Fingolimod is a new oral administrated sphingosine 1-phosphate receptor modulator, approved for treatment of multiple sclerosis (MS) patients.1,2 There is evidence that fingolimod acts by preventing lymphocyte egress from lymph nodes, spleen and thymus by antagonistic action on the sphingosine 1-phosphate receptors. These receptors regulate proliferation, differentiation, survival, chemotaxis and diapedesis.3,4 Although an increase in opportunistic infections has not been documented in clinical trials,1,2 current experience suggests that fingolimod might increase the risk of severe herpetic infections. Fatal cases of herpetic infections have occurred in two patients on fingolimod.5 Recently, a case of varicella zoster encephalitis and vasculopathy has been reported in a VZV seropositive patient receiving fingolimod.6 Usually authors discontinue the novel therapy indefinitely and switch to another disease modulating drug. In all reported cases there was diminished lymphocytes blood count on admission laboratory tests.6–8 We present a case of polydermatomal zoster with genital involvement occurring in a MS patient four months after the initiation of fingolimod therapy.
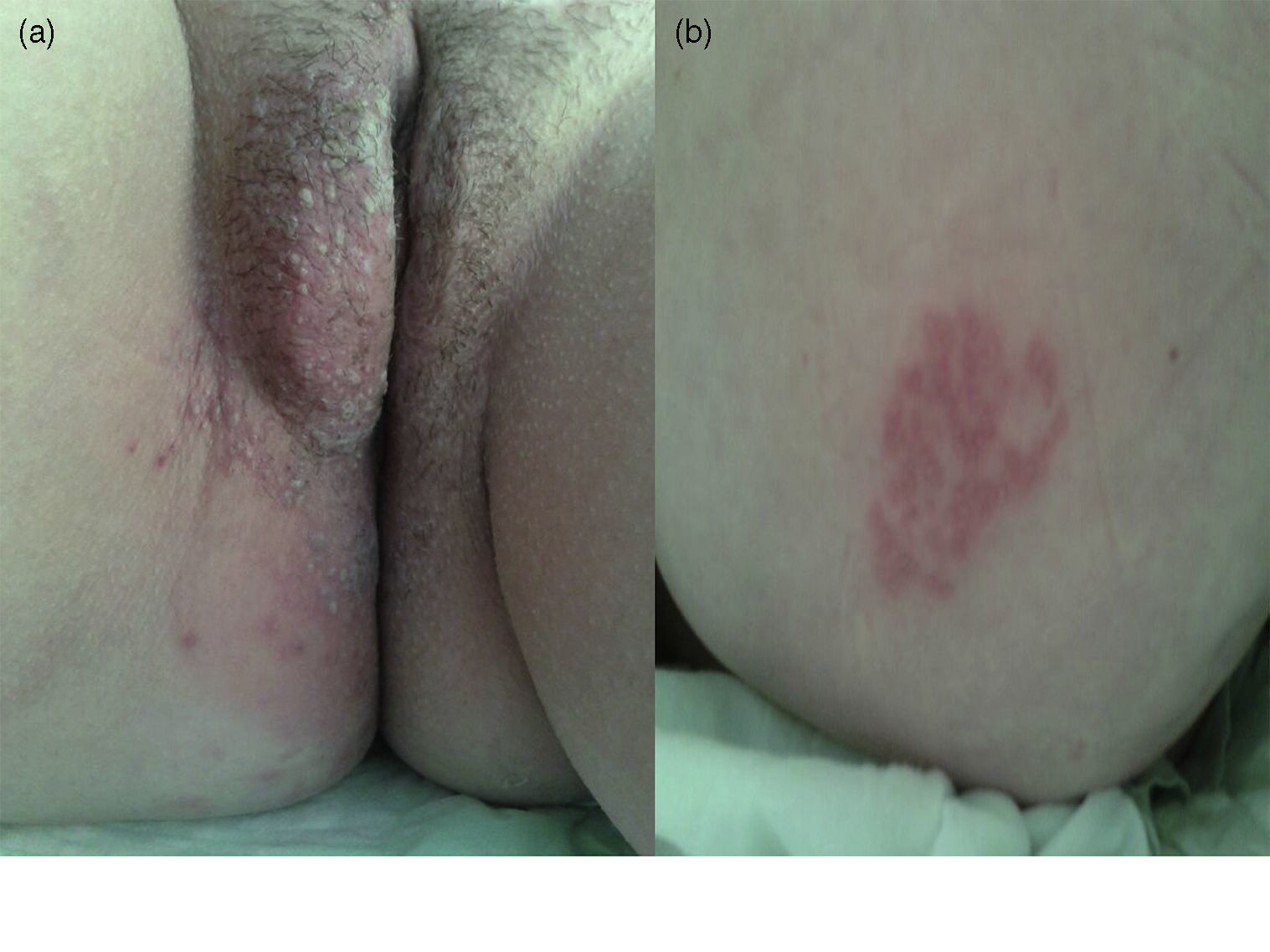
Case reportA 38-year-old woman was admitted to the emergency department because of severe genital and anal pain of one-week duration. The patient was diagnosed with relapsing-remitting MS 12 years before. During the last 6 years she had been receiving interferon switched to fingolimod 4 months before because of continued relapses. She had positive baseline serology for varicella-zoster virus (VZV). The Expanded Disability Scale Score was 3.0. On admission the physical examination revealed temperature of 38°C and characteristic vesicular lesions involving the right vulva, perineal and right gluteal regions, corresponding to L1–L2 and S2–S5 dermatomes (Fig. 1a and b). Varicella-zoster virus DNA was detected by polymerase chain reaction testing of vesicular fluid. On admission the total lymphocyte count was 300 per mL. Fingolimod was suspended immediately and intravenous acyclovir was given for 14 days (10mg per kg every 8h). Complete lymphocytes recovery (1300 per mL) was observed 7 days later. A maintenance therapy with famcyclovir 500mg every 12h was given for two months. Lesions resolved completely in 2 months. One week after discharge the patient initiated treatment with glatiramer acetate, but in less than 8 weeks she presented a new MS relapse characterized by left hemianesthesia, for which high dose methylprednisolone was prescribed during 5 days and 4 weeks taper.
DiscussionThis patient developed polydermatomal herpes zoster in the setting of fingolimod-related lymphopenia expected due to intrinsic action of the new drug.3,4 The temporal association of VZV infection after the introduction of fingolimod without other predisposing factors suggests a causal relationship between fingolimod and zoster reactivation. The mechanism of facilitation of severe herpes zoster reactivation by fingolimod remains unclear. It could be caused by lowering the peripheral number of specific T cell subtype necessary to control VZV replication.9 Until more data are available, a cautious approach might be to determine VZV serology before starting fingolimod, and if the result is negative some authors recommend pre-treatment vaccination.2,6,8,10 In the light of the current data we consider prudent to discontinue fingolimod in patients who display any symptoms which may suggest potentially life threatening infectious complications. Moreover, there was one case of reactivation of VZV in a patient treated with fingolimod6 who, despite treatment with oral antiviral for zoster, developed severe VZV encephalitis. In the case reported here, intravenous treatment for polydermatomal zoster was started immediately and this could be at least in part responsible for the favourable outcome. Publication of more cases and further registries are needed for better safety assessment.