The life expectancy of HIV-infected individuals has dramatically improved with potent antiretroviral therapies. However, organ-specific toxicities of some antiretrovirals and persistent inflammation and immune activation due to residual virus replication account for a high burden of age-associated comorbidities in the HIV population.
MethodsThe prevalence of overt cardiovascular, renal and bone diseases as well as their major risk factors were cross-sectionally examined during the year 2014 in the VACH cohort, a large nationwide population of HIV-infected individuals in Spain.
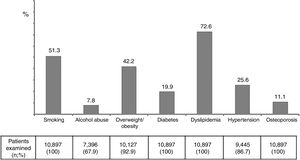
ResultsA total of 10,897 HIV-infected patients were examined. Seventy-one point four percent were male and the mean age was 48 years. Mean time since HIV diagnosis was 15.8 years and mean time on antiretroviral therapy was 13.1 years. The proportion of patients with undetectable viral load was 87.1%, whereas 65.7% had CD4 counts>500 cells/mm3. Overall, cardiovascular, renal and bone disease were recorded in 4.7%, 5.9% and 2.8%, respectively. The prevalence of major risk factors was as follows: smoking 51.3%, alcohol abuse 7.8%, overweight/obesity 42.2%, diabetes 19.9%, dyslipidaemia 72.6%, hypertension 25.6%, and osteoporosis 11.1%. In the subset of patients older than 55 years-old (18%), all figures for overt disease and their major risk factors were significantly greater.
ConclusionMajor age-related medical conditions and most of their risk factors are highly prevalent in HIV-infected individuals on long-term antiretroviral therapy in Spain. Preventive actions, including careful selection of antiretroviral agents, should be prioritized in the ageing HIV population.
La esperanza de vida de las personas infectadas por el VIH ha mejorado con la terapia antirretroviral. Sin embargo, la toxicidad de algunos antirretrovirales, la inflamación persistente y la activación inmune explican la alta carga de comorbilidades asociadas con el envejecimiento en la población con VIH.
MétodosLa prevalencia de enfermedad cardiovascular, renal y ósea, así como sus principales factores de riesgo, se analizó en una cohorte de individuos infectados por el VIH en un estudio realizado en España durante el año 2014.
ResultadosSe examinaron un total de 10.897 pacientes infectados por VIH. Varones el 74,1%, edad media 48 años. El tiempo medio transcurrido desde el diagnóstico del VIH fue de 15,8 años y el tiempo medio de tratamiento antirretroviral fue de 13,1 años. La proporción de pacientes con carga viral indetectable fue del 87,1%, mientras que el 65,7% tenían recuentos de CD4>500 células/mm3. La enfermedad cardiovascular, renal y ósea se encontró en el 4,7, 5,9 y 2,8%, respectivamente. La prevalencia de los principales factores de riesgo fue: fumar 51,3%, abuso de alcohol 7,8%, sobrepeso/obesidad 42,2%, diabetes 19,9%, dislipidemia 72,6%, hipertensión 25,6% y osteoporosis 11,1%. En el grupo de pacientes mayores de 55 años (18%), la prevalencia de comorbilidades y sus principales factores de riesgo fue significativamente mayor.
ConclusiónLas principales comorbilidades relacionadas con la edad y la mayoría de los factores de riesgo asociados son muy prevalentes en las personas infectadas por el VIH que reciben tratamiento antirretroviral en España. Las acciones preventivas, incluida la selección cuidadosa de agentes antirretrovirales, deben ser priorizadas en la población con VIH que está envejeciendo.
Significant improvements in HIV therapies and increased antiretroviral treatment access have shifted HIV disease from being considered a life-threatening condition to become a chronic illness.1,2 This new paradigm brings new challenges in the management of the HIV population, as patients are living longer at the cost of prolonged exposure to antiretroviral drugs along with the harmful effects of persistent immune activation and systemic inflammation driven by residual virus replication.3,4 Accordingly, long-term drug toxicities and high rates of age-associated comorbidities have become leading causes of non-AIDS morbidity and premature death in the HIV population.4,5 In the START trial, non-AIDS illnesses were more frequent in patients that deferred initiation of antiretroviral therapy than in those treated earlier, with most clinical events occurring in patients with CD4 counts above 500 cells/mm3.6 In this context, it is important to characterize the prevalence and risk factors for major age-associated comorbidities in the ageing HIV population.
Certain antiretrovirals are associated with organ-specific toxicities, adding harm on the top of common illnesses that are age-related, such as diabetes and hypertension.7 In this regard, cardiovascular and renal diseases have become leading causes of death among elderly HIV patients on long-term antiretroviral therapy.3,5 In the international D:A:D study, abacavir, and ritonavir-boosted atazanavir or lopinavir were independent predictors of cardiovascular events in HIV-positive persons.8 Likewise, in the EuroSIDA cohort that examined nearly 7000 HIV-positive individuals, exposure to abacavir and boosted indinavir were significantly associated with an increasing risk for developing cardiovascular disease.9 Moreover, in both cohorts exposure to tenofovir disoproxil fumarate (TDF) instead of abacavir was associated with development of chronic kidney disease.10–12 More recently, boosted darunavir exposure was also linked to increased cardiovascular disease risk.13 Only when a better management of risk factors is ensured, benefits on the incidence of cardiovascular and/or renal complications are attained.14
The aim of our study was to assess the rate and predictors of cardiovascular, renal and bone disease in a large cohort of HIV-infected individuals on antiretroviral therapy followed longitudinally in Spain.
Patients and MethodsThe VACH study is an open, prospective, multicenter cohort of HIV-infected adults consecutively attended since year 1997 at 23 hospital-based HIV clinics distributed across Spain (http://vach.es/). Up to the end of year 2014, a total of 15,559 subjects had been recruited.
Patients were included in the cohort provided that they were at least 16 years-old and gave informed consent to participate in the study. All analyses conducted using the VACH cohort conformed to Spanish laws and regulations regarding confidentiality, patient autonomy, data protection and medical research. Despite absence of a centralized laboratory, all of the VACH-associated clinics used the same viral load assay and definition criteria for laboratory test abnormalities throughout the study period.
All patients having at least one visit during year 2014 were included in the current analysis. Secondarily, a comparative cross-sectional examination of the same individuals seen 4 years earlier (2010) was performed.
Information on major comorbidities and their risk factors were collected electronically. They included cardiovascular disease (CVD) events (myocardial infarction, invasive coronary procedures, stroke or death associated to CVD); chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR) below 60mL/min/1.73m2 using the CKD-EPI formula;15 and documented bone fractures at any location.
Data on risk factors was recorded following definitions proposed by international guidelines,16–21 and included arterial hypertension (systolic blood pressure ≥140mm Hg and/or diastolic blood pressure ≥90mm Hg, or taking anti-hypertensive drugs).17 Overweight and obesity were defined for body mass indexes above 25 and 30, respectively. Diabetes was defined as fasting glucose>125mg/dL, or taking oral antidiabetic drugs or insulin.18 Dyslipidemia was defined as elevated fasting total cholesterol ≥240mg/dL and/or elevated triglycerides ≥200mg/dL.19 High alcohol consumption was considered for a daily intake above 3 units (42g). Osteoporosis was defined for bone mineral density loss above 2.5 in the T score using densitometry, regardless age.20
A history of prior CVD events included having had coronary heart episodes, stroke, or peripheral arterial ischemia. Globally, atherosclerotic CVD was defined grouping four syndromes: 1) coronary heart disease (myocardial infarction, angina pectoris, heart failure, and coronary death); 2) cerebrovascular disease (stroke and transient ischemic attack); 3) peripheral arterial disease (intermittent claudication and significant limb ischemia); and 4) aortic aneurysm (abdominal and/or thoracic).
The Framingham risk score was used to determine individual chances of developing CVD events within 10 years.21 It includes age, gender, LDL-cholesterol, HDL-cholesterol, blood pressure, diabetes, and smoking. Individuals were categorized as having low (<10%), moderate (10-20%) or high (>20%) risk of myocardial infarction or coronary death at 10 years.
Resembling the Framingham risk score for predicting future CVD events, the D.A.D. study group built a predictive model for developing renal impairment in HIV-infected individuals.22 Older age, intravenous drug use, hepatitis C coinfection, lower baseline eGFR, female gender, lower CD4 count nadir, hypertension, diabetes, and CVD were independent predictors of renal impairment. The adjusted incidence rate ratios of these nine categorical variables were scaled and summed to create a risk score. The chance for developing CKD within the next 5 years was 1:393 (0.25%), 1:47(2.13%) and 1:6 (16.67%) in the low (score <1), medium (score 1-4) and high (score ≥5) risk groups, respectively.
Statistical analysis. A cross-sectional examination was made in year 2014, recording the number of patients/events. For qualitative data, absolute and relative frequencies were used. Percentages were obtained based on the total number of subjects with non-missing values unless specified otherwise. Significance was compared using the chi-square test. For quantitative data, we used means (+/- standard deviations) for normally distributed continuous variables, and median (with interquartile ranges) for continuous variables with a skewed distribution.
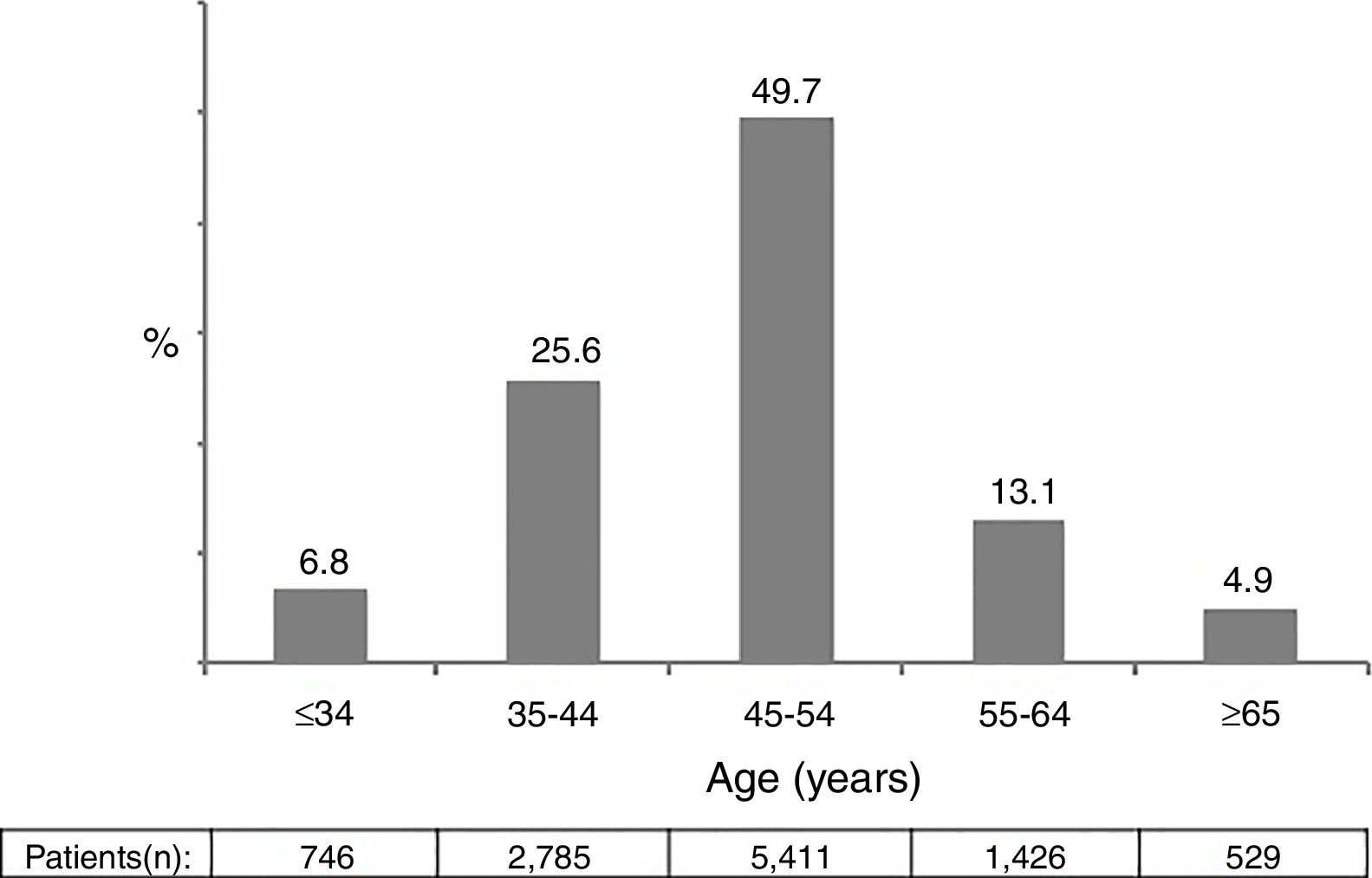
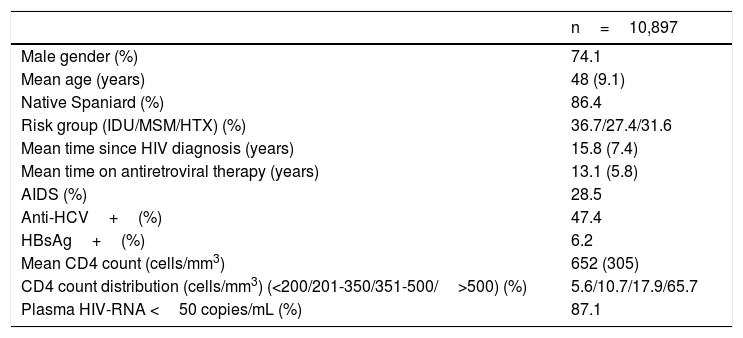
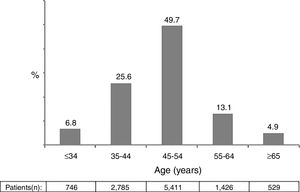
ResultsA total of 10,897 patients met the inclusion criteria and were included in the analysis. This was the population attending at least one visit during year 2014. The main epidemiological and clinical characteristics of these patients are recorded in Table 1. Overall, 74.1% were male, 86.4% native Spaniards, and 36.7% former injection drug users. The mean time since HIV diagnosis was 15.8 years. Overall, 87.1% of patients had plasma HIV-RNA below 50 copies/ml. The mean CD4 count was 652 cells/mm3. Nearly two thirds of patients had more than 500 cells/mm3. Figure 1 records the distribution of patients according to age. Of note, 18% of subjects in year 2014 were ≥55 years-old.
Main characteristics of the study population.
| n=10,897 | |
|---|---|
| Male gender (%) | 74.1 |
| Mean age (years) | 48 (9.1) |
| Native Spaniard (%) | 86.4 |
| Risk group (IDU/MSM/HTX) (%) | 36.7/27.4/31.6 |
| Mean time since HIV diagnosis (years) | 15.8 (7.4) |
| Mean time on antiretroviral therapy (years) | 13.1 (5.8) |
| AIDS (%) | 28.5 |
| Anti-HCV+(%) | 47.4 |
| HBsAg+(%) | 6.2 |
| Mean CD4 count (cells/mm3) | 652 (305) |
| CD4 count distribution (cells/mm3) (<200/201-350/351-500/>500) (%) | 5.6/10.7/17.9/65.7 |
| Plasma HIV-RNA <50 copies/mL (%) | 87.1 |
IDU, injection drug user; MSM, men having sex with men; Heterosex, heterosexuals
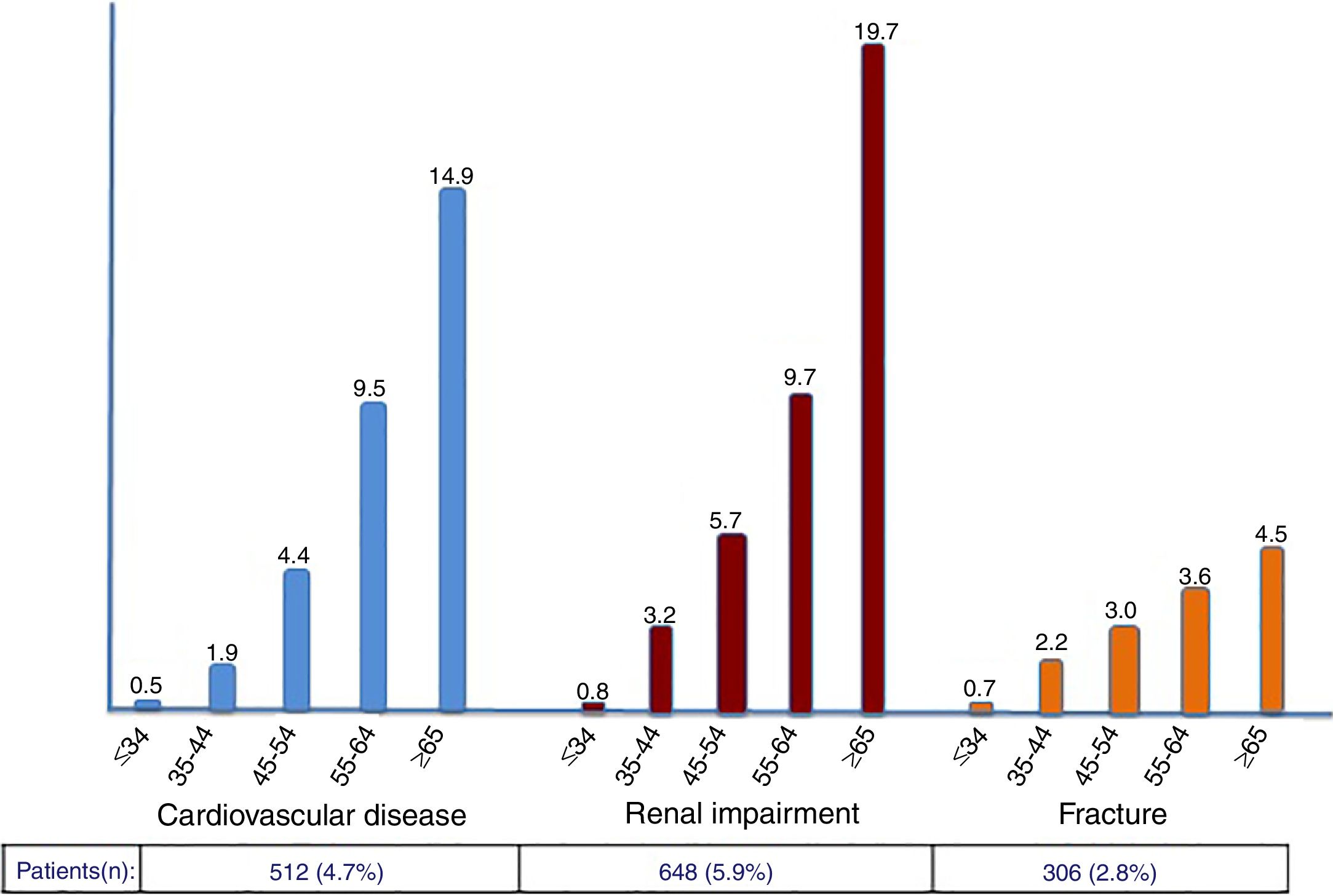
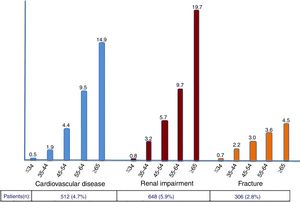
The rate of prior CVD events, CKD and fractures in the study population was 4.7%, 5.9% and 2.8%, respectively. Compared to 4 years earlier, significant increases were noticed (42%, 24% and 40%, respectively). As expected, incident and cumulative events were more frequent in older patients, with more pronounced rates occurring after 55 years-old (Figure 2).
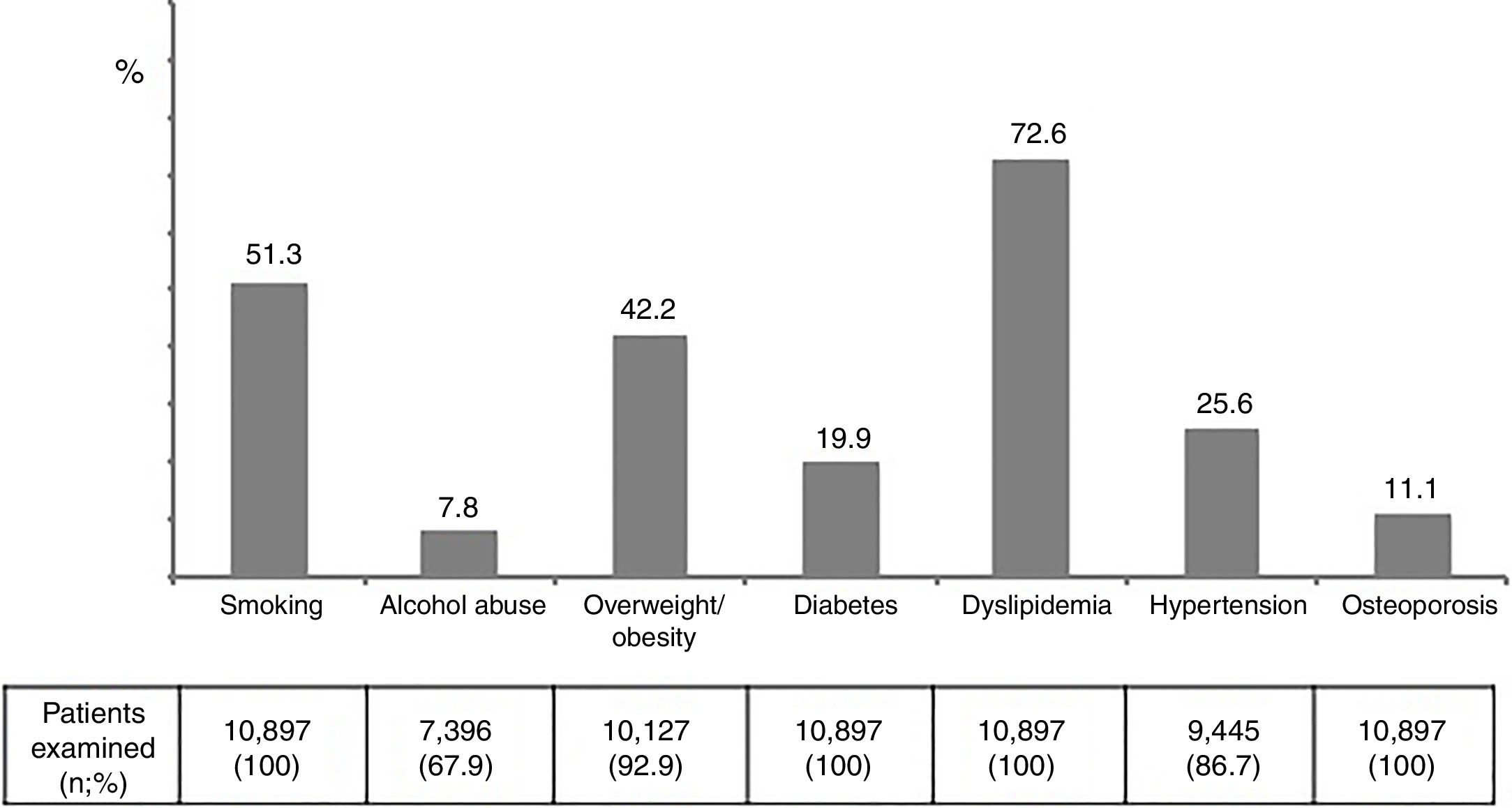
The prevalence of major risk factors was as follows: alcohol abuse 7.8%, overweight/obesity 42.2%, diabetes 19.9%, dyslipidemia 72.6%, hypertension 25.6%, and osteoporosis 11.1% (Figure 3). Interestingly and compared to 4 years earlier, while smoking and alcohol abuse had declined, all age-related metabolic complications had increased, being changes of 13%, 29% and 45% for dyslipidemia, diabetes and hypertension. These trends were generally more pronounced in the subset of patients older than 55 years-old (Figure 1supplementary).
Prediction of cardiovascular diseaseThe proportion of patients with distinct Framingham risk scores is depicted in Figure 2supplementary. The estimated risk of coronary heart disease within 10 years was overall low, moderate or high in 56.9%, 31.1% and 12% of patients, respectively. Of note, roughly three quarters of patients older than 55-years-old depicted moderate-high Framingham risk scores.
Prediction of renal insufficiencyThe proportion of patients with high risk for progressing to CKD using the D:A:D risk score is shown in Figure 3supplementary. In the subset of patients older than 55 years-old, high risk for developing renal impairment within the next 5 years was 53.3%.
Interestingly, half of patients with moderate/high risk for progressing to CKD had also moderate/high risk of coronary heart events within the next 10 years. Globally, 37% of patients had simultaneously moderate/high risk of both CKD and CHD.
Prediction of bone diseaseOverall, 2.8% of patients had had a fracture. However, it had occurred in 8.1% in the subset older than 55 years-old. Osteoporosis was globally recorded in 11.1% of patients, but was present in 18.6% in those older than 55 years-old. Information for DEXA was recorded for the last testing available, which on average was within two years.
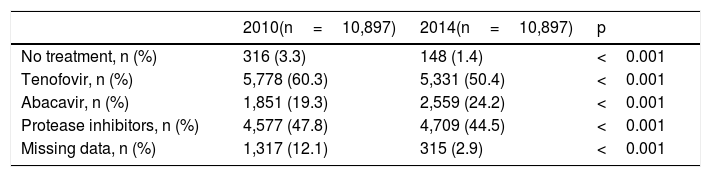
Antiretroviral therapyAll but 148 (1.4%) of patients were on antiretroviral therapy. The reasons for remaining drug-naïve were very low or undetectable viremia along with high CD4 counts (long-term non-progressors or elite controllers), and/or patient's decision to reject treatment. For treated patients, the mean time on antiretroviral therapy was of 13.1 years. The most common used antiretroviral agents were tenofovir (50.4%), abacavir (24.2%) and boosted protease inhibitors (44.5%).
Table 2 shows the proportion of patients on tenofovir, abacavir and boosted protease inhibitors at two different periods. It is noteworthy that tenofovir use had drop roughly from 60% in 2010 to 50% in 2014, whereas abacavir use increased from 19% to 24% during the same period. Boosted protease inhibitors were roughly taken by 48% of patients in 2010 and remained prescribed in 45% in 2014.
Antiretroviral therapy in the study population comparing two different periods.
| 2010(n=10,897) | 2014(n=10,897) | p | |
|---|---|---|---|
| No treatment, n (%) | 316 (3.3) | 148 (1.4) | <0.001 |
| Tenofovir, n (%) | 5,778 (60.3) | 5,331 (50.4) | <0.001 |
| Abacavir, n (%) | 1,851 (19.3) | 2,559 (24.2) | <0.001 |
| Protease inhibitors, n (%) | 4,577 (47.8) | 4,709 (44.5) | <0.001 |
| Missing data, n (%) | 1,317 (12.1) | 315 (2.9) | <0.001 |
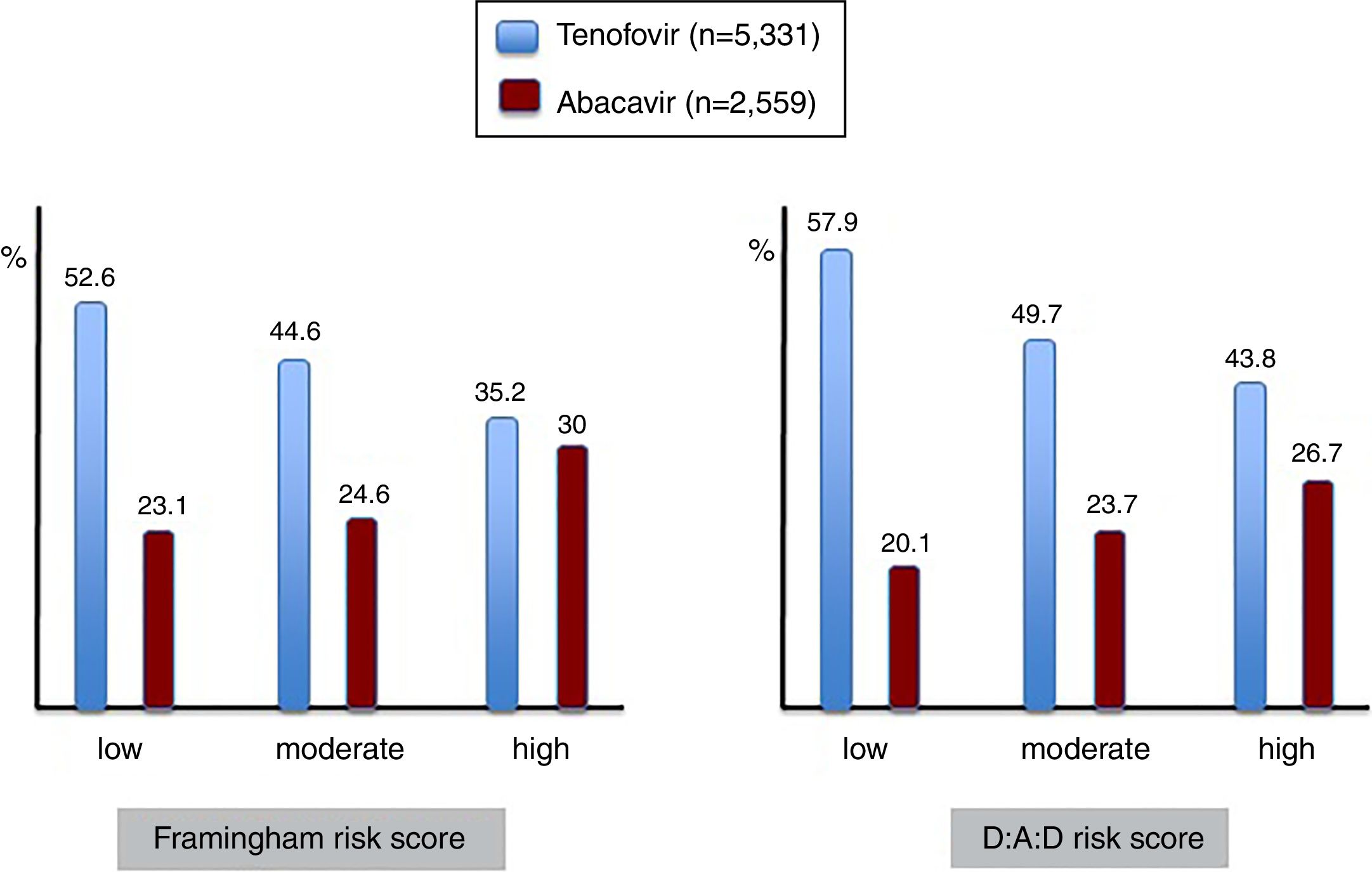
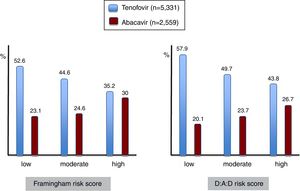
Given the association between the most frequently used nucleos(t)ide analogues and major comorbidities, the prescription of tenofovir and abacavir was examined in patients showing different risk scores for cardiovascular disease and renal insufficiency (Figure 4). It is interesting to note that abacavir was more frequently prescribed in the subset of patients with high compared to moderate or low Framingham risk scores, given that some studies had found an association between abacavir use and myocardial infarction. Moreover, less than 15% of patients with high CDV risk on abacavir had renal impairment, and therefore other reasons than avoiding nephrotoxicity guided antiretroviral choice in most patients.
DiscussionThis study is the first epidemiological investigation about the rate of major comorbidities and their risk factors in a large population of adult HIV-infected patients on long-term antiretroviral therapy in Spain. Multiple clinics across the whole country contributed to this cohort, enrolling roughly 15% of the estimated 100,000 individuals diagnosed with HIV living in Spain at the time this cross-sectional study was conducted.
The overall rate of prior CVD was 4.7% whereas the prevalence of CKD was 5.9%. Fractures had occurred in 2.8%. Classical conditions associated with cardiovascular, renal or bone disease, such as hypertension, diabetes, and obesity were relatively frequent in the study population. However, it was aging, a non-modifiable risk factor, that played the most significant role, largely accounting for the high rate of non-infectious comorbidities. Indeed, roughly three quarters of patients older than 55-years-old depicted moderate-high Framingham risk scores. These figures are particularly worrisome taking into consideration that the mean age of the population enrolled in the VACH cohort was 48 years-old. Likewise, the risk for developing renal impairment within 5 years was above 50% in this oldest group. Of note, 37% of patients had simultaneously high risk for developing cardiovascular and kidney diseases. Thus, it seems worthy to reinforce preventive actions on modifiable risk factors, such as tobacco and overweight/obesity by promoting healthy life style, diet and exercise. Furthermore, earlier interventions should prioritize the management of dyslipidemia, hypertension and diabetes. The benefit of better management of risk factors for reducing the incidence of CVD events has recently been pointed out.14
While dramatic improvements in antiretroviral therapy have allowed for better virologic and immunologic outcomes, the high and increasing prevalence of common age-related comorbidities and their risk factors should require prompt attention, including adequate choice of safer antiretroviral agents, considering their long-term toxicity profile and the fact that treatment is currently recommended for everyone infected and as soon as possible.23
In our study population, tenofovir was more than two-fold more frequently prescribed than abacavir as nucleos(t)ide analogue. However, abacavir use significantly increased since year 2010 approaching its prescription to one quarter of patients by 2014. This was most likely explained by the fact that older patients had more often renal impairment and concerns on TDF-associated nephrotoxicity prompted clinicians to shift their therapy.
Around 45% of patients were treated with protease inhibitors. It is noteworthy the relatively high rate of patients that received abacavir and/or protease inhibitors, since their use has been associated with an increased rate of CVD events.24,25 Whereas abacavir may enhance vascular thrombosis through platelet activation,24 boosted protease inhibitors may increase CVD risk largely by producing metabolic abnormalities, including insulin resistance and dyslipidemia.25
Whereas 30% of HIV patients with high cardiovascular risk were on abacavir, roughly 44% of those with high risk for renal insufficiency were on TDF. Most antiretroviral treatment guidelines suggest that abacavir should be avoided or used with caution in patients with high cardiovascular risk whereas TDF is not recommended in patients with high risk of renal impairment.23 The involvement of abacavir in myocardial infarction, however, remains controversial. Moreover, we should keep in mind that our HIV patients started these treatments when guidelines were different from the current ones. Furthermore, treatment options some years ago were limited in comparison with the multiple drugs with improved safety profile that are currently available. Clearly, the choice of safer options for individuals with either heart or renal risk scores should be prioritized.
The recent advent of tenofovir alafenamide (TAF) represents an attractive alternative option,26 as replacement for those on TDF and potentially for switching from abacavir. Until recently, TDF was discouraged in older HIV patients given its toxicity on the kidneys and bone mineralization, both of which have ameliorated significantly with the new formulation that depicts less systemic drug exposure.26 With respect to boosted protease inhibitors, unboosted integrase inhibitors are recommended as preferred initial treatments, because their safer metabolic profile.26 This advise is particularly important for subjects with CVD risk factors and high CVD Framingham risk scores.
Along with preventive efforts on CVD events, caution is warranted when using drugs known to be potentially harmful for the kidneys, such as TDF and/or ritonavir-boosted protease inhibitors.11,27 The improved life expectancy of HIV-infected individuals is inexorably leading to a growing population of HIV-positive elderly patients 1 and therefore choosing antiretrovirals with a safer renal toxicity profile would represent an important advantage.27 In this regard, the advent of TAF is an important step forward that would reduce concerns on tubular damage and bone mineral loss linked to long-term TDF therapy.26 Other alternatives that would minimize kidney damage may include nucleos(t)ide-sparing regimens, such as the recently approved dual combination of dolutegravir-rilpivirine for virologic suppressed patients.23 Moreover, promising results have been reported with dolutegravir-lamivudine in drug-naive patients 28 as well as with long-acting intramuscular dual therapy.29
Our study has several limitations largely due to its observational and cross-sectional design, which makes it strictly descriptive. Firstly, pro-active interventions could not be tested, and selection biases could have occurred without noticing. Secondly, exposure to different antiretrovirals was more extensively examined for a few agents and prior exposure time lengths were not considered, being the relative contribution as time dependent not weighted. However, the sample size is large and many clinics contributed, so the data are very representative of the whole Spanish HIV population. Furthermore, the assessment of comorbidities and their risk factors was carried out using objective and widely used criteria, which makes the data reliable and the survey useful for examining time trends in Spain and making comparisons with HIV patients at other regions.
A third limitation of our study is that age strata were made empirically trying to balance the size of distinct groups. In this regard, a threshold in 55 years-old for considering people older could be considered as too premature. However, similar approaches have been taken by others testing HIV populations with an average age younger than 45 years-old, as it was in our cohort. Finally, although aging, a non-modifiable risk factor, played the most significant role, largely accounting for the high rate of non-infectious comorbidities in our study, the relative weight of different risk factors was not compared, and aging by itself is associated with a higher proportion of other risk factors for CVD, kidney and bone disease.
In summary, our results highlight that common age-related comorbidities and their risk factors are highly prevalent in HIV-infected patients on long-term antiretroviral therapy in Spain. Nevertheless, tobacco remained the number one cause of preventable death in our cohort. In other words, antiretroviral treatment has improved so much that HIV-infected persons that smoke cigarettes are at greater risk of dying of lung cancer than of AIDS.30 Therefore, at the top of our agenda must be helping our patients to stop smoking, an effort too often forgotten. Lastly, attention must be given to selection of the most convenient antiretroviral drugs, considering aging and comorbidities of individual patients.
FinanciaciónEl estudio fue financiado por Gilead España.
Conflicto de interesesLos autores declaran no tener conflictos de intereses relacionados con la autoría y publicación del presente artículo.
Queremos agradecer a todos los participantes de la cohorteVACH, así como a Valeska Andreozzi (ExigoConsultores, Lisboa, Portugal) por su excelente asesoramiento científico.