To describe the clinical presentation of a large number of Q fever endocarditis (QFE) and its management considering the role of serology.
Patients and methodsEighty-three patients with definite QFE (56 native and 27 prosthetic valve) with a long-term follow-up after stopping treatment (median: 48 months) were included. Final outcome (cure or relapse) was compared according with the serological titre at the end of therapy: less than 1:400 of phase I Ig G antibodies by indirect immunofluorescence (group 1, N=23) or more than 1:400 (group 2, N=30).
ResultsEleven patients (13.2%) died from QFE and other 8 died for other reasons not related to endocarditis during follow-up. Surgery was performed in 61 (73.5%) patients and combined antimicrobial treatment was long (median: 23 months, IQR: 12 – 36). Seven relapses were observed, but five of them had received an initial incomplete antibiotic regimen. In patients who completed the programmed treatment (range: 12 – 89 months), serological titres at the end of therapy were not useful for predicting the final outcome: one relapse in each group.
ConclusionsQFE requires a prolonged antimicrobial treatment, but serological titres are not useful for determining its duration.
Describir la presentación clínica de la endocarditis por fiebre Q (EFQ) y su manejo terapéutico, así como el papel de la serología en este aspecto.
Pacientes y métodoSe incluyeron 83 casos de EFQ definidas (56 nativas y 27 protésicas) con un seguimiento prolongado después de la finalización del tratamiento (mediana de 48 meses). La evolución final (curación o recidiva) se comparó dividiendo los casos en dos grupos según el título serológico al final del tratamiento: menos de 1:400 para Ac Ig G en fase I mediante inmunofluorescencia indirecta (grupo 1, N=23) o más de 1:400 (grupo 2, N=30).
ResultadosOnce pacientes (13.2%) murieron por EFQ y otros 8 lo hicieron durante el seguimiento por diversas razones no relacionadas con la endocarditis. Fueron operados 61 (73.5%) pacientes y el tratamiento antimicrobiano fue muy prolongado (mediana: 23 meses, RIQ: 12 – 36). Siete pacientes recidivaron al cesar el tratamiento, pero cinco de ellos no habían completado el inicialmente programado. En los pacientes que sí completaron el tratamiento antimicrobiano (rango: 12 – 89 meses), los títulos serológicos observados al final del mismo no fueron útiles para predecir la evolución final, observándose una recidiva en cada uno de los grupos.
ConclusionesLa EFQ requiere un tratamiento antimicrobiano prolongado, pero los títulos serológicos no son un instrumento útil para determinar su duración
Q fever is a world-wide zoonosis caused by Coxiella burnetii. Infections in humans can be acute or chronic, and endocarditis is the predominant form of this last type of infection.1 Because C. burnetii is an obligate intracellular bacteria that does not grow in current synthetic media, the diagnosis frequently relies on cardiac findings, evidence of an inflammatory process (with non specific and many times silent clinical manifestations), and the detection of specific antibodies (phases II and I), that often delay the definite recognition for several months.2–4 The importance of this negative blood culture endocarditis is controversial. An incidence of 3 to 5% of all infectious endocarditis has been reported in the United Kingdom and France,5–8 and it is also frequently described in Spain, Canada and Australia,9–15 but rarely reported in Germany or United States.16,17 Moreover, when the disease was first described, a high number of relapses and deaths were reported, perhaps due to inappropriate treatments.6,8,18 Today, an earlier diagnosis and the use of combined and prolonged antibiotic drug therapy have probably changed the final outcome. However, some issues in treatment of Q fever endocarditis are still debated nowadays, including the appropriate regimen, the role of combinative therapy, the therapeutic duration and the role of valve replacement. The little number of cases collected in a single centre and the need of a prolonged follow-up after the end of treatment for detecting late relapses have made somewhat difficult the establishment of evidence-based guidelines The aim of this study has been to answer some of these aspects by the analysis of a great number of cases collected in our country with uniform criteria.
Patients and methodsSubjects and settingPatients included in this study were diagnosed as QFE between 1979 to 2006 at 13 tertiary and University hospitals in Spain, which are reference centres for cardiac surgery. A retrospective questionnaire recording epidemiological, clinical, analytical, echocardiographic, microbiological and therapeutic data was fulfilled by the physician that had attended the patient in each centre. Several of these cases are described in detail elsewhere.9–13 For analysis of diagnosis time, surgery and mortality, we have considered two periods: 1979 - 1994 (first period) and 1995 – 2006 (second period).
DefinitionsQ fever endocarditis was defined following the modified Duke criteria for including endocarditis caused by Coxiella burnetii.19
Cure was defined when, after cessation of treatment, the patient remained asymptomatic with no alteration in analytical data and no elevation in his serological titres of phase I antibodies (Ab) up to one year.
Relapse was defined as the reappearance of symptoms (fever, malaise, spleen or liver enlargement, etc.), alteration in analytical data (anaemia, thrombocytopenia, rise of liver enzymes, etc.) after excluding any other reason, and a 4-fold increase of IgG phase I titres after the end of treatment and during the whole follow-up, or the isolation of Coxiella burnetii from cardiac tissue, if surgery was performed later.
Late prosthetic valve endocarditis was defined when the diagnosis was done more than 12 months after valve surgery.
Diagnostic proceduresTransthoracic echocardiography (TTE) was routinely performed and transesophageal studies (TEE) were done after 1990, when the results of TT echo were inconclusive or an intracardiac complication (i.e.: paravalvular abscess) was suspected.
Serological studies were performed in all cases in each centre, and were considered diagnostic of chronic Q fever if antibodies (Ab) to phase I antigens (Ag) were greater than or equal to 1:800 for IgG or 1:50 for IgA, by indirect immunofluorescence test (IFI).20 For the purpose of the study, paired samples of each patient were revised for definitive titres.
Valve cusps and vegetations were stained with Giemsa, haematoxylin-eosin and in some cases studied by transmission electronic microscopy.
In some cases, isolation of C. burnetii from valve cusps was performed using cell culture as described previously,11 or by shell vial assay as described by Raoult et al,21 including also samples from blood. The analysis by polymerase chain reaction (PCR) of blood and cardiac tissue was performed as described by Stein and Raoult.22
Follow up and serological evaluationTreatment and duration of it was decided by the attending physicians. In order to obtain the best bioavailability of doxycycline, this drug was given separated from foods and use of antacids was not permitted. After the end of treatment, patients were evaluated every 3-4 months with physical examination, general laboratory analysis and serological determinations. Taking into account only the patients with complete follow-up (more than one year after the end of treatment) and serological samples available, we established two groups for the analysis regarding the serological titres in the final outcome: Group 1 were patients with phase I Ig G Ab less than 1:400 by IFI at the end of treatment and Group 2 were patients with still phase 1 Ig G Ab ≥ 1:400.
Statistical analysisDescriptive statistics for continuous variables were summarized in terms of means and standard deviation or medians and interquartile ranges (IQR). Categorical variables were reported in terms of number and percentage of patients. Chi-square or Fisher's test and Kruskal-Wallis test were used to compare variables. A difference was considered significant when p<0.05.
ResultsEighty-three patients were reported from six different regions of Spain: Andalusia (N=35), Catalonia (N=16), Madrid (N=15), Extremadura (N=9), Cantabria (N=8) and Basque country (N=1). Overall, QFE represented the 1.6% of 5,004 episodes of infective endocarditis recorded. The number of cases reported was similar in each period: 43 in the first period and 40 in the second one, and the prevalence in each centre ranged between 0.2% and 4.8%. Mean age was 44.8±14 years (range: 11 - 82) and 78.3% of patients (n=65) were males. Fifty-six cases (67.4%) were native valve endocarditis and 27 late prosthetic endocarditis (14 metallic and 13 biological valves), with a median time since implantation of 45 months (IQR: 24 - 72).
Most patients (n=42) with native valve endocarditis had predisposing valvulopathy: rheumatic disease (n=16), bicuspid aortic valve (n=12), other congenital diseases (n=7), mitral valve prolapse (n=3), aortic sclerosis (n=3) and atrial myxoma (n=1). In 10 cases (12%) a previous episode of endocarditis by a different microorganism was registered. The aortic valve was affected in 49 patients (59%), mitral valve in 27 (32.5%), and both valves in five. In one patient vegetations were detected above a myxoma and over an interventricular communication in another one. Fifty-five patients (66.2%) had risk factors for acquisition (table 1), although only in 18% it was occupational (farmers or other continuous contact with animals). Seventeen patients (21%) had a potential immunosuppressive disease: 5 patients with severe liver disease (one of them with liver transplantation), 3 with advanced neoplasm, 3 with chronic renal insufficiency, 3 with chronic pulmonary disease and frequent use of steroids, 2 with alcoholic condition and one patient with HIV infection.
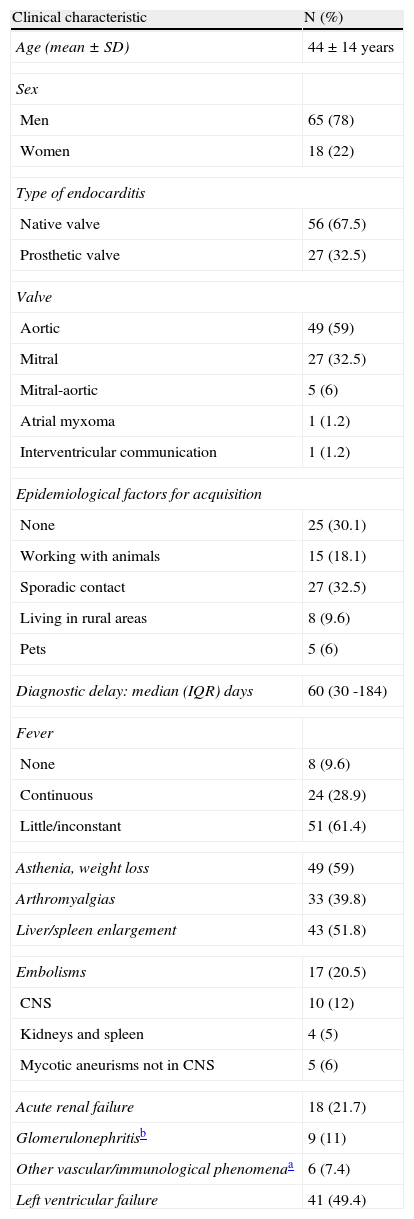
Main clinical characteristics and analytical data of cases.
| Clinical characteristic | N (%) |
| Age (mean±SD) | 44±14 years |
| Sex | |
| Men | 65 (78) |
| Women | 18 (22) |
| Type of endocarditis | |
| Native valve | 56 (67.5) |
| Prosthetic valve | 27 (32.5) |
| Valve | |
| Aortic | 49 (59) |
| Mitral | 27 (32.5) |
| Mitral-aortic | 5 (6) |
| Atrial myxoma | 1 (1.2) |
| Interventricular communication | 1 (1.2) |
| Epidemiological factors for acquisition | |
| None | 25 (30.1) |
| Working with animals | 15 (18.1) |
| Sporadic contact | 27 (32.5) |
| Living in rural areas | 8 (9.6) |
| Pets | 5 (6) |
| Diagnostic delay: median (IQR) days | 60 (30 -184) |
| Fever | |
| None | 8 (9.6) |
| Continuous | 24 (28.9) |
| Little/inconstant | 51 (61.4) |
| Asthenia, weight loss | 49 (59) |
| Arthromyalgias | 33 (39.8) |
| Liver/spleen enlargement | 43 (51.8) |
| Embolisms | 17 (20.5) |
| CNS | 10 (12) |
| Kidneys and spleen | 4 (5) |
| Mycotic aneurisms not in CNS | 5 (6) |
| Acute renal failure | 18 (21.7) |
| Glomerulonephritisb | 9 (11) |
| Other vascular/immunological phenomenaa | 6 (7.4) |
| Left ventricular failure | 41 (49.4) |
| Analytical data | N (%) |
| Anaemia (haemoglobin<12g/dl) | 58 (70) |
| Leucocytosis (>10.000/mm3) | 16 (19.3) |
| Thrombocytopenia (<100.000/mm3) | 24 (29) |
| Elevated erythrocyte sedimentation (>15mm) | 63 (76) |
| Hepatitisc | 16 (19.3) |
| Leukocyturia | 3 (3.6) |
| Haematuria | 15 (18.1) |
| Proteinuria | 8 (9.6) |
CNS: Central Nervous System.
Clinical features and analytical data are presented in table 1. Q fever endocarditis presented as a prolonged febrile illness of unknown origin in 30 patients (36.1%), in 18 (21.7%) the reason for hospitalization was congestive heart failure without etiological diagnosis, 5 patients presented with a major embolism in Central Nervous System, and in only 30 patients the first diagnosis was negative blood culture infective endocarditis. Fever was frequently (61.7%) of low-grade or intermittent, and time since the first symptoms until definitive diagnosis was long (median: 60 days, IQR: 30 - 184) with a greater, although not significant, delay in the first period of the study (90 vs. 58 days). Cardiac embolisms were infrequent compared with other endocarditis (20.5%) and conversely, we registered 9 cases of immune glomerulonephritis. Anaemia, thrombocytopenia, haematuria and elevated liver enzymes were the main alterations reported.
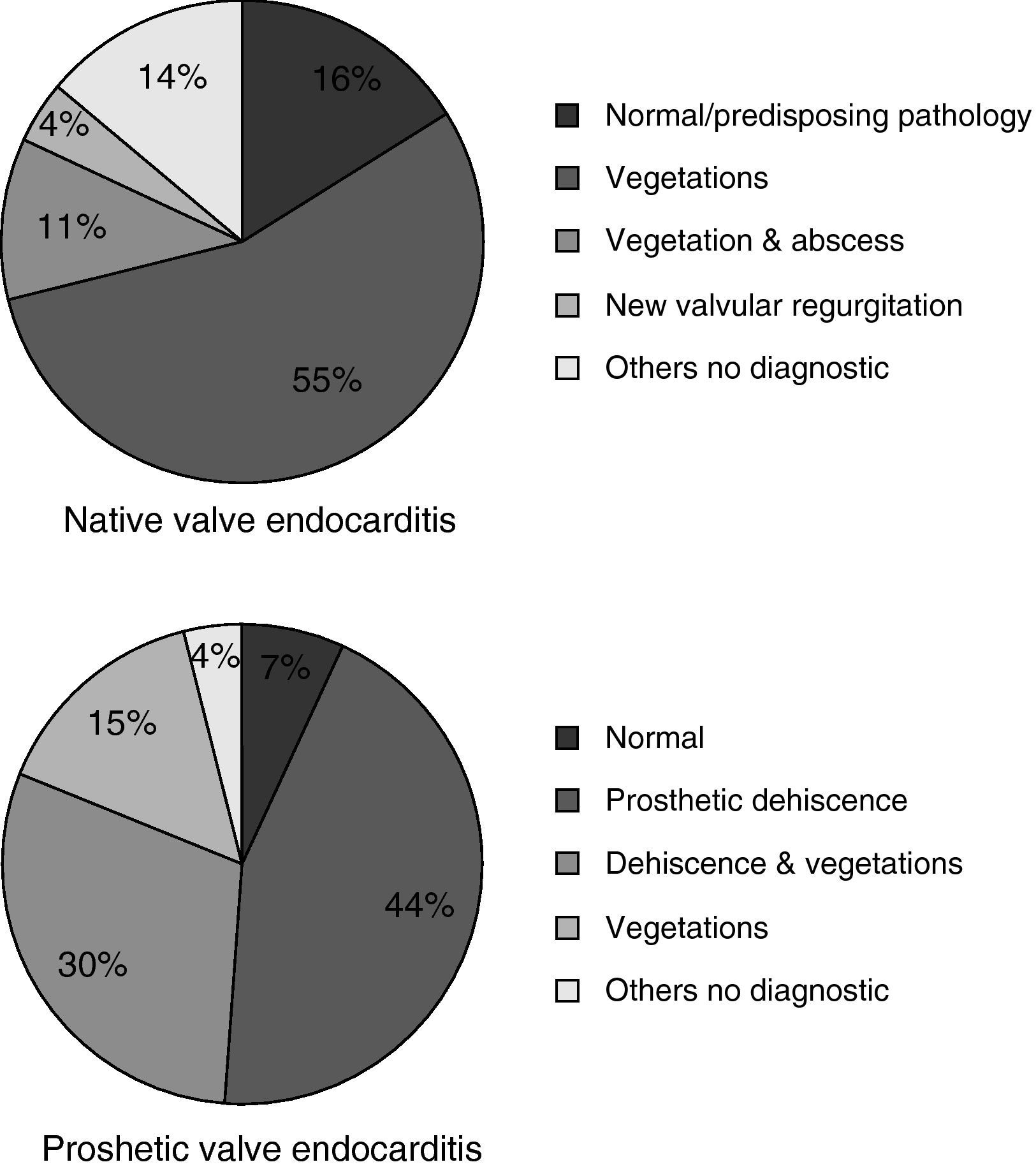
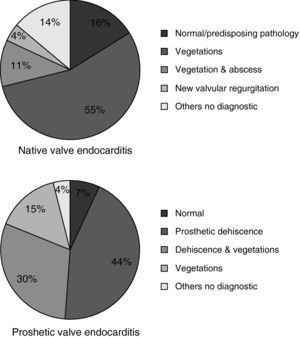
Echographic studies were performed in all patients: 55 patients with TT study, 2 with TE and 26 with both studies. In 65 patients (78.3%) the study was considered diagnostic of IE, more frequently in prosthetic valve endocarditis (fig. 1). Twenty-eight patients (33.7%) experienced intracardiac complications: aortic abscesses (N=20), aortic root aneurisms (N=9), valve perforation or rupture (N=8) and an aortocaval fistula (N=1). Pericardic effusion was observed in two cases (2.4%).
Sixty-one patients (73.5%) were operated with slight differences between the two periods (79% vs. 62.5%). In 47 of them, cardiac surgery was performed during the first hospitalization and in 14 during the follow-up, mainly for left ventricular failure (N=41), prosthesis dehiscence (N=9) and/or intracardiac complications (N=11). A second operation was performed in 9 patients for prosthetic dehiscence, and three patients suffered more than two procedures during the follow-up. Eighteen valves were stained with Giemsa and processed for electronic microscope, with microorganisms seen in five samples (27.7%). Cell cultures were used in nine cases and they were positive in six (66.6%). Finally, PCR was performed in four valves and was positive in all of them.
Antimicrobial therapy was in general long, with a median time of 23 months (IQR: 12 – 36). Fourteen patients were treated with doxycycline alone and this drug was combined with cotrimoxazole (N=19), rifampicin (N=4), quinolones (N=35), changing combinations (N=7) or chloroquine (N=4). No differences in evolution (cure/relapse) or mortality (7% with monotherapy and 14.4% with combined therapy) were observed regarding the type of treatment used, and the main secondary effect was photosensitivity, reported in nine patients (10.8%).
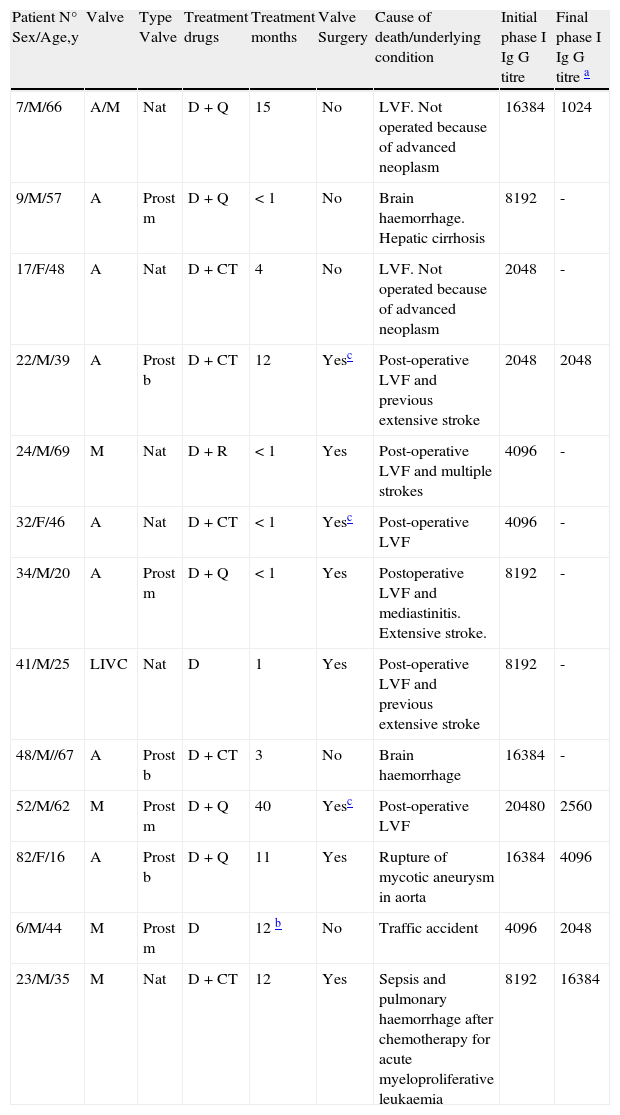
Thirteen patients died while on treatment (N=12) or before completing one year of follow-up after cessation (N=1). In 11 cases (13.2%), mortality was related to endocarditis: 6 patients with surgery (10.1%) and 5 without surgery (22.7%), with no significant differences between periods (16.2% in the first period and 11.1% in the second one). Reasons were: left ventricular failure (N=8, five in the early postoperative period after valve reposition, four of them with neurological complications), brain haemorrhage (N=2) and rupture of an aortic mycotic aneurysm (N=1). The other two patients died from a traffic accident (patient n° 6) and from secondary effects of chemotherapy for malignancy within the first year (patient n° 23). Valve (aortic/mitral), type of endocarditis (native/prosthetic), age, comorbidity, delay in diagnosis, type of treatment (single or combinative) and duration of it, and surgery were not related to mortality (data not shown).The only prognostic factor related to mortality was embolic or haemorrhagic events in Central Nervous System: 13 patients, six of them (46.2%) died vs. five deaths (7.5%) in patients without brain damage (P<0.01). Detailed data are shown in Table 2.
Patients with endocarditis-related death and non related death before completing one year of follow-up.
| Patient N° Sex/Age,y | Valve | Type Valve | Treatment drugs | Treatment months | Valve Surgery | Cause of death/underlying condition | Initial phase I Ig G titre | Final phase I Ig G titre a |
| 7/M/66 | A/M | Nat | D+Q | 15 | No | LVF. Not operated because of advanced neoplasm | 16384 | 1024 |
| 9/M/57 | A | Prost m | D+Q | < 1 | No | Brain haemorrhage. Hepatic cirrhosis | 8192 | - |
| 17/F/48 | A | Nat | D+CT | 4 | No | LVF. Not operated because of advanced neoplasm | 2048 | - |
| 22/M/39 | A | Prost b | D+CT | 12 | Yesc | Post-operative LVF and previous extensive stroke | 2048 | 2048 |
| 24/M/69 | M | Nat | D+R | < 1 | Yes | Post-operative LVF and multiple strokes | 4096 | - |
| 32/F/46 | A | Nat | D+CT | < 1 | Yesc | Post-operative LVF | 4096 | - |
| 34/M/20 | A | Prost m | D+Q | < 1 | Yes | Postoperative LVF and mediastinitis. Extensive stroke. | 8192 | - |
| 41/M/25 | LIVC | Nat | D | 1 | Yes | Post-operative LVF and previous extensive stroke | 8192 | - |
| 48/M//67 | A | Prost b | D+CT | 3 | No | Brain haemorrhage | 16384 | - |
| 52/M/62 | M | Prost m | D+Q | 40 | Yesc | Post-operative LVF | 20480 | 2560 |
| 82/F/16 | A | Prost b | D+Q | 11 | Yes | Rupture of mycotic aneurysm in aorta | 16384 | 4096 |
| 6/M/44 | M | Prost m | D | 12 b | No | Traffic accident | 4096 | 2048 |
| 23/M/35 | M | Nat | D+CT | 12 | Yes | Sepsis and pulmonary haemorrhage after chemotherapy for acute myeloproliferative leukaemia | 8192 | 16384 |
A: aortic valve; M: mitral valve; LIVC: left interventricular communication Nat: native valve endocarditis; Prost: prosthetic valve endocarditis; m: mechanical prosthesis; b: bioprosthesis; D: doxycycline; R: rifampicin, CT: cotrimoxazole; Q: quinolones, H: hydroxychloroquine. LVF: Left ventricular failure.
Seven patients did not complete the follow-up while on treatment and were lost at 1, 2, 3, 4, 6, 22 and 48 months because they moved to other regions and six patients are still on treatment (6, 24, 26, 27, 32, an 34 months) and are also not evaluable. Five patients initially received incomplete treatment (all of them less than 3 months) and promptly relapsed, but they re-initiated it and were then available for a long follow-up. Overall, 57 patients completed the programmed therapy and have a median follow-up of 33 months (IQR: 12 - 57) after the ending of it. Fifty-five were considered cured (96.5%), after a median therapy time of 24 months (IQR: 16.5 – 36.5) and two patients relapsed (see below). During the extended follow-up 7 patients died (patients n° 15, 26, 37, 40, 53, 62 and 71), but all of them have been at less two years without symptoms of QFE after ending treatment and mortality was clearly related to other reasons: 3 from malignancies, one from end renal insufficiency, one from lung emphysema and two in the surgery of a new endocarditis by Streptococcus viridans. Serological data from these patients are also provided in Tables 3 and 4.
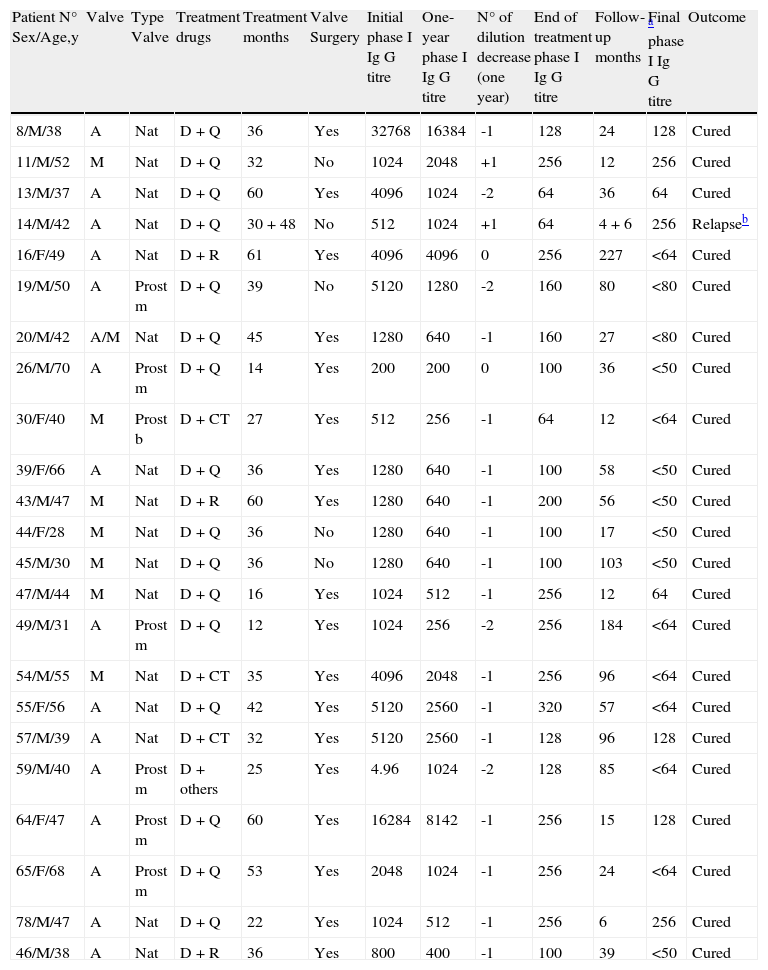
Characteristics of patients in Group 1 (End of treatment phase I Ig G titre <1:400 by IFI).
| Patient N° Sex/Age,y | Valve | Type Valve | Treatment drugs | Treatment months | Valve Surgery | Initial phase I Ig G titre | One-year phase I Ig G titre | N° of dilution decrease (one year) | End of treatment phase I Ig G titre | Follow-up months | Final a phase I Ig G titre | Outcome |
| 8/M/38 | A | Nat | D+Q | 36 | Yes | 32768 | 16384 | -1 | 128 | 24 | 128 | Cured |
| 11/M/52 | M | Nat | D+Q | 32 | No | 1024 | 2048 | +1 | 256 | 12 | 256 | Cured |
| 13/M/37 | A | Nat | D+Q | 60 | Yes | 4096 | 1024 | -2 | 64 | 36 | 64 | Cured |
| 14/M/42 | A | Nat | D+Q | 30+48 | No | 512 | 1024 | +1 | 64 | 4 + 6 | 256 | Relapseb |
| 16/F/49 | A | Nat | D+R | 61 | Yes | 4096 | 4096 | 0 | 256 | 227 | <64 | Cured |
| 19/M/50 | A | Prost m | D+Q | 39 | No | 5120 | 1280 | -2 | 160 | 80 | <80 | Cured |
| 20/M/42 | A/M | Nat | D+Q | 45 | Yes | 1280 | 640 | -1 | 160 | 27 | <80 | Cured |
| 26/M/70 | A | Prost m | D+Q | 14 | Yes | 200 | 200 | 0 | 100 | 36 | <50 | Cured |
| 30/F/40 | M | Prost b | D+CT | 27 | Yes | 512 | 256 | -1 | 64 | 12 | <64 | Cured |
| 39/F/66 | A | Nat | D+Q | 36 | Yes | 1280 | 640 | -1 | 100 | 58 | <50 | Cured |
| 43/M/47 | M | Nat | D+R | 60 | Yes | 1280 | 640 | -1 | 200 | 56 | <50 | Cured |
| 44/F/28 | M | Nat | D+Q | 36 | No | 1280 | 640 | -1 | 100 | 17 | <50 | Cured |
| 45/M/30 | M | Nat | D+Q | 36 | No | 1280 | 640 | -1 | 100 | 103 | <50 | Cured |
| 47/M/44 | M | Nat | D+Q | 16 | Yes | 1024 | 512 | -1 | 256 | 12 | 64 | Cured |
| 49/M/31 | A | Prost m | D+Q | 12 | Yes | 1024 | 256 | -2 | 256 | 184 | <64 | Cured |
| 54/M/55 | M | Nat | D+CT | 35 | Yes | 4096 | 2048 | -1 | 256 | 96 | <64 | Cured |
| 55/F/56 | A | Nat | D+Q | 42 | Yes | 5120 | 2560 | -1 | 320 | 57 | <64 | Cured |
| 57/M/39 | A | Nat | D+CT | 32 | Yes | 5120 | 2560 | -1 | 128 | 96 | 128 | Cured |
| 59/M/40 | A | Prost m | D+others | 25 | Yes | 4.96 | 1024 | -2 | 128 | 85 | <64 | Cured |
| 64/F/47 | A | Prost m | D+Q | 60 | Yes | 16284 | 8142 | -1 | 256 | 15 | 128 | Cured |
| 65/F/68 | A | Prost m | D+Q | 53 | Yes | 2048 | 1024 | -1 | 256 | 24 | <64 | Cured |
| 78/M/47 | A | Nat | D+Q | 22 | Yes | 1024 | 512 | -1 | 256 | 6 | 256 | Cured |
| 46/M/38 | A | Nat | D+R | 36 | Yes | 800 | 400 | -1 | 100 | 39 | <50 | Cured |
A: aortic valve; M: mitral valve; Nat: native valve endocarditis; Prost: prosthetic valve endocarditis; m: mechanical prosthesis; b: bioprosthesis; D: doxycycline; R: rifampicin, CT: cotrimoxazole; Q: quinolones, H: hydroxychloroquine.
Characteristics of patients in Group 2 (End of treatment phase I Ig G titre ≥1:400 by IFI).
| Patient N° Sex/Age,y | Valve | Type Valve | Treatment drugs | Treatment months | Valve Surgery | Initial phase I Ig G titre | One-year phase I Ig G titre | N° of dilution decrease (one year) | End of treatment phase I Ig G titre | Follow-up months | Final a phase I Ig G titre | Outcome |
| 1/M/32 | M | Nat | D+CT | 10 | Yes | 1024 | 1024 | 0 | 1024 | 57 | 512 | Cured |
| 2/M/28 | A | Nat | D+R | 12 | No | 512 | 512 | 0 | 512 | 24 | 512 | Cured |
| 4/M/11 | A | Nat | D | 18 | Yes | 2048 | 1024 | -1 | 1024 | 42 | 64 | Cured |
| 5/F/35 | M | Prost b | D | 18 | Yes | 2048 | 1024 | -1 | 1024 | 6 | 1024 | Cured |
| 12/M/24 | A | Nat | D+Q | 24 | Yes | 8192 | 8192 | 0 | 8192 | 36 | 8192 | Cured |
| 15/M/48 | A | Nat | D+others | 51 | Yes | 4096 | 2048 | -1 | 1024 | 39 | 1024 | Cured |
| 18/M/30 | A | Nat | D+Q | 43 | Yes | 51200 | 51200 | 0 | 3200 | 137 | 2560 | Cured |
| 21/M/37 | A | Nat | D+CT | 24 | No | 8192 | 2048 | -2 | 2048 | 6 | 1024 | Cured |
| 27/M/37 | M | Nat | D+Q | 24 | Yes | 3200 | 3200 | 0 | 1625 | 25 | 3200 | Cured |
| 28/M/49 | M | Nat | D+Q | 16 | Yes | 3200 | 128000 | +4 | 128000 | 235 | 400 | Cured |
| 29/F/28 | M | Prost b | D+Q | 12 | Yes | 1280 | 640 | -1 | 640 | 12 | 1280 | Cured |
| 31/M/43 | A/M | Prost b | D+CT | 12 | Yes | 1280 | 640 | -1 | 640 | 9 | 80 | Cured |
| 33/M/40 | A/M | Nat | D+CT | 18 | Yes | 1024 | 1024 | 0 | 1024 | 12 | 128 | Cured |
| 35/F/42 | M | Nat | D | 12 | No | 5120 | 5120 | 0 | 5120 | 43 | 128 | Cured |
| 40/M/26 | A | Prost m | D+Q | 12 | Yes | 1024 | 1024 | 0 | 1024 | 35 | 128 | Cured |
| 42/M/39 | A | Prost b | D+CT | 12+60 | Yes | 2048 | 2048 | 0 | 2048 | 204+56 | 128 | Relapseb |
| 50/M/50 | M | Prost m | D+CT | 37 | Yes | 40960 | 20480 | -1 | 10240 | 27 | 320 | Cured |
| 51/M/47 | M | Prost m | D+Q | 23 | Yes | 40960 | 20480 | -1 | 10240 | 6 | 1024 | Cured |
| 53/M/47 | A | Nat | D+CT | 12 | Yes | 2560 | 2560 | 0 | 2560 | 75 | 600 | Cured |
| 61/M/40 | A | Prost b | D+others | 48 | Yes | 16284 | 8142 | -1 | 512 | 51 | 256 | Cured |
| 62/M/62 | A/M | Prost b | D+others | 23 | No | 4096 | 8192 | +1 | 16384 | 25 | 1024 | Cured |
| 63/M/29 | A | Nat | D+CT | 24 | Yes | 4096 | 2048 | -1 | 1024 | 25 | 256 | Cured |
| 67/M/45 | A | Nat | D+H | 16 | Yes | 3200 | 1600 | -1 | 1600 | 12 | 1600 | Cured |
| 69/M/41 | M | Nat | D+Q | 18 | No | 2048 | 1024 | -1 | 1024 | 96 | 256 | Cured |
| 71/M/69 | A | Nat | D+Q | 24 | Yes | 81920 | 20475 | -2 | 640 | 48 | 160 | Cured |
| 75/M/59 | A | Nat | D+Q | 89 | Yes | 10240 | 10240 | 0 | 2560 | 12 | 640 | Cured |
| 76/M/36 | A | Nat | D+others | 17 | Yes | 10240 | 1280 | -3 | 640 | 33 | 160 | Cured |
| 77/M/61 | A | Prost b | D+Q | 26 | No | 2560 | 5120 | +1 | 5120 | 9 | 320 | Cured |
| 80/M/53 | A | Nat | D+CT | 24 | Yes | 4096 | 2048 | -1 | 2048 | 24 | 512 | Cured |
| 81/M/23 | M | Nat | D | 24 | No | 320 | 640 | +1 | 640 | 36 | 640 | Cured |
A: aortic valve; M: mitral valve; Nat: native valve endocarditis; Prost: prosthetic valve endocarditis; m: mechanical prosthesis; b: bioprosthesis; D: doxycycline; R: rifampicin, CT: cotrimoxazole; Q: quinolones, H: hydroxychloroquine.
Regarding the impact of serology in the final outcome, four patients were not evaluable because routinely serological titres were not performed. Twenty-three patients were inscribed in Group 1 (final titre of phase I Ab<1:400), 17 of them (74%) were operated and the median time of treatment was 36 months (IQR: 12 – 55), with one relapse. This patient was not operated and received 30 months of treatment with an early relapse (4 months after cessation) despite repeated phase I Ab titres of 1:256. He has recently completed a new treatment of 48 months and now is on follow-up. In Group 2 (Ab≥1:400) thirty patients were inscribed, 23 of them were operated (76%) and the median time of treatment was 23 months (IQR: 12 -24), with one relapse. This patient had a mitral-aortic endocarditis with aortic valve replacement, and received antimicrobial treatment for 12 months (final phase I titre: 1:2048). After this, serological titres progressive fell, but eight years later he developed clinical symptoms again, titres ascended and mitral valve was severely affected. He was operated again and mitral valve was replaced (Giemsa stain positive), but aortic valve prosthesis was not affected. A complete description of both groups is presented in Tables 3 and 4. In general, antibodies titres fell very slowly and negative values were obtained after 4-5 years depending of the initial titre. Transitory mild elevations (two to four-fold increases) were sometimes observed with concomitant non related infections in patients upon treatment (2 respiratory and 1 urinary infection; data not shown) and also with cardiac surgery (patient n° 28). We did not find any relationship between their decline in the first year and the outcome and neither with the administered treatment.
DiscussionQ fever endocarditis is the most serious complication of Coxiella burnetii infection and has been observed in 1-16% of reported cases of Q fever,23,24 but it can be much higher in patients with predisposing valvulopathy or prosthetic valves.15,25 In Spain, seroprevalence for Coxiella burnetii depends on the type of population and geographic zone, ranging from 1 to 46%.26 Moreover, acute Q fever is frequently reported as the main cause of fever of intermediate duration in the South27 and the predominance of chronic cases from this region in this study can be due to a high proportion of population at risk in rural areas, and perhaps to under-diagnosis in other regions. However, it is surprising the low number of cases reported in the North, in which several outbreaks of the acute form has been described.28 Perhaps different C. burnetii strains could explain, in part, these differences.29
QFE is one of the most indolent form of infective endocarditis and it is not unusual for the infection to be mildly symptomatic for years before it is diagnosed. This factor could explain the considerable diagnostic delay observed in this report. Identified exposure to potential infective sources ranged from 29% to 75%30 and predisposing valvulopathy is almost invariably present,25 as it is reported in our cases. Clinical signs and symptoms in this study do not differ from others,2,6–8,15,18 being QFE a disease with lower embolic events and higher immunologic features compared with other aetiologies.31 Echocardiography is frequently inconclusive in this entity because cardiac vegetations tend to be small and localized beneath endothelial surfaces. Furthermore, pathologic features can be easily confused with non-infectious degenerative valve damage, for which a transesophageal study is recommended.32 In this series, echocardiography was diagnostic in a higher proportion of prosthetic valves, probably because they were considered positive if a new prosthetic dehiscence was detected, which is frequently observed due to the prolonged course of the disease.33
In the literature, chronic Q fever is sometimes described as a severe illness with a spontaneous death rate that may exceed 65%.30 With appropriate antibiotic therapy, associated mortality can be reduced, but the microorganism is difficult to eradicate, and a long course of treatment is necessary. Objective evaluation of antibiotic efficacy, in vivo or in vitro, is troublesome due to the rarity of the disease, the need for a very long follow-up, and the absence of well established in vitro models or animal experimentation. Doxycycline is considered the mainstay of medical therapy, but relapses are frequent upon cessation of treatment, prompting the development of combination regimens.34 Rifampicin, cotrimoxazole and quinolones have been used, but without controlled comparison. The only study claiming a statistically significant advantage with combination therapy (doxycycline plus quinolones vs. doxycycline alone) included patients who died during the first month of therapy, and differences can be explained because of a reduced time of diagnosis and a better surgical approach in the late period of the study in which combination therapy was recommended.8 Furthermore, viable C. burnetii was isolated from excised valves despite 9 and 12 months of treatment, confirming in vitro data that have shown that the pH within the acidified phagosomal compartment in which intracellular C. burnetii resides, may be responsible for the lack of bactericidal activity of many antibiotics.35 In the same way, a correlation between serum doxycycline concentration and the decrease of serological titres in patients with QFE was also observed36–38 and the combination of doxycycline with hydroxychloroquine (an alkalinizing agent of phagolysosomes) has shown in vitro bactericidal activity against C. burnetii.39 In a clinical study involving 35 patients with QFE,40 this combination reduced the median duration of treatment by two years compared to treatment with doxycycline and ofloxacin, and there were no relapses after 18 months of therapy in the group that received hydroxychloroquine-doxycycline, although two cases were observed in this arm when treatment was shortened (10 months). In our retrospective study, hydroxychloroquine-doxycycline was used only in the latest cases and the short number of cases in each group made impossible to find statistical differences. The length of therapy was then critical, because relapses can occur many years later,41,42 as we could see in one of our cases. Relapses are sometimes difficult to detect because the slow progression and the protean clinical manifestation in patients with pre-existing valve disease, which may be misinterpreted as the spontaneous course of primary valve involvement. Recommendations have varied between 1 year to lifelong administration,18 and other authors have proposed the monitoring of Ig G levels in the setting that treatment could be stopped if phase I Ig G levels dropped below certain values. A cut-off of 1:200 for Ig G phase 1antibodies was firstly proposed43 and it is still recommended in recent European Guidelines for the management of endocarditis.44 However, this cut-off had been elevated to 1:400 and 1:800 by the same authors after a minimum of three years of treatment if hydroxychloroquine is not used.8,40 In the same way, a decline greater than two-fold dilutions on the first year of treatment has also been considered a good outcome predictor that is correlated with a lower MIC for doxycycline and a higher concentration of this drug in plasma.37,38 However, it is difficult to assume that these plasmatic concentrations parallels with high intracellular levels that are the main characteristic of doxycycline. Moreover, a carefully evaluation of the data shows that there is a considerable overlap between the values and that all cases were treated more than two years with no relapses. In our report, most of the patients were treated monitoring phase I Ig G levels as were the current recommendations therefore. However, the decrease of IgG phase I antibodies until three years was rare and not related with cures or relapses. In fact, the occurrence of relapse based on serological data was unpredictable in our study, and treatment guided by monitoring phase I Ig G levels was a median of 1 year longer, without differences in mortality or relapses, as have been reported by others.45 Furthermore, most relapses occurred with inappropriate treatments of less than 3 months, but a considerable number (N=17) of our patients cured with short treatments (12 – 18 months) and no factor was related to relapse when a minimum of 12 months was completed. High levels of antibodies probably reflect a polyclonal immune response that can be enhanced in a non-specific manner when other injuries (infection or surgery) occur, as we have observed with several patients in this study. Perhaps the difference in titres decline observed by Rolain et al37 must be due to the use of hydroxychloroquine, a potent anti-inflammatory drug that is widely used in rheumatic practice.
Of interest, in this report 70% of patients were operated and the two relapses observed were in non-excised valves. The contribution of valve replacement to the odds for cure is doubtful, since relapses following surgery have been widely described8,41,42. However, surgery in this study was protective (less mortality) and although indicated only for haemodynamic reasons, it probably contributed to a high number of recoveries with shorter treatments (median time: 23 months) when removing a considerable part of the infected tissue. In fact, 15 of 17 patients with the shortest treatments (12 – 18 months) were operated on. Moreover, mortality in this report was low compared with early descriptions.6,18 This fact has been pointed by others46 and probably reflects a better knowledge and more appropriate medical and surgical management nowadays.
Finally, we must remark that this is one of the largest series of Q fever endocarditis ever reported in the literature, with a very long follow-up. Epidemiological, clinical and analytical data have been collected in an uniform mode and interesting information is given. A long treatment (2 -3 years) seems necessary for avoiding relapses and perhaps surgery could shorten it, although the retrospective nature and variability of our work do not permit well-based recommendations regarding time and drugs used. Testing laboratories and serological reagents are different and inter laboratories discrepancies were not assessed, and furthermore, in the first years serology to Bartonella spp was not routinely performed and it is possible that non-operated cases with lower titres have been misdiagnosed due to cross-reactions with that microorganism.47 However, our report reflects current clinical practice: international criteria for diagnosis were used, close clinical follow-up were performed by the same physicians, and multiple (with controls titres) serological determinations were obtained for a long period for each patient, in the same laboratory. Most of the patients in this cohort were cured and this was confirmed by a prolonged follow-up, but there was not any specific test that made it possible to conclude that a patient had definitely been cured and, in our study, serology did not predict clinical outcome in chronic Q fever as neither do in other zoonosis (i.e.: brucellosis). In our opinion, only time of treatment is critical in this setting, and the monitoring of phase I antibodies titres as a guide for stopping antimicrobials only warranted a longer treatment in this study, with more cumulative toxicity.
Grant support information: This study was supported in part by the following grants: from the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008), from the Fundación Privada Máximo Soriano Jiménez, Barcelona, Spain for the grant supporting the Hospital Clínic Endocarditis database (Dr. Miro). Dr. J.M. Miró was a recipient of a Research Grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) and the Conselleria de Salut de la Generalitat de Catalunya, Barcelona (Spain).
Conflict of interestThe authors have no conflicts of interest to declare
Arístides de Alarcón MD PhD, Rafael Luque-Márquez MD, Maria Victoria Mogollón MD, Amalia Martín (Hospital Universitario Virgen del Rocío, Sevilla); Ana del Rio MD PhD, Asuncion Moreno MD PhD, Carlos A. Mestres MD, PhD, Carlos Paré MD PhD, Cristina Garcia de la Maria PhD, Elisa De Lazzari MSs, Francesc Marco MD PhD, Jose M Gatell MD PhD, José M. Miró MD, PhD, Manuel Almela MD, Manuel Azqueta MD, Maria Jesús Jiménez-Expósito MD PhD, Natividad de Benito MD PhD, Noel Perez MD, Xavier Claramonte MD, Yolanda Armero MD (Hosp. Clinic – IDIBAPS, Universidad de Barcelona);Isabel Sanfeliu MD PhD (Consorci Hospitalari Parc Taulí, Sabadell, Barcelona); Manuel Anguita MD PhD (Hospital Universitario Reina Sofía, Córdoba), Pilar Tornos MD PhD, Vicenc Falcó MD PhD, Benito Almirante MD PhD, Albert Pahissa MD PhD, Joan Gavaldá MD, PhD (Hospital Universitario Vall d¿Hebron, Barcelona); Juan Galvez-Acebal MD, Miguel A Muniaín MD PhD, Jesús Rodríguez-Baño (Hospital Universitario Virgen Macarena, Sevilla); Agustín Muñoz-Sanz MD, Ph D, Araceli Vera Tomé MD, Francisco Féliz Rodríguez MD (Hospital Universitario Infanta Cristina, Badajoz); Maria del Carmen Fariñas MD PhD, Ana Arnaíz MD PhD, JM Revuelta, JM Bernal, JF Nistal, JF Gutiérrez-Díez, MA Bernal (Hospital Universitario Marqués de Valdecilla, Santander); Manuel Fernández-Guerrero MD, Ph (Fundación Jiménez Díaz, Madrid); Isidre Vilacosta MD PhD, Maria del Carmen Manzano MD, Ph (Hospital San Carlos, Madrid); Patricia Muñoz MD PhD, Emilio Bouza MD PhD, Mercedes Marín MD PhD, M Desco, MA García-Fernández, M Martínez-Selles, MC Menárquez, M Moreno, B Pinilla, A Pinto, V Ramallo, M Rodríguez-Creixems, I Tamallo, JL Vallejo, F Díaz (Hospital Universitario Gregorio Marañón, Madrid); Jose Miguel Montejo MD Ph (Hospital Universitario de Cruces. Bilbao); Carmen Hidalgo-Tenorio MD Ph (Hospital Universitario Virgen de las Nieves, Granada); José María Ramos-Rincón (Hospital General de Elche).
Arístides de Alarcón, Rafael Luque-Márquez, Juan Gálvez-Acebal, and Carmen Hidalgo-Tenorio are also members of the Grupo para el Estudio de Infecciones Cardiovasculares de la Sociedad Andaluza de Enfermedades Infecciosas.
Members of the Spanish Q Fever Endocarditis Group are listed in the Appendix A.