To investigate a Serratia marcescens (S. marcescens) outbreak in a Neonatal Unit in a tertiary university hospital.
MethodsDescriptive study of children admitted to the Unit with S. marcescens infection from November 2012 to March 2013. Conventional microbiological methods for clinical and environmental samples were used. The clonal relationship between all available isolates was established by molecular methods. A multidisciplinary team was formed, and preventive measures were taken.
ResultsS. marcescens was isolated from 18 children. The overall attack rate was 12%, and the case fatality rate in the Intensive Care Unit was 23.5%. The most prevalent types of infections were pneumonia (6), conjunctivitis (6), and bloodstream infection (5). Clinical isolates and environmental isolates obtained from an incubator belonged to a unique clone. The clonal relationship between all S. marcescens strains helped us to identify the possible source of the outbreak.
ConclusionIsolation of S. marcescens from stored water in a container, and from the surface of an incubator after cleaning, suggests a possible environmental source as the outbreak origin, which has been perpetuated due to a failure of cleaning methods in the Unit. The strict hygiene and cleaning measures were the main factors that contributed to the end of the outbreak.
Investigar un brote por Serratia marcescens (S. marcescens) en una unidad de neonatología en un hospital universitario de tercer nivel.
MétodoEstudio descriptivo de los pacientes ingresados en la Unidad de noviembre de 2012 a marzo de 2013. Se usaron métodos microbiológicos convencionales de muestras clínicas y ambientales. La relación clonal de los aislados disponibles se llevó a cabo mediante estudio molecular. Se formó un equipo multidisciplinar a partir del cual se tomaron las medidas preventivas.
ResultadosSe aisló S. marcescens en 18 niños. La tasa global de ataque fue del 12% y la letalidad en la Unidad de Cuidados Intensivos llegó al 23,5%. Los tipos de infección más frecuentes fueron la neumonía (6), conjuntivitis (6) y bacteriemia (5). Tanto las muestras clínicas como las ambientales obtenidas de una incubadora pertenecían a un único clon. La relación clonal entre todas las cepas de S. marcescens permitió identificar la posible fuente de infección del brote.
ConclusiónEl aislamiento de S. marcescens en agua almacenada en un contenedor y en la superficie de una incubadora tras su limpieza sugiere una posible fuente ambiental como el origen del brote, perpetuado por fallos en los métodos de limpieza en la Unidad. El cumplimiento estricto de la higiene de manos y mejora en la limpieza fueron los principales factores que contribuyeron a la finalización del brote.
Serratia marcescens is considered an opportunistic pathogen able to produce serious infections in Neonatal Intensive Care Units (NICU) with high morbidity and mortality, and the latter could reach 44% in low birthweight.1
S. marcescens can survive in damp environments and colonize the respiratory and/or gastrointestinal tract, as well as in hands of patients and healthcare workers, accounting for 5–15% of all nosocomial infections in NICU, and has been isolated for a long time in some nosocomial outbreaks.2,3
Strict preventive measures are required to control these infections, primarily because of the great difficulty to eradicate this bacterium from these Units during an outbreak. Secondly, some of these strains have the ability to be resistant to multiple antibiotics which may hinder their treatment, and this fact could have severe consequences in low birthweight infected patients.4 In this paper, we describe the epidemiological investigation of a S. marcescens nosocomial outbreak at the NICU that allowed us to identify a possible environmental source and undertake the preventive measures to control it, as well as to define areas for improvement in order to prevent the appearance of new cases.
MethodsArea and period of studySince November 2012 to March 2013, a S. marcescens nosocomial outbreak occurred at the Neonatal Unit (NU) of the University Hospital Virgen del Rocío in Seville, which it is also a regional tertiary reference center for other provinces of Andalusia (Spain). The NU is newly built since 2011, and is subdivided into three areas: General Neonatology (32 beds), Intermediate Care Unit (ICU) with 16 beds, and Neonatal Intensive Care Unit (NICU) with 12 beds.
This Unit served a total of 1790 inpatients in 2011, with an average stay of 8.7 days per patient. None of the three Units share health personnel except the pediatric postgraduate residency training on duty.
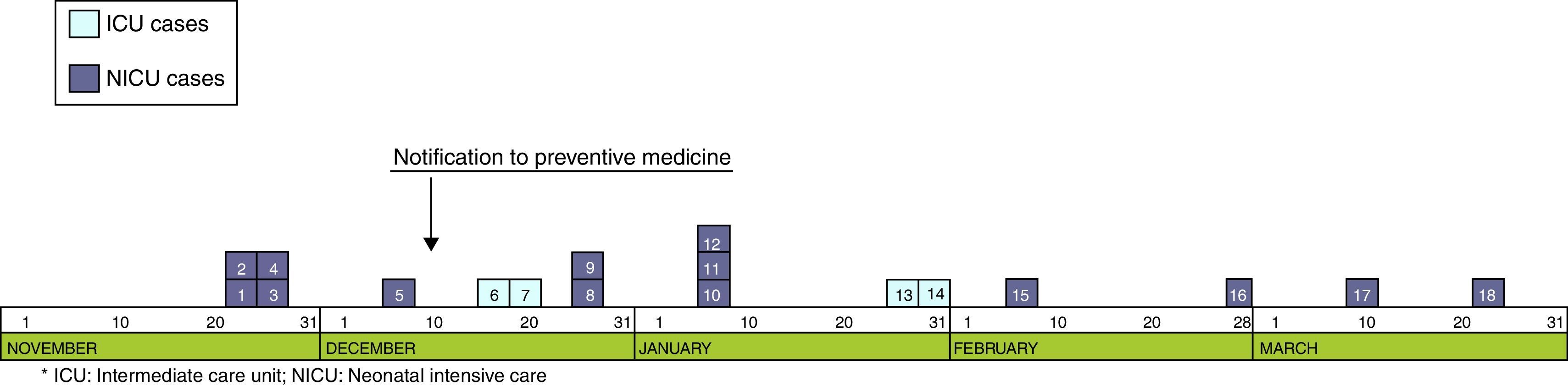
Epidemiological researchThe 11th of December 2012, the outbreak was declared when five cases were already diagnosed in the NICU. Subsequently, more cases were identified at the NICU and ICU, with a total of 18 children involved, and the last case being detected in March 2013.
Study population included all patients infected and admitted to NICU and ICU from the first detected case. Case definition was any patient admitted to NICU or ICU, since November 2012 to March 2013, with a S. marcescens positive culture.
According to the site of infection and the clinical symptoms, we classify children as confirmed cases (when S. marcescens was isolated from sterile fluid samples and/or from non-sterile sites and clinical symptoms were present) and colonized/carrier cases (when the bacteria was isolated from non-sterile sites in the absence of signs and/or symptoms of infection).
The Service of Preventive Medicine collected cases data by individual inquiry protocol. Variables including personal identifiable information, intrinsic risk factors and those associated with infection and related procedures were collected.
Environmental studyDuring 2013, an extensive environmental study, which included 42 samples, was conducted to search for a potential source of S. marcescens.
Microbiological studyEnvironmental and clinical samples were processed following the Proceedings of the Spanish Society of Clinical Microbiology and Infectious Diseases, SEIMC (Environmental Microbiological 2nd Edition (42), 2012) and incubated for 7 days. Environmental samples were collected at three different dates: November 2012, February 2013 and December 2013.
The S. marcescens isolates were preliminarily identified by mass spectrometry (MALDI-TOF-MS Brucker®, Microflex LT instrument software and database Flexcontrol Biotyper 3.0 2.0 (Bruker Daltonics)). Isolates were stored at −70° until the realization of molecular study.
The fenotypical identification and susceptibility testing was performed with MicroScan Walkaway system (Beckman Coulter Inc.). Susceptibility testing was performed by standard methods in accordance with the European Committee on Antimicrobial Susceptibility Testing – (EUCAST) performance standards.
On January 29, 2013 a study of conjunctival and rectal carriage of S. marcescens to the 14 inpatient children of the Unit was performed. Pulsed field electrophoresis (PFEG) analysis on all the available strains isolated from clinical and environmental samples was performed, following the modified protocol described by Shi Zhi-yuan et al., using the SpeI restriction enzyme.5 Electrophoresis patterns obtained were captured with GelDoc™ XR+with Image Lab™ Software. These patterns were compared using the Bioinformatics FPQuest™ Software system, BIO-RAD. Dendogram was performed by the Dice coefficient and UPGMA (tolerance 2%), and the pulsotypes obtained were interpreted according to the Tenover criteria.6
Statistical analysisThe attack rate (AR) or S. marcescens infection incidence of inpatient children at the Unit (number of new cases among all patients at risk), the case fatality rate (number of deaths among infected) and S. marcescens mortality (deaths of all patients at risk) were calculated. The risk population was considered in all the inpatient children at the affected Units during the outbreak period.
A time, place and person descriptive analysis of the cases was performed. Categorical variables were represented by frequency tables and quantitative numerical summaries [mean and standard deviation, median and interquartile range (IQR)].
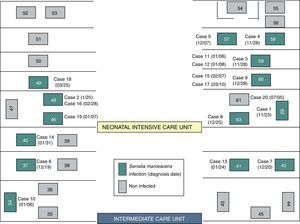
ResultsOutbreak descriptionOn the 23rd of November, the outbreak started at the NICU, and since then, clusters of new cases were detected until March 25 of 2013, when the last case was diagnosed. In the first period, from November 23rd to December 7th, a cluster of five cases with a high AR (41.6%) and mortality (40%) was identified in NICU (Fig. 1). Lately, new cases appeared intermittently with longer period among them, and the last case was detected on March 25, 2013. The global AR throughout the epidemic period has been 12%, ranging from 9.3% in ICU to 13.5% in NICU.
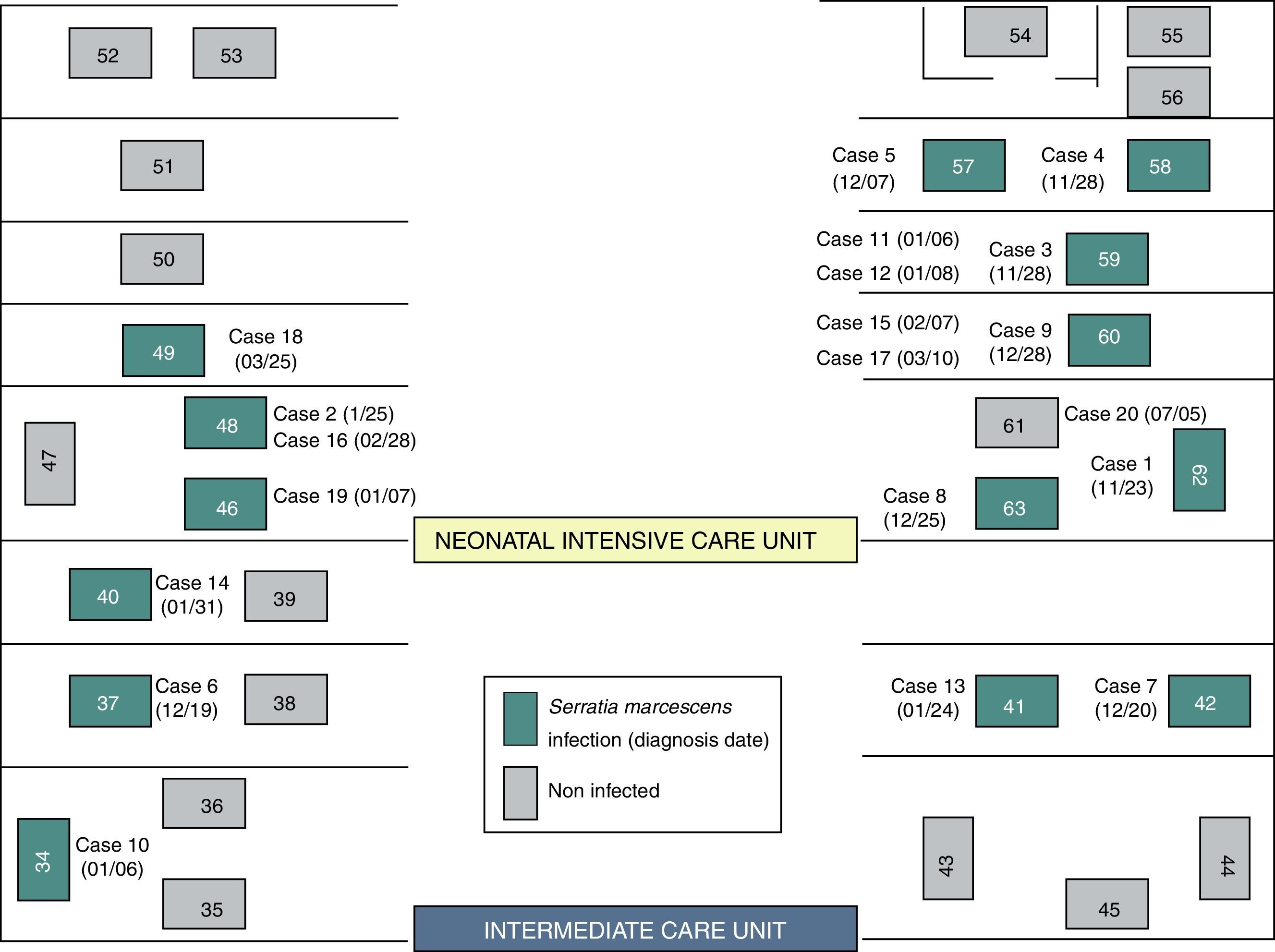
Although two Units have been affected, the outbreak began at the NICU where most of the new cases were detected throughout all the epidemic period including 13 (72.2%) of the 18 cases. Ten cases (55.6%) were located at the area where the incubators 57–63 were set (Fig. 2). In the second half of December, five cases were diagnosed at the ICU after the transfer of two cases from NICU to ICU.
S. marcescens was isolated from 16 infected and 2 colonized patients, 13 male (72.2%) and 5 female children (27.8%), with a median age of 24 days (IQR 12–45). Eighty-seven percent of patients were at the Unit since its birth, and the patient's median stay in the Unit when the infection succeeds was of 19 days (IQR 11–46). Prematurity (56%) was the first cause for admission followed by congenital malformation (22.3%). Sixty-seven percent received antibiotic therapy (median 14 days; IQR 7–25.5), and the most commonly used antibiotics were ampicillin, gentamicin, vancomycin and meropenem.
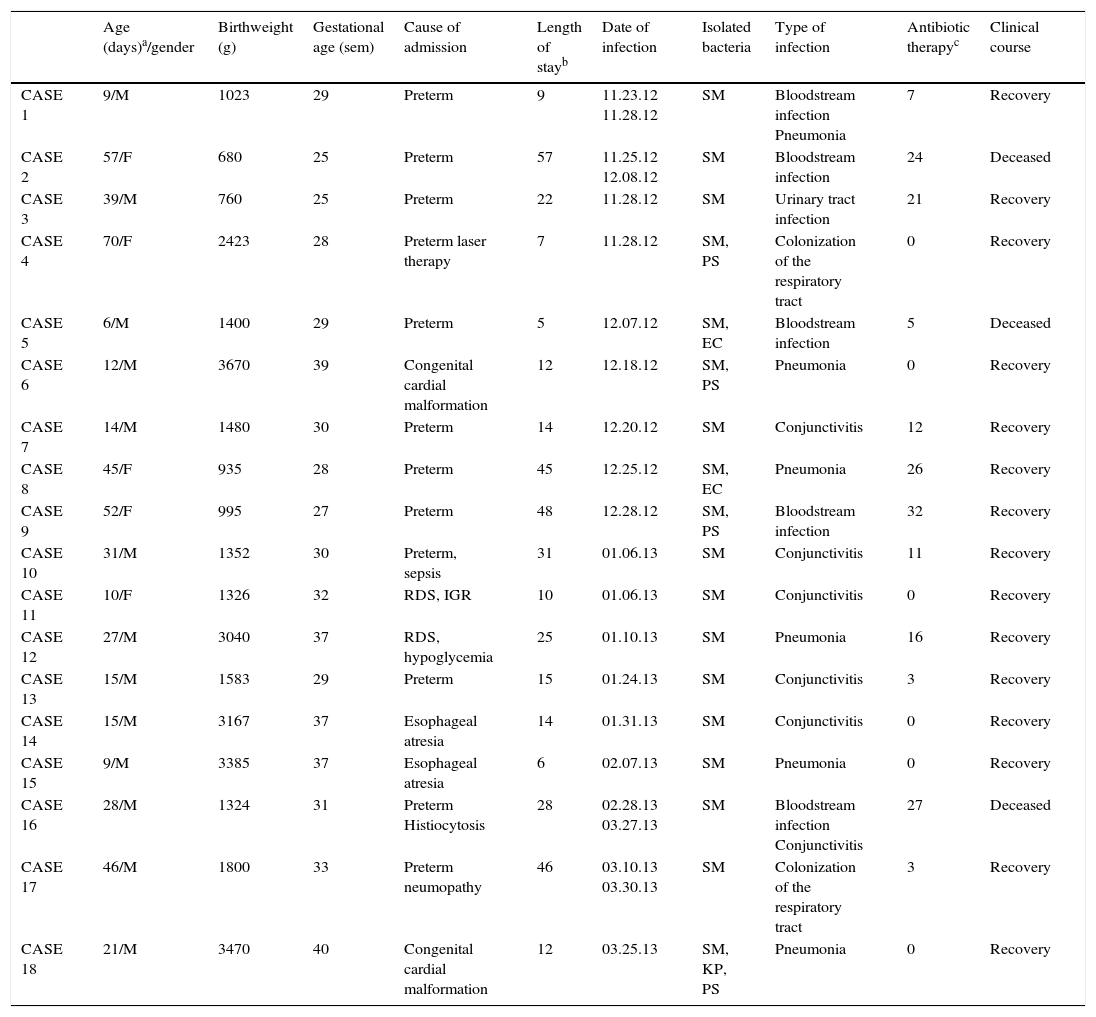
The most prevalent clinical disease patterns were distributed as follows: six pneumonia, six conjunctivitis, five bloodstream infection and one urinary tract infection. In six cases, there was coinfection with other enterobacteria (Table 1). Additionally, in six cases, respiratory tract colonization was present.
Description of patients with Serratia marcescens infection.
| Age (days)a/gender | Birthweight (g) | Gestational age (sem) | Cause of admission | Length of stayb | Date of infection | Isolated bacteria | Type of infection | Antibiotic therapyc | Clinical course | |
|---|---|---|---|---|---|---|---|---|---|---|
| CASE 1 | 9/M | 1023 | 29 | Preterm | 9 | 11.23.12 11.28.12 | SM | Bloodstream infection Pneumonia | 7 | Recovery |
| CASE 2 | 57/F | 680 | 25 | Preterm | 57 | 11.25.12 12.08.12 | SM | Bloodstream infection | 24 | Deceased |
| CASE 3 | 39/M | 760 | 25 | Preterm | 22 | 11.28.12 | SM | Urinary tract infection | 21 | Recovery |
| CASE 4 | 70/F | 2423 | 28 | Preterm laser therapy | 7 | 11.28.12 | SM, PS | Colonization of the respiratory tract | 0 | Recovery |
| CASE 5 | 6/M | 1400 | 29 | Preterm | 5 | 12.07.12 | SM, EC | Bloodstream infection | 5 | Deceased |
| CASE 6 | 12/M | 3670 | 39 | Congenital cardial malformation | 12 | 12.18.12 | SM, PS | Pneumonia | 0 | Recovery |
| CASE 7 | 14/M | 1480 | 30 | Preterm | 14 | 12.20.12 | SM | Conjunctivitis | 12 | Recovery |
| CASE 8 | 45/F | 935 | 28 | Preterm | 45 | 12.25.12 | SM, EC | Pneumonia | 26 | Recovery |
| CASE 9 | 52/F | 995 | 27 | Preterm | 48 | 12.28.12 | SM, PS | Bloodstream infection | 32 | Recovery |
| CASE 10 | 31/M | 1352 | 30 | Preterm, sepsis | 31 | 01.06.13 | SM | Conjunctivitis | 11 | Recovery |
| CASE 11 | 10/F | 1326 | 32 | RDS, IGR | 10 | 01.06.13 | SM | Conjunctivitis | 0 | Recovery |
| CASE 12 | 27/M | 3040 | 37 | RDS, hypoglycemia | 25 | 01.10.13 | SM | Pneumonia | 16 | Recovery |
| CASE 13 | 15/M | 1583 | 29 | Preterm | 15 | 01.24.13 | SM | Conjunctivitis | 3 | Recovery |
| CASE 14 | 15/M | 3167 | 37 | Esophageal atresia | 14 | 01.31.13 | SM | Conjunctivitis | 0 | Recovery |
| CASE 15 | 9/M | 3385 | 37 | Esophageal atresia | 6 | 02.07.13 | SM | Pneumonia | 0 | Recovery |
| CASE 16 | 28/M | 1324 | 31 | Preterm Histiocytosis | 28 | 02.28.13 03.27.13 | SM | Bloodstream infection Conjunctivitis | 27 | Deceased |
| CASE 17 | 46/M | 1800 | 33 | Preterm neumopathy | 46 | 03.10.13 03.30.13 | SM | Colonization of the respiratory tract | 3 | Recovery |
| CASE 18 | 21/M | 3470 | 40 | Congenital cardial malformation | 12 | 03.25.13 | SM, KP, PS | Pneumonia | 0 | Recovery |
Clinical evolution was favorable in 15 cases but three of the bloodstream infection cases died. The overall case fatality rate for the entire epidemic period was 16.6% (23% in NICU) and the death rate of infection in S. marcescens exposed population was 2% (3% in NICU).
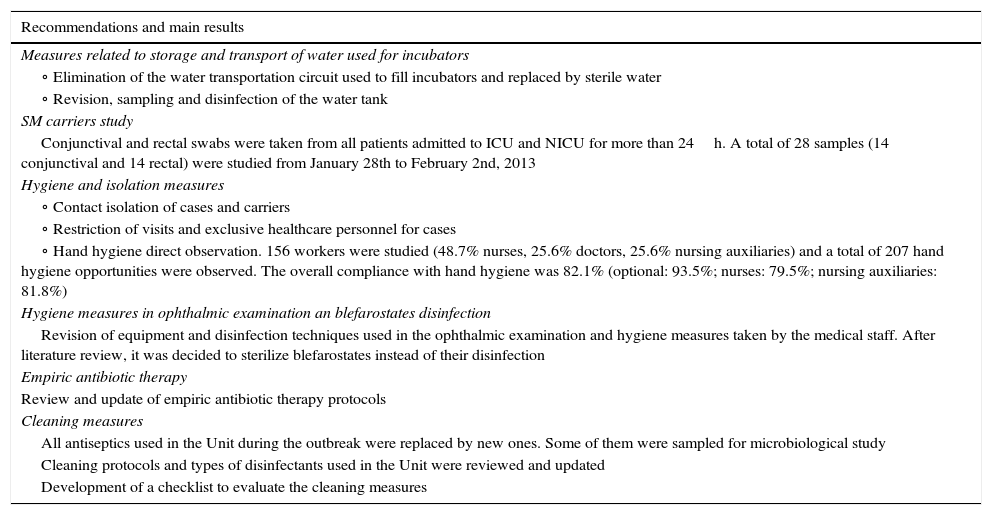
Preventive measuresThe first preventive measures adopted to prevent cross-transmission of infection were prospective surveillance of patients admitted to NICU, extreme cleaning measures and emphasized the strict compliance hand washing on healthcare workers.7
Other preventive measures such as conducting environmental microbiological study, review and logging cleaning steps, contact isolation, restriction of visits, study of S. marcescens carriers, review of empirical antibiotic policy at the NICU and direct observation of hand hygiene of healthcare workers were subsequently taken (Table 2).
Preventive measures undertaken to control the outbreak.
| Recommendations and main results |
|---|
| Measures related to storage and transport of water used for incubators |
| ∘ Elimination of the water transportation circuit used to fill incubators and replaced by sterile water |
| ∘ Revision, sampling and disinfection of the water tank |
| SM carriers study |
| Conjunctival and rectal swabs were taken from all patients admitted to ICU and NICU for more than 24h. A total of 28 samples (14 conjunctival and 14 rectal) were studied from January 28th to February 2nd, 2013 |
| Hygiene and isolation measures |
| ∘ Contact isolation of cases and carriers |
| ∘ Restriction of visits and exclusive healthcare personnel for cases |
| ∘ Hand hygiene direct observation. 156 workers were studied (48.7% nurses, 25.6% doctors, 25.6% nursing auxiliaries) and a total of 207 hand hygiene opportunities were observed. The overall compliance with hand hygiene was 82.1% (optional: 93.5%; nurses: 79.5%; nursing auxiliaries: 81.8%) |
| Hygiene measures in ophthalmic examination an blefarostates disinfection |
| Revision of equipment and disinfection techniques used in the ophthalmic examination and hygiene measures taken by the medical staff. After literature review, it was decided to sterilize blefarostates instead of their disinfection |
| Empiric antibiotic therapy |
| Review and update of empiric antibiotic therapy protocols |
| Cleaning measures |
| All antiseptics used in the Unit during the outbreak were replaced by new ones. Some of them were sampled for microbiological study |
| Cleaning protocols and types of disinfectants used in the Unit were reviewed and updated |
| Development of a checklist to evaluate the cleaning measures |
SM: Serratia marcescens; ICU: Intermediate Care Unit; NICU: Neonatal Intensive Care Unit.
Twenty-three S. marcescens strains were isolated, 18 from clinical samples and 5 from environmental samples. Among the later, two of them were from the incubator surfaces, one from the water container and likewise, two S. marcescens strains from the pipes of two different sinks.
None of the 28 cultured samples to detect S. marcescens carriage from inpatient children were positive.
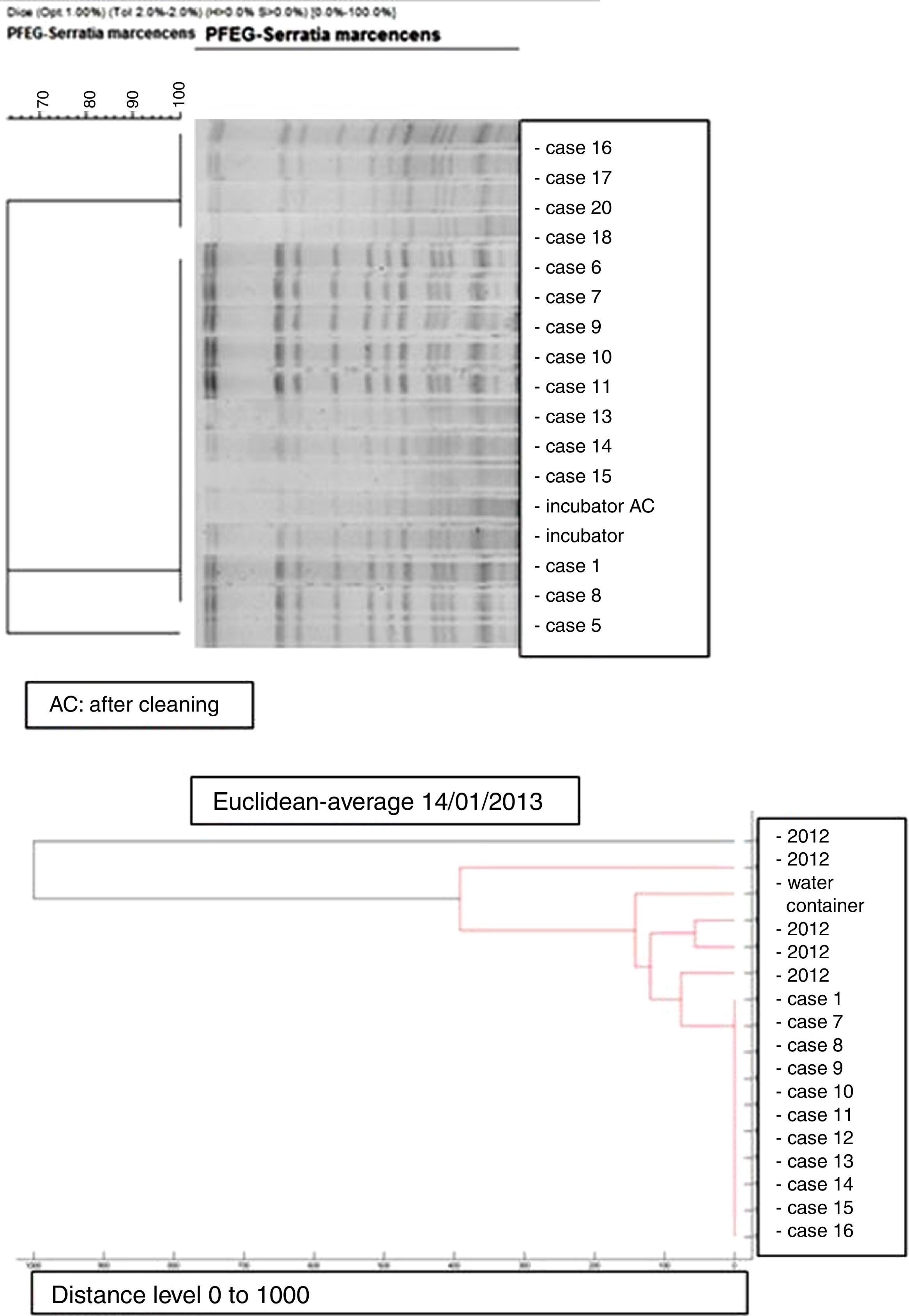
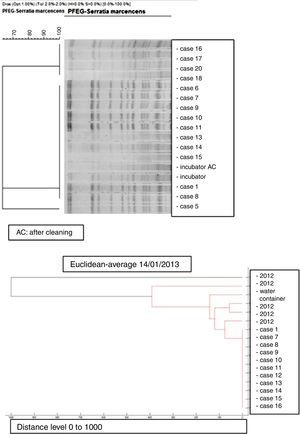
Microbiology and molecular studyAll the S. marcescens isolates were resistant to ampicillin, amoxicillin/clavulanate, cephalothin, cefazolin, amikacin and tobramycin and susceptible to ciprofloxacin, cefepime, imipenem, ertapenem, gentamicin, piperacillin/tazobactam, cotrimoxazole and tigecycline. The dendrogram created by mass-spectrometry analysis software grouped the first seven strains of the outbreak into one cluster, and allowed us to rule out any clonal relationship with previous isolates (Fig. 3).
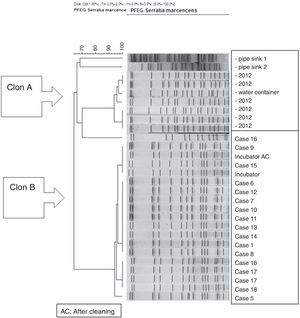
PFGE analysis of 17 Serratia marcescens strains including outbreak cases and incubator strains (before and after cleaning) and the dendrogram of the proteins profiles created by mass-spectrometry analysis of 18 strains of Serratia marcescens (including 11 outbreak cases – one strain per patient, two environmental samples and five clinical isolates of the year 2012. Correlation distance measure with the average linkage Algorithm. Maldi-TOFF. Bruker™.
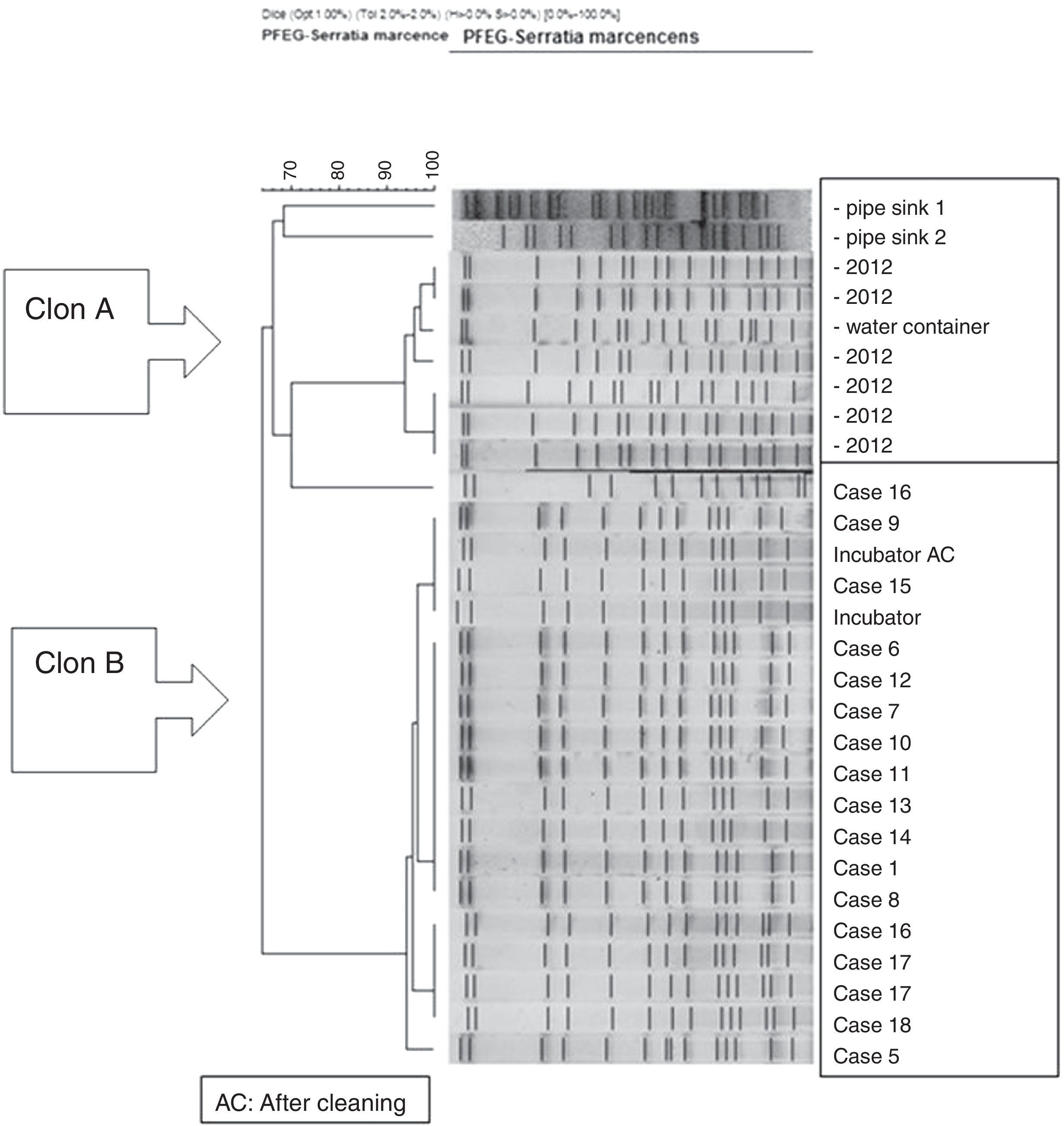
The molecular study identified two main lineages of S. marcescens (Clon A and B). The Clon A included all the 2012 strains isolated before the outbreak's declaration and the strain isolated from the water container (this strain was not identical to those from the clinical strains though highly related – 97%, suggesting a common lineage). The Clon B included all the strains involved in the outbreak cases and those isolated from the incubator, before and after cleaning (Fig. 4).
DiscussionIn recent years, S. marcescens has been frequently involved in outbreaks at NICU that are characterized for its quick spread, causing severe complications, and with a highly variable incidence and duration.3,8,9
Our work, in contrast with other studies, emphasized the high number of confirmed cases in a short period of time (4 months) and the high lethality of the infection in the first epidemic wave (close to 40%), especially in bloodstream infection cases, exceeding what is reported in other outbreaks.4,10,11
Risk factors that contribute to severe forms of S. marcescens infections in neonates are: low gestational age and birthweight, longer hospital stays, prior antibiotic therapy and use of invasive devices such as central or percutaneous venous catheter, orotracheal intubation or parenteral nutrition.2,8,9,12–14 In our study, more than half of the infected patients shared the aforementioned factors such as birthweight less than 1500g, gestational age less than 35 weeks and prior prolonged antibiotic therapy and length of stay.
An environmental source is not detected in most of the studies, and it is assumed that patients act as a reservoir of infection, and through cross-transmission the bacteria spreads to other patients.3,8,14 However, in our study, although we cannot unequivocally identify the source, there are some facts that suggest their existence. Firstly, because of the clonal relationship between the water container S. marcescens, strain with those isolated from the blood cultures in months prior to the outbreak. Secondly, no rectal, neither conjunctival S. marcescens carriers, were detected during the outbreak. Finally, the epidemic curve distribution with a high number of cases in the first outbreak wave suggest that a common environmental source initiated it, and the infected patients acted later as reservoir of the bacteria. The S. marcescens ability to survive in the gastrointestinal and/or respiratory tract of infected patients for long periods of time also supports this hypothesis, as can be demonstrated in the 16th and 17th patients, where the same strain was isolated twice, one month apart (Table 2), and by the skin persistence of S. marcescens in the hands of healthcare workers that could have contributed to maintain its spread.1,8,15,16
Additionally, the effect of overcrowding and understaffing may have contributed to the persistence of the outbreak in time as previously described.8 In the first epidemic period, where the largest number of cases and higher lethality occurred, there was a temporal absence of four resident physicians in the Intensive and Intermediate Care Units.
Rapid implementation of recommended preventive measures, designed by the multidisciplinary team, is essential in controlling the outbreak and its progression. The contact isolation of cases, exclusiveness of healthcare workers attending the cases, reinforcement on hand hygiene and cleaning are the most referred measures by other authors.3,9
Moreover, the availability of PFEG and mass spectrometry played an important role in the outbreak investigation. It allowed us to demonstrate that all cases belonged to a single strain which was the same as that isolated from the water container. This contrasts with other studies where different strains are involved.4,10,12,13
Although it is usually assumed that ICU are adequately cleaned, it is known that only about 40% of the areas close to the patient are being disinfected according to the protocols.17 As it happened in our study, where two identical S. marcescens strains were isolated before and after the incubator cleaning, it highlighted a failure in disinfection methods and prompted us to review and update the cleaning protocols and develop an evaluating method to ensure its compliance.
Carling et al. claimed that the thoroughness of disinfection can be improved by up to 82%, which may result in a decrease of 40% in the transmission of multiresistant microorganisms.17 Furthermore, several guidelines suggest that hospitals should monitor the cleaning tasks, especially on surfaces close to the patient and healthcare workers, and it should be performed by trained personnel.18–21
The high number of cases of conjunctivitis also led us to review the disinfection methods of the blefarostates used in the newborn ophthalmologic examinations and decided to sterilize them instead of their disinfection (they usually where disinfected and reused in different patients in the same day).
Other control measures applied in S. marcescens outbreaks reported in the literature have been the closure of the Unit and the study of S. marcescens carriers among healthcare workers.13,14,22 However, the closure of the Unit not always guarantees the absence of new cases after reopening, and there is only one study published in 2006 that detected the same strain of S. marcescens which caused the outbreak in the hands of a healthcare worker.16 In our study, hand hygiene (HH) compliance of healthcare workers was higher than what has been published in a recent systematic review, whose median value is at a 40%,23 compared to the 80% obtained in our study. However, we should take in to account that the observation method was made during the outbreak investigation, a situation that could motivate workers to be stricter with the HH. This fact, combined with the absence of S. marcescens carriers, led to the decision of not studying the bacterial contamination of the hands of the Unit staff.
In conclusion, we emphasize the importance of molecular study and training of the multidisciplinary team who were determining factors in the control and management of the outbreak, both for the development of the study hypothesis and mechanism of transmission of infection, and the implementation of the appropriate preventive measures.
Based on lessons learned, we highlight the importance of monitoring the cleaning measures in ICU, since we tend to minimize their importance in the transmission of nosocomial infection.
FundingNo financial support was given for this study.
Conflict of interestAll authors report no conflicts of interest relevant to this article.