In this prospective observational study performed in 12 hospitalized patients with proven or suspected invasive fungal infection treated for a mean of 14 days with micafungin (MCF), 8 of whom with pre-existing liver function impairment, plasma levels of MCF at steady state were not correlated with liver function tests at the beginning of treatment. Liver function remained stable or even improved in all patients, except in one in which MCF was discontinued due to liver toxicity.
Se trata de un estudio prospectivo observacional en 12 pacientes ingresados en un hospital con sospecha o infección fúngica confirmada tratados con micafungina (MCF) durante una media de 14 días, 8 de los cuales con presencia de alteración hepática preexistente. Los niveles plasmáticos de MCF en el estado estacionario no se correlacionaron con los valores basales de las pruebas hepáticas al inicio del tratamiento. La función hepática permaneció estable o incluso mejoró en todos los pacientes excepto en uno de ellos en el que el tratamiento con MCF fue interrumpido debido a la presencia de toxicidad hepática.
Invasive fungal infections in hospitalized patients are a major source of mortality and morbidity.1 Widespread and prolonged use of fluconazole has been associated with an increase of nosocomial non-albicans Candida spp. bloodstream infections2 and conventional amphotericin B with nephrotoxicity and severe adverse events.3,4 Micafungin (MCF) is an echinocandin antifungal agent that inhibits the synthesis of 1,3-β-d-glucan with activity against Candida and Aspergillus species. It has previously been shown to be non-inferior to both liposomal amphotericin B and caspofungin for the treatment of invasive candidiasis and candidemia and it has demonstrated efficacy for the prophylaxis of invasive fungal infections5,6 and the treatment of esophageal candidiasis.7 Potential hepatotoxicity is an adverse event reported for the different antifungal families, with pooled estimates of the percentage of patients with elevated liver enzymes requiring stopping treatment of 3.0% for MCF, 7% for caspofungin, 9.3% for fluconazole, 14.1% for amphotericin B formulations, 17.4% for itraconazole, and 19.7% for voriconazole.8 MCF displays linear pharmacokinetics (PK)9 and preliminary studies in subjects with hepatic impairment indicates that dose adjustment is not required.10
MethodsA prospective, observational study was designed to assess the potential drug-induced liver injury of MCF during its clinical use in routine daily practice, and to evaluate a possible linear correlation between baseline liver function tests and MCF plasma levels. The study population consisted of 12 hospitalized patients with proven or suspected invasive fungal infections treated with MCF. Plasma levels of MCF were measured at steady state (at least 4 days after starting MCF treatment) using a validated highly performed liquid chromatography (HPLC) method (Waters Alliance Systems). Two determinations of MCF plasma levels per patient were performed (Cmaxss or peak and Cminss or trough). Data recorded were age; sex; body mass index (BMI); liver function tests, including serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, alkaline phosphatase, and Child-Pugh score at the beginning and at the end of MCF treatment; MCF-related data, including daily dose, dose/kg actual body weight, duration of treatment (days), Cmaxss and Cminss; concomitant use of other hepatotoxic and cholestatic drugs; MCF withdrawal due to drug-induced liver disease; and crude mortality during treatment with MCF. In this study, liver toxicity was defined as the presence of serum levels of AST and ALT more than five times the upper limit of the normal range, alkaline phosphatase levels more than two-fold the upper limit of the normal range, and total bilirubin more than three times the upper limit of the normal range and/or a value >2.5mg/dL.11,12 Bivariate correlations between liver function tests at the beginning and at the end of MCF treatment as well as MCF levels at steady state were analyzed using the Spearman's rho coefficient.
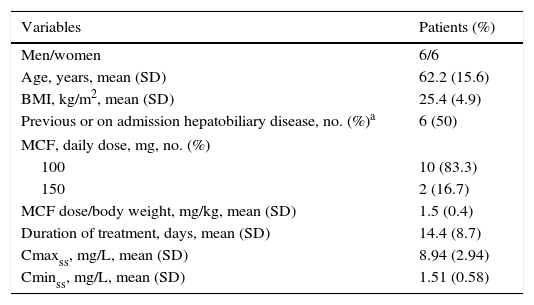
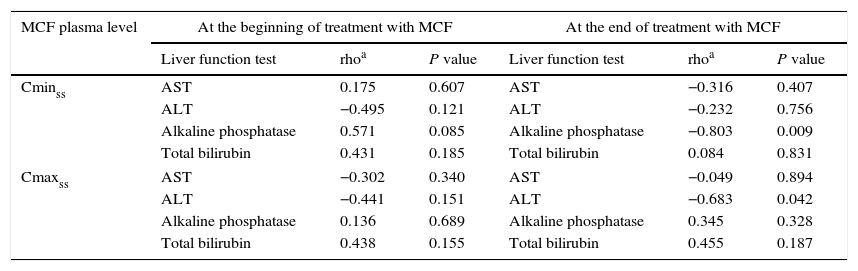
ResultsThere were 6 men and 6 women, with a mean (standard deviation, SD) age of 62.2 (15.6) years, and a mean BMI of 25.4 (4.9) kg/m2. The clinical and pharmacokinetic characteristics of these patients are shown in Table 1. History of liver disease was recorded in 6 (50%) patients. The majority of patients (83.3%) were treated with a daily dose of MCF of 100mg. The mean duration of treatment was 14.4 (8.7) days. The mean Cmaxss was 8.94 (2.94) mg/L and the mean Cminss 1.51 (0.58) mg/L. As shown in Table 1, there were 8 (66.7%) patients with altered liver function tests at the beginning of MCF treatment as compared with 5 patients at the end of it, although all of these 5 patients had been concomitantly treated with other drugs that can induce cholestasis, such as paracetamol, linezolid, imipenem, ceftriaxone, cloxacillin, erythromycin, and others. No linear correlation was observed between MCF plasma levels (Cmaxss nor Cminss) and the values of different liver parameters (AST, ALT, total bilirubin and alkaline phosphatase) at the beginning of treatment (Table 2). At the end of treatment with MCF, serum levels of alkaline phosphatase correlated inversely with Cminss and ALT values with Cmaxss, with a correlation coefficient (rho) of −0.803 (P=0.009) and −0.683 (P=0.042), respectively (Table 2).
Characteristics of 12 patients with proven or suspected invasive fungal infection treated with micafungin (MCF).
| Variables | Patients (%) |
|---|---|
| Men/women | 6/6 |
| Age, years, mean (SD) | 62.2 (15.6) |
| BMI, kg/m2, mean (SD) | 25.4 (4.9) |
| Previous or on admission hepatobiliary disease, no. (%)a | 6 (50) |
| MCF, daily dose, mg, no. (%) | |
| 100 | 10 (83.3) |
| 150 | 2 (16.7) |
| MCF dose/body weight, mg/kg, mean (SD) | 1.5 (0.4) |
| Duration of treatment, days, mean (SD) | 14.4 (8.7) |
| Cmaxss, mg/L, mean (SD) | 8.94 (2.94) |
| Cminss, mg/L, mean (SD) | 1.51 (0.58) |
| Altered liver function tests | ||
|---|---|---|
| At the start of MCF therapy | At the end of MCF therapy | |
| Patients, no. (%) | 8 (66.7) | 5 (41.7) |
| AST, IU/L>5 upper normal limit | 1 (8.3) | 0 |
| ALT, IU/L>5 upper normal limit | 0 | 0 |
| Alkaline phosphatase, IU/L, >2 upper normal limit | 5 (41.7) | 3 (25) |
| Total bilirubin, mg/dL, >3 upper normal limit | 2 (16.7) | 2 (16.7) |
| Child-Pugh A | 3 (25) | 5 (41.7) |
| Child-Pugh B | 7 (58.3) | 5 (41.7) |
| Child-Pugh C | 2 (16.7) | 2 (16.7) |
Data expressed as patients and percentages (in parenthesis) unless otherwise stated.
Acute pancreatitis and biliary lithiasis (n=1); acute pancreatitis and acute cholangitis (n=1); Biliary lithiasis (gallbladder and common duct) (n=1); HCV chronic infection (genotype A1) (n=1); Alcohol-related liver cirrhosis Child-Pugh C (n=1); HCV-related cirrhosis Child-Pugh B, hepatocellular carcinoma BCLC A4 (n=1).
Results of correlation analysis between MCF plasma levels and liver functions tests at the beginning and at the end of treatment.
| MCF plasma level | At the beginning of treatment with MCF | At the end of treatment with MCF | ||||
|---|---|---|---|---|---|---|
| Liver function test | rhoa | P value | Liver function test | rhoa | P value | |
| Cminss | AST | 0.175 | 0.607 | AST | −0.316 | 0.407 |
| ALT | −0.495 | 0.121 | ALT | −0.232 | 0.756 | |
| Alkaline phosphatase | 0.571 | 0.085 | Alkaline phosphatase | −0.803 | 0.009 | |
| Total bilirubin | 0.431 | 0.185 | Total bilirubin | 0.084 | 0.831 | |
| Cmaxss | AST | −0.302 | 0.340 | AST | −0.049 | 0.894 |
| ALT | −0.441 | 0.151 | ALT | −0.683 | 0.042 | |
| Alkaline phosphatase | 0.136 | 0.689 | Alkaline phosphatase | 0.345 | 0.328 | |
| Total bilirubin | 0.438 | 0.155 | Total bilirubin | 0.455 | 0.187 | |
Also, there were no differences in Cmaxss and Cminss between patients with and without altered liver function tests at the beginning of MCF treatment as well as between patients with and without altered liver function tests at the end of MCF treatment (Table 3). The crude mortality was 16.7% (n=2). One patient (8.3%) died during MCF treatment. The cause of death was hemodynamic instability with severe metabolic acidosis and cardiorespiratory arrest. Also, MCF discontinuation was necessary due to drug-induced adverse event (increase in bilirubin level of 19mg/dL) in one patient. This patient showed persistent positive culture for Candida spp. After withdrawal of MCF, anidulafungin was administered but serum bilirubin levels continued to increase up to 47.7mg/dL. In none of the patients worsening of liver function parameters was observed, with the exception of this patient with increased bilirubin level.
Differences in plasma levels of MCF between patients with and without altered liver function tests either at the beginning or at the end of MCF treatment.
| MCF plasma levels | Altered liver function tests at the start of MCF treatment | P value | |
|---|---|---|---|
| Present | Absent | ||
| Cmaxss, mg/L, mean (SD) | 8.5 (7.8) | 9.3 (8.7) | 0.660a |
| Cminss, mg/L, mean (SD) | 1.47 (1.5) | 1.56 (1.3) | 0.792b |
This prospective observational study carried out in a small clinical series of 12 patients, 8 (66.7%) of which had altered function tests at the beginning of MCF treatment, shows that MCF is a safe option for the treatment of invasive fungal infection, even for those patients with pre-existing liver function impairment. Liver function remained stable or even improved in all but one MCF-treated patients. In only one patient MCF was discontinued due to liver toxicity. However, this patient with invasive candidiasis showed persistent positive blood cultures for Candida and elevated serum bilirubin levels that even increased after discontinuation of MCF and switching to treatment with anidulafungin. Data from randomized double blind clinical trials performed in patients with baseline normal liver function tests have reported elevations of alkaline phosphatase, AST and ALT in only 2.7, 2.3 and 2.0 of the patients, respectively.13 A favorable clinical safety profile for MCF similar to other echinocandins has been demonstrated in different studies, including analysis a large database (a pooled clinical trial data set of 3028 patients that received at least one dose of MCF).14
In a recent study of the pharmacokinetics of MCF in 8 healthy volunteers and 8 patients with severe liver dysfunction treated with a single dose of MCF of 100mg, pharmacokinetic parameters were lower in subjects with severe hepatic dysfunction, except for clearance, which was higher in these subjects. However, the magnitude of the differences was not considered to be clinically meaningful.15
In this study, MCF plasma levels at steady state showed no correlation with hepatic parameters at the beginning of MCF treatment. Also, Cmaxss and Cminss were similar in patients with and without altered liver function tests both at starting and at the end of MCF treatment. These data support the usefulness of MCF in patients with liver dysfunction and invasive fungal infections.
FundingNone.
Conflict of interestThe authors declare no conflict of interest.
The authors thank Marta Pulido, MD, for editorial assistance.