameters were assumed to follow a log-normal distribution with absence of covariance. The values used for fu and F during the simulation exercise were the typical population estimates. Unbound plasma concentrations were simulated without residual error.
For amoxicillin and amoxicillin/clavulanate, pharmacodynamic exposures for simulated dose regimens were assessed as follows. The time that free drug concentrations were maintained above the MIC (ƒT>MIC) was determined using the Splus software (Insightful, Seattle, WA). The provability of target attainment (PTA) was calculated by counting the subjects who achieved ƒT>MIC for at least 50% of the dosing interval. The cumulative fraction of response (CFR) for each dose administration regimen was calculated by multiplying the PTA at each MIC by the fraction of organism susceptible at that concentration of the respective MIC distribution. The sum of those individual products is the CFR10, and can be interpreted as the probability of successful treatment of infections caused by bacteria with a specific susceptibility pattern in the population studied.
In the case of ceftriaxone, in determining the amount that free plasma concentrations need to exceed MICs to achieve bacteriological eradication, the frequency of times above the MIC for 24, 48, 72, 96, and 120hours was evaluated. CFR was calculated as the probability to achieve ƒT>MIC considering the MIC distribution.
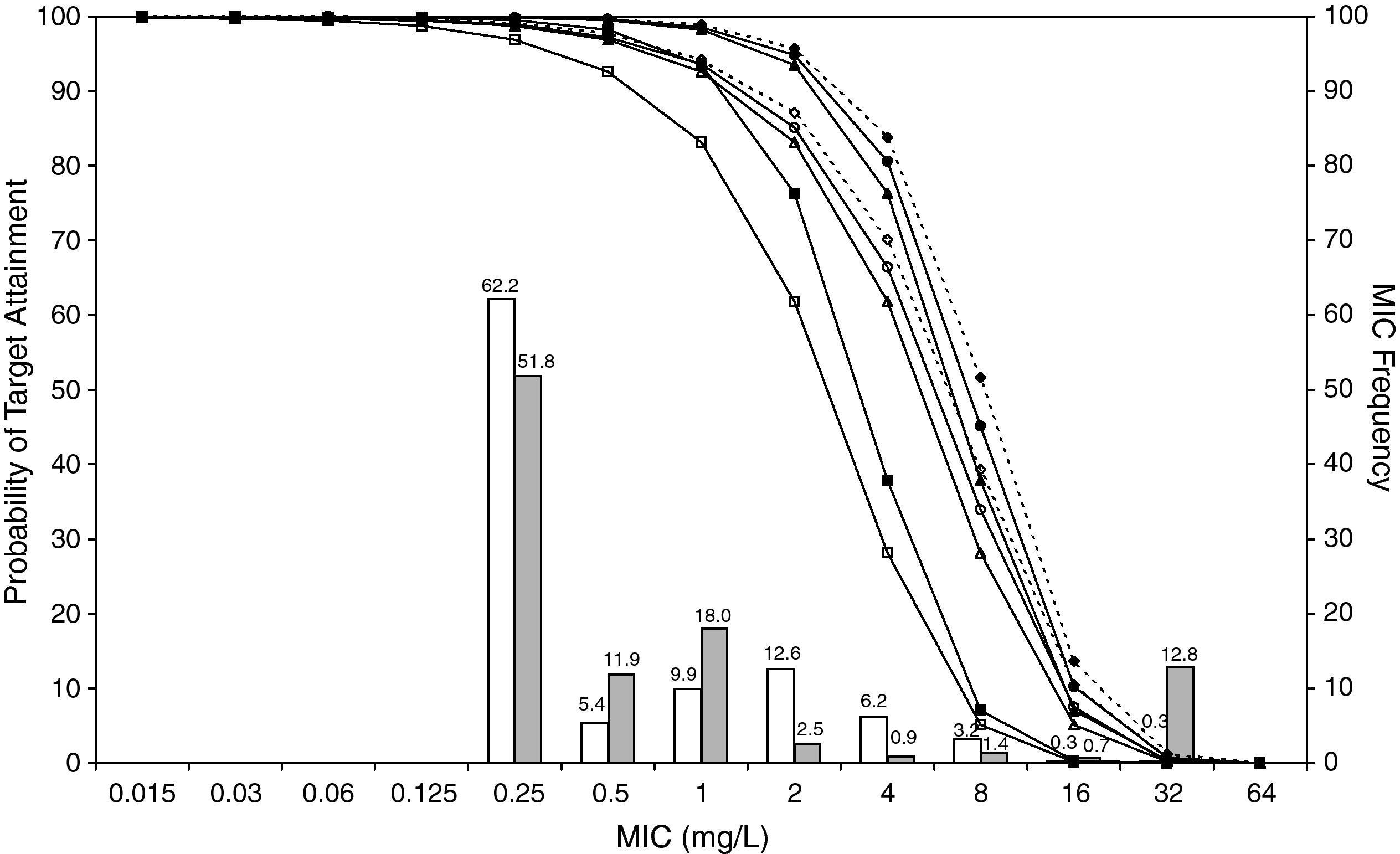
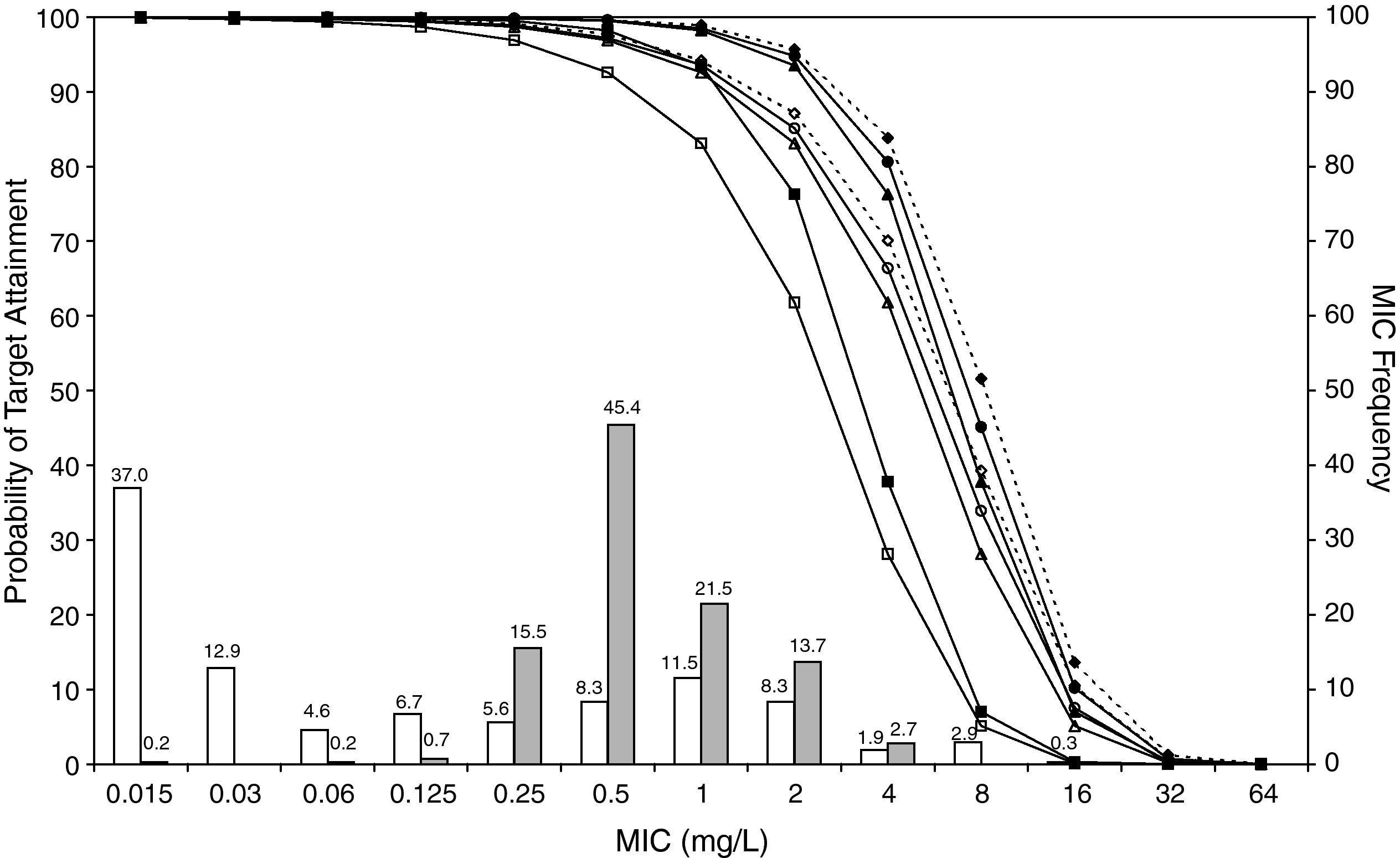
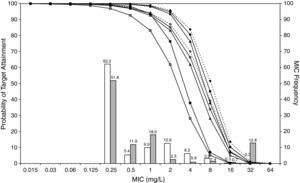
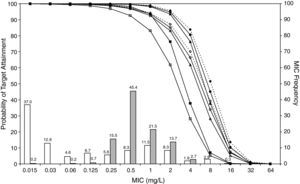
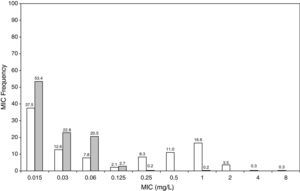
ResultsFigures 1 and 2 show the antimicrobial susceptibility of S. pneumoniae and H. influenzae paediatric strains to amoxicillin and amoxicillin/clavulanate, respectively. According to breakpoints recommended by CLSI for non-meningeal infections11, both were very active against S. pneumoniae with susceptibilities >90%. Amoxicillin/clavulanate was also very active against H. influenzae, with a susceptibility of 100%, but amoxicillin was less active. Among H. influenzae isolates, 15.5% were β-lactamase positive-ampicillin resistant strains and 2.7% were β-lactamase negative ampicillin resistant strains, presenting diminished susceptibility to ampicillin with MIC≥2mg/L.
MIC distribution of amoxicillin against 373 paediatric strains of S. pneumoniae (white bars) and 438 paediatric strains of H. influenzae (grey bars) and probability of target attainment as a function of the MIC for 5000 simulated subjects given amoxicillin. (Open diamond: 50mg/kg every 12h; filled diamond: 33mg/kg every 8h; open circle: 45mg/kg every 12h; filled circle: 30mg/kg every 8h; open triangle: 40mg/kg every 12h; filled triangle: 27mg/kg every 8h; open square: 20mg/kg every 12h; filled square: 13mg/kg every 8h). The chosen target was 50% of the dosing interval of free-amoxicillin plasma concentrations to be in excess of the MIC.
MIC distribution of amoxicillin/clavulanate against 373 paediatric strains of S. pneumoniae (white bars) and 438 paediatric strains of H. influenzae (grey bars) and probability of target attainment as a function of the MIC for 5000 simulated subjects given amoxicillin/clavulanate. (Open diamond: 50mg/kg every 12h; filled diamond: 33mg/kg every 8h; open circle: 45mg/kg every 12h; filled circle: 30mg/kg every 8h; open triangle: 40mg/kg every 12h; filled triangle: 27mg/kg every 8h; open square: 20mg/kg every 12h; filled square: 13mg/kg every 8h). The chosen target was 50% of the dosing interval of free-amoxicillin plasma concentrations to be in excess of the MIC.
The results of the analysis of the PTA by MIC for both antibiotics are also shown in Figures 1 and 2. The achieved PTA with high doses (≥80mg/kg/day) was >80% up to an MIC of 2mg/L. The PTA with the lowest doses was also higher than 80% up to an MIC of 1mg/L.
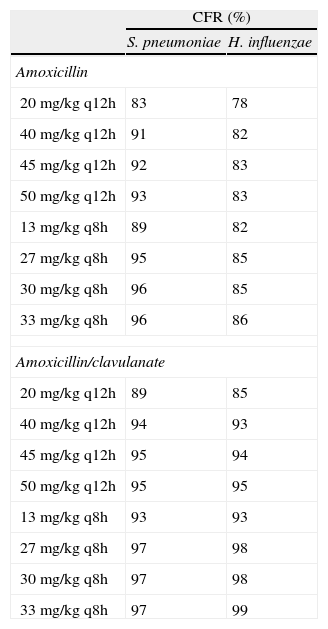
Table 1 shows the assessment of CFR for amoxicillin and amoxicillin/clavulanate. When amoxicillin was evaluated, CFR varied from 83% to 96% against S. pneumoniae, and from 78% to 86% against H. influenzae. For amoxicillin/clavulanate, CFR was always >85%.
Expected cumulative fractions of response (CFR) for amoxicillin and amoxicillin/clavulanate. The target chosen was 50% of unbound concentration above the MIC. For amoxicillin/clavulanate, only amoxicillin doses are indicated.
| CFR (%) | ||
| S. pneumoniae | H. influenzae | |
| Amoxicillin | ||
| 20 mg/kg q12h | 83 | 78 |
| 40 mg/kg q12h | 91 | 82 |
| 45 mg/kg q12h | 92 | 83 |
| 50 mg/kg q12h | 93 | 83 |
| 13 mg/kg q8h | 89 | 82 |
| 27 mg/kg q8h | 95 | 85 |
| 30 mg/kg q8h | 96 | 85 |
| 33 mg/kg q8h | 96 | 86 |
| Amoxicillin/clavulanate | ||
| 20 mg/kg q12h | 89 | 85 |
| 40 mg/kg q12h | 94 | 93 |
| 45 mg/kg q12h | 95 | 94 |
| 50 mg/kg q12h | 95 | 95 |
| 13 mg/kg q8h | 93 | 93 |
| 27 mg/kg q8h | 97 | 98 |
| 30 mg/kg q8h | 97 | 98 |
| 33 mg/kg q8h | 97 | 99 |
q12h, every 12h; q8h, every 8h.
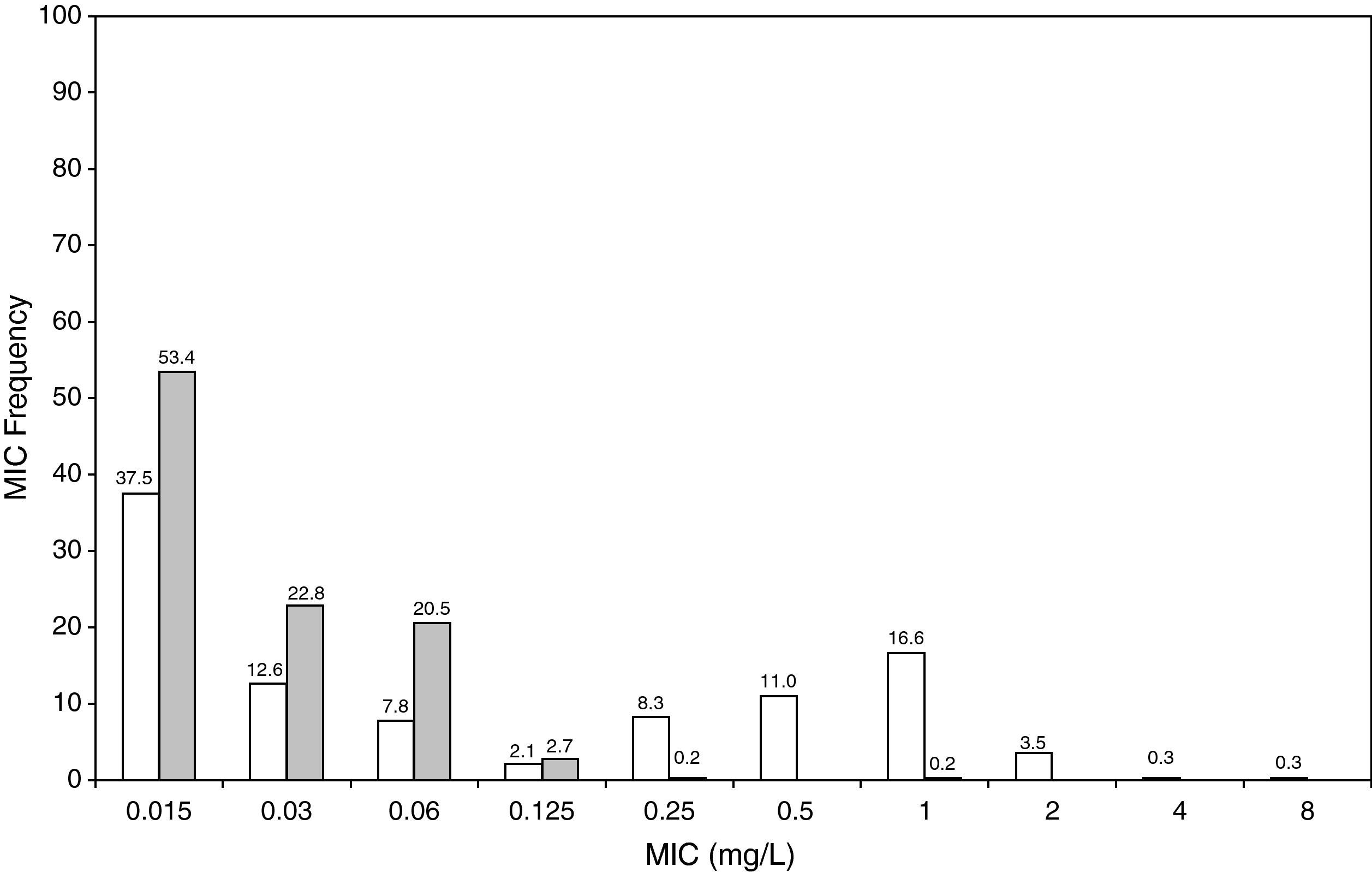
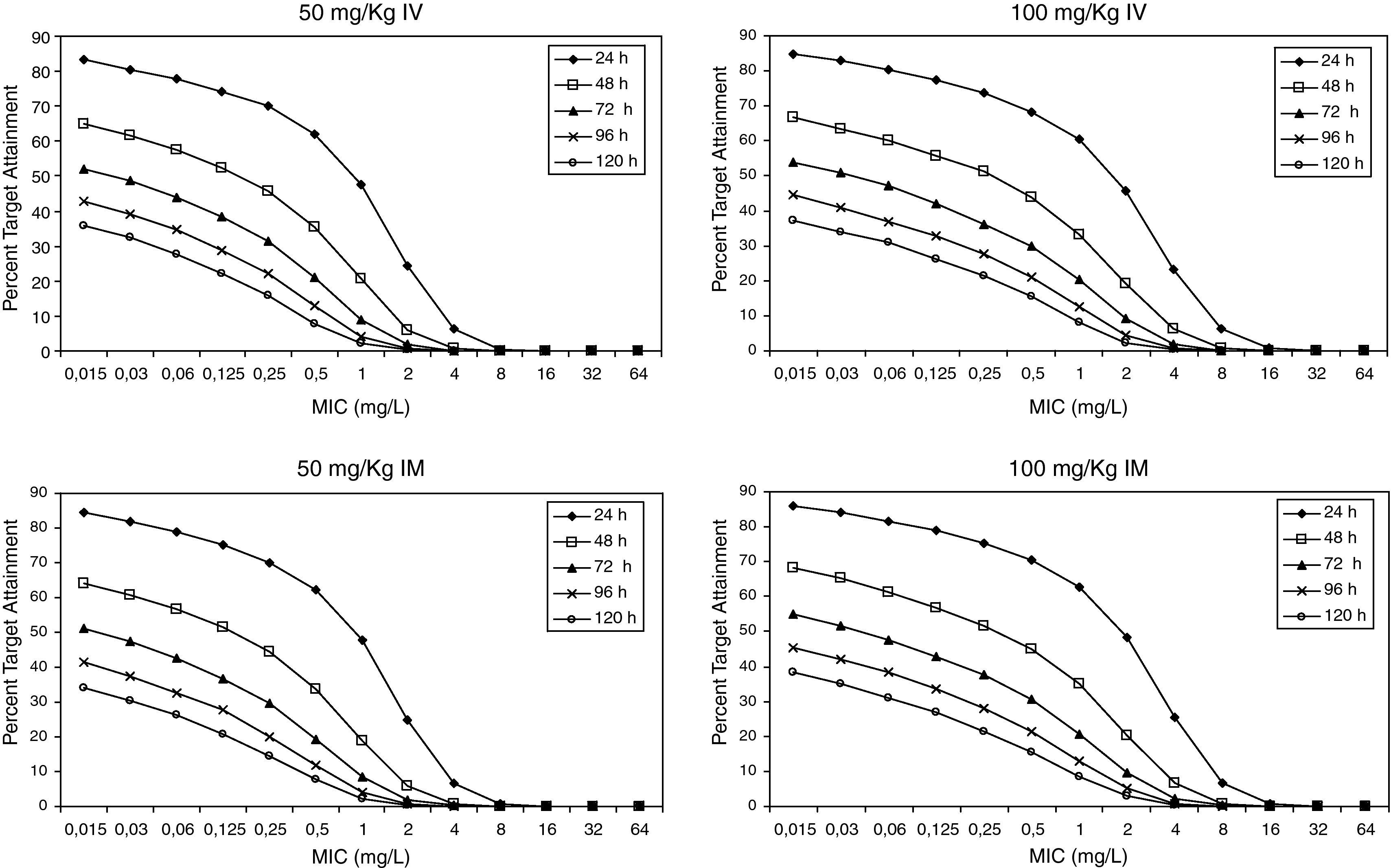
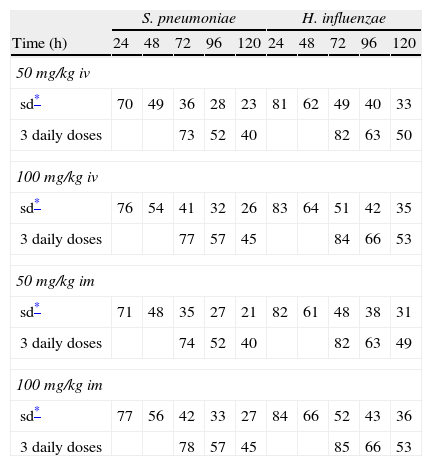
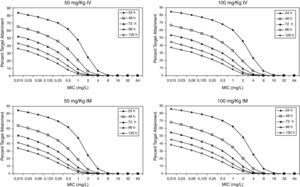
Figure 3 shows the antimicrobial susceptibility for ceftriaxone. This antibiotic was very active against S. pneumoniae and H. influenzae, with susceptibilities of 96% and 100%, respectively. Figure 4 shows ceftriaxone target attainment to maintain free drug concentration above MIC at 24, 48, 72, 96 and 120hours for all posologies. When a single dose of 50mg/kg im ceftriaxone was simulated, the proportion of virtual patients with free plasma concentrations that exceeded the MICs of 0.015, 0.03, 0.06, 0.125 and 0.25mg/L at 24hours was 85%, 83%, 80%, 77% and 73%, respectively. These values decreased further as time after dosing increased (at 48, 72, 96 and 120hours post-dose). Slightly more favourable results were achieved with the highest dose (100mg/kg iv or im). Considering the antimicrobial susceptibility of H. influenzae to ceftriaxone (99.8% isolates presented MICs ≤0.25mg/L), all dose regimens provided ƒT>MIC longer than 24hours in more than 70% of the patients. However, a significant number of strains had MICs of 1mg/L (16.6%) or 2mg/L (3.5%) against S. pneumoniae. In these cases, target attainment at 24hours decreased significantly (50% for 50mg/kg and 60% for 100mg/kg). Consecutively, CFR values were higher for H. influenzae than for S. pneumoniae (Table 2). When the 3-day regimen is considered, the probability to achieve ƒT>MIC for 72, 96 and 120hours significantly increased compared to the single dose.
Expected cumulative fractions of response (CFR) for ceftriaxone. The target chosen was ƒT>MIC at 24, 48, 72, 96 and 120h.
| S. pneumoniae | H. influenzae | |||||||||
| Time (h) | 24 | 48 | 72 | 96 | 120 | 24 | 48 | 72 | 96 | 120 |
| 50 mg/kg iv | ||||||||||
| sd* | 70 | 49 | 36 | 28 | 23 | 81 | 62 | 49 | 40 | 33 |
| 3 daily doses | 73 | 52 | 40 | 82 | 63 | 50 | ||||
| 100 mg/kg iv | ||||||||||
| sd* | 76 | 54 | 41 | 32 | 26 | 83 | 64 | 51 | 42 | 35 |
| 3 daily doses | 77 | 57 | 45 | 84 | 66 | 53 | ||||
| 50 mg/kg im | ||||||||||
| sd* | 71 | 48 | 35 | 27 | 21 | 82 | 61 | 48 | 38 | 31 |
| 3 daily doses | 74 | 52 | 40 | 82 | 63 | 49 | ||||
| 100 mg/kg im | ||||||||||
| sd* | 77 | 56 | 42 | 33 | 27 | 84 | 66 | 52 | 43 | 36 |
| 3 daily doses | 78 | 57 | 45 | 85 | 66 | 53 | ||||
Considering that AOM is typically treated empirically, the treatment of choice should target the most frequently isolated pathogens. In this study PK/PD simulations were performed to evaluate different dose regimens of amoxicillin, amoxicillin/clavulanate and ceftriaxone, taking into account the antimicrobial susceptibility of paediatric strains of the two main pathogens responsible for the disorder in Spain, S. pneumoniae and H. influenzae, together with the pharmacokinetic variability in paediatric population.
When PK/PD principles are employed, PK and PD profiles of antimicrobials at infection site should be taken into account. However, we have used unbound plasma drug concentrations to perform simulations because they can accurately predict middle ear fluid (MEF) concentrations, as has been explained previously in the literature12–15. Moreover, when the parameter that predicts efficacy is determined, it is by using the plasma levels as a surrogate for what is happening at the site of infection. If a robust relationship can be found between bacterial inhibition and killing and plasma PK/PD, the model may be considered validated16.
In the treatment of infectious diseases one may accept a risk of treatment failure in 10% to 20% of children for infections that are not life-threatening and have low morbidity17. To achieve a CFR ≥90% against S. pneumoniae, high-dose amoxicillin was needed, at least 80mg/kg/day, although with all posologies target expectation was higher than 80%. However, when H. influenzae is the pathogen involved, high-dose amoxicillin had an 82-86% likelihood of achieving the target pharmacodynamic exposure, but CFR ≥90% was never achieved. When considering amoxicillin/clavulanate, a CFR<90% was only obtained when 20mg/kg q12h was simulated.
Current AOM management guidelines recommend high-dose amoxicillin as the first-line drug of choice in children18. However, the probability of a successful outcome of high-dose amoxicillin against H. influenzae calculated using the Global Respiratory Antimicrobial Surveillance Project (GRASP) database19 was lower than 65%. This value, lower than those obtained in our study, could be explained by the regional susceptibility patterns. Amoxicillin susceptibility against S. pneumoniae and H. influenzae was documented in 80.6% and 54.5% of isolates from the GRASP study, whereas in SAUCE 3 it was 90.1% and 81.7%, respectively. These discrepancies justify different recommendations for empirical antibiotic treatment. Considering our results, high-dose amoxicillin should be confirmed as the first-line choice for children with AOM in Spain (CFR>80%). In patients who have severe illness, and in those for whom additional coverage for H. influenzae is desired, selected therapy should be amoxicillin/clavulanate. Studies carried out in the US19 and Israel4, recommend high-dose amoxicillin/clavulanate (90mg/kg/day), but 13mg/kg q8h provides a high probability of achieving the requisite pharmacodynamic exposure in Spain (CFR>90%).
The strains not covered by either amoxicillin or amoxicillin/clavulanate would be responsible for the failure of the treatment with these agents. Dagan4 observed that apart from amoxicillin/clavulanate, ceftriaxone was the only agent that successfully prevented bacterial persistence for the pathogens involved in AOM. For this drug, the target of continuously achieving 36hours of free concentrations in tonsil tissue that exceeded the MIC has been considered as the criterion for defining microbiological success in the treatment of tonsillopharingitis9. A target for treatment of AOM has not been established; this is why we calculated the probability to achieve plasma free drug concentrations above the MIC at 24, 48, 72, 96 and 120h.
Leibovitz et al20 showed a 48% bacteriological failure rate with a single 50mg/kg ceftriaxone dose against penicillin-susceptible S. pneumoniae. Our results do not reflect such a high probability of treatment failure, as a susceptibility of 96% was documented in SAUCE 3, whereas 17-20% of strains isolated in the Leibovitz study were non-susceptible to ceftriaxone.
Estimated CFRs of ceftriaxone for H. influenzae are lower than expected, if we consider that 100% of the Spanish strains are susceptible (MICs≤1mg/L). This could be due to the fact that the target we chose may not be the most suitable one. The time above the MIC during the 24-h dosing interval for MEF is much greater than the ƒT>MIC in serum13. Therefore, a more favourable scenario could be expected if we consider MEF concentrations instead of unbound plasma concentrations. Hence, the search for an adequate target for ceftriaxone in AOM is necessary, as Blumer established for tonsillopharingitis9 and availability of clinical data on microbiological success rate is very important in order to contrast these results.
Recently studies19,20 have documented that a 3-day ceftriaxone regimen is significantly superior to a 1-day one in the treatment of non-responsive AOM caused by penicillin-resistant S. pneumoniae. We showed that CFR is still higher than 70% for S. pneumoniae 72h after starting a 3-day treatment, while it decreased significantly after 24h with a single dose. However, pharmacodynamic exposure target for both one-day and three-day dose regimens should be better established and confirmed with clinical data.
In spite of the results reported above, the following issues should be considered. Firstly, paediatric pharmacokinetic data of antibiotics are scarce and are obtained from a non-homogeneous population in relation to age. This leads to large interindividual variability, which was included in our pharmacokinetic model. Secondly, we used microbiological data from paediatric isolates, but not all of them were recovered from patients with otitis media. The reason for including strains from MEF and from the lower respiratory tract is that the microorganisms from MEF are more resistant, since in Spain, samples from MEF are only collected from the more severe cases or recurrence of AOM. The selection of these strains will provide biased information on the success of empirical treatments. The use of strains from the lower respiratory tract will provide more realistic information of susceptibility of strains causing AOM. Thirdly, this study did not consider the changing microbiology of AOM after widespread use of heptavalent pneumococcal vaccine (PCV7), which has been described in different countries. PVC7 has not been included into the vaccination schedule in Spain. If this occurred, the coverage could be similar as for any other vaccine included in the vaccination schedule (95%). In that case, this could result in a shift in frequency of causative bacterial pathogens responsible for AOM, and the results observed with this simulation exercise would have to be recalculated. Finally, this study has been developed using susceptibility data representative of bacteria causing non-complicated AOM in Spain. Consequently, caution should be taken before applying these findings to complicated/refractory infections or to other countries with different pathogen distribution data or different susceptibility patterns. Moreover, susceptibility data are based on a collection of isolates from the SAUCE 3 project, obtained during 2000-2001 and they may have changed since then. In the event of significant modifications in susceptibilities from new available data, new PK/PD evaluations should be performed in order to detect changes in the efficacy profiles of the antimicrobials.
In conclusion, considering the current susceptibility of bacterial pathogens most frequently isolated in AOM in Spain, high-dose amoxicillin should be the first-line choice for children with uncomplicated or non-refractory infection. Amoxicillin/clavulanate will provide the highest CFR (>90%) against both S. pneumoniae and H. influenzae. Differences with other studies may be explained by variations in antibiotic susceptibility patterns between countries. Results obtained with one-day ceftriaxone regimen indicate that it would be insufficient to achieve an acceptable bacteriological success rate if S. pneumoniae is responsible for the infection. Administration of 3 daily consecutive doses increases bacteriological eradication. Additional clinical evaluations will be necessary to establish the best target attainment for the treatment of AOM with ceftriaxone.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors would like to thank the Medical Department of GlaxoSmithKline for providing specific MIC distributions obtained from the SAUCE 3 surveillance.
Martín Herrero is an employee of GSK, without any other financial interests in the company.