Despite the advanced antibiotic therapies, sepsis continues being a clinical entity with high morbidity and mortality. The ozone/oxygen mixture (O3/O2) has been reported to exhibit positive effects on immunity. The aim of our study was to analyze whether (O3/O2) combined with amoxicillin/clavulanate has any influence on the morbidity and mortality of septic rats.
MethodsWe used 48 Sprague-Dawley rats randomly allocated to 6 groups (n=8): healthy (C), septic (I), healthy+ozone therapy (O3), septic+amoxicillin/clavulanate (AMC), septic+amoxicillin/clavulanate+ozone therapy (AMC/O3) and septic+ozone therapy (I/O3). O3/O2 was administered rectally at increasing O3 concentrations during 10 days prior to the onset of sepsis model (intraperitoneally injection of fecal material) or saline administration in healthy control rats. Later (post-inoculation), 3 days per week, O3 was also administered. Vital signs were recorded, and microbiological, hematological and histopathological studies were performed.
ResultsThe number of surviving animal/total was higher in AMC (8/8) than in AMC/O3 (4/8) p=0.077. The percentage of surviving animals with pneumonia was higher in AMC/O3 than in AMC (100% vs 37.5%). In dead animals, AMC/O3 rats had a significantly higher percentage of lesions: Cardiac lesions, pulmonary hemorrhages and pleuritis (100%) and serositis/peritonitis (75%). Only Escherichia coli (2 different biotypes) was isolated from blood and/or peritoneal fluid from all infected groups. A significant decrease in the percentage of band neutrophils from the surviviors belonging to AMC/O3vs AMC was observed (p<0.05).
ConclusionRectal pre-treatment with O3/O2 aggravates clinic status in septic rats treated with amoxicillin/clavulanate.
A pesar de los avances en terapia antibiótica, la sepsis sigue siendo una entidad clínica con alta morbimortalidad. Se ha publicado que la mezcla ozono/oxígeno (O3/O2) presenta efectos beneficiosos sobre el sistema inmunológico. El objetivo de este estudio es analizar si (O3/O2) combinado con amoxicilina/clavulánico tiene efectos en la morbimortalidad de ratas sépticas.
MétodosUtilizamos 48 ratas Sprague-Dawley distribuidas aleatoriamente en 6 grupos (n=8): sanas (C), sépticas (I), sanas+ozonoterapia (O3), sépticas+amoxicilina/clavulánico (AMC), sépticas+amoxicilina/clavulánico+ozonoterapia (AMC/O3) y sépticas+ozonoterapia (I/O3). (O3/O2) se administró por vía rectal a concentraciones crecientes de O3 los 10días previos a la instauración del modelo de sepsis (inyección intraperitoneal de material fecal) o de la administración de solución salina, en las ratas control. Posteriormente (postinoculación) se continuó administrando (O3/O2), 3días por semana. Registramos los signos vitales y realizamos estudios microbiológicos, histopatológicos y hematológicos.
ResultadosEl número de supervivientes/total fue mayor en AMC (8/8) que en AMC/O3 (4/8), p=0,077. El porcentaje de supervivientes con neumonía fue mayor en AMC/O3 que en AMC (100% vs 37,5%). Entre los fallecidos, AMC/O3 tenía un porcentaje mayor de lesiones: cardiacas, hemorragias pulmonares y pleuritis (100%) y serositis/peritonitis (75%). A partir de la sangre y/o líquido peritoneal de los grupos infectados se aislaron exclusivamente Escherichia coli (2biotipos diferentes). Observamos una disminución significativa en el porcentaje de neutrófilos en banda en las supervivientes pertenecientes a AMC/O3vs AMC (p<0,05).
ConclusiónEl tratamiento rectal previo con (O3/O2) agrava el estado clínico en ratas sépticas tratadas con amoxicilina/clavulánico.
Despite the huge advanced antibiotic therapies, supportive treatments and technological facilities, sepsis continues to be a clinical entity with high morbidity and mortality.1 The pathophysiology of sepsis involves complex interactions between host organs and the invading pathogen. The lung is the organ which is affected initially, and sepsis leads to acute lung injury.2 Sepsis and particularly septic shock induce the production of large amounts of free radicals in a non-regulated mode associated with high-oxidative potential damage.3 Several generating sources of free radicals have been detected in sepsis and septic shock, demonstrating the presence of oxidative stress, early production of reactive oxygen species (ROS), and development of multiple system organ dysfunction.4–8
The ozone/oxygen mixture has been reported to exhibit several effects on the immune system, such as the modulation of phagocytic activity.9 This gas may stimulate host defenses against microbes, mainly by oxidative reactions10 enhancing pro-inflammatory cytokine release.11,12 It has been reported to be effective in osteomyelitis, peritonitis or in vascular disorders.12 Unfortunately, these studies have been usually performed without adequate control groups, not fulfilling standards of evidence based medicine.13 Thus, currently ozone treatment is not recognized in traditional medicine and it is subsumed under complementary (alternative) medicine.14 However, the interest in ozone as a therapeutic or prophylactic agent has been renewed since the reports that it is a bio-molecule, produced by neutrophils as part of adaptive immunity,15,16 thus being the most important host defense against bacterial pathogens.
Whether ozone pre-treatment is beneficial in peritonitis is unclear, since there are contradictory reports of its immunological effects.17 Recent studies18,19 have shown that ozone therapy reduced tissue oxidative stress, regulated the systemic inflammatory response, and abated cellular infiltration to the lung. Those studies support the use of the ozone therapy as adjuvant therapy to antibiotherapy in protecting the lung against septic injury. However, an aggravating effect of ozone pre-treatment on the systemic inflammatory response in a sepsis rat model and a decreased survival has been also reported.20 These contradictory findings reinforce the need of studies testing the efficacy of ozone therapy on septic animals, to clarify its mode of action. The aim of our study was to analyze whether the rectal pre-conditioning with ozonized oxygen combined with amoxicillin/clavulanate (AMC) treatment, has any influence on the morbidity/mortality of septic rats.
Materials and methodsThis study was approved by the Ethic Committee of the Hospital Universitario de Gran Canaria Dr. Negrín, and was performed in compliance with standard operating and quality procedures following published guidelines (OECD Principles on Good Laboratory Practice [as revised in 1997], Council Directive of 22 September 2010 on the protection of animals used for experimental and other scientific purposes [2010/63/UE]).
Rats and housingWe used 54 female Sprague-Dawley rats weighing 270–320g (Charles River Laboratories, Barcelona, Spain). Food (R.02-E Standard Diet, Prolabor, Barcelona, Spain) and tap water were available ad libitum. Ambient temperature was 21±1°C and relative humidity was 55±5% with an air change rate of 15 times/h. Routine microbiological monitoring, revealed no evidence of infection or parasitosis with common murine or rats pathogens.
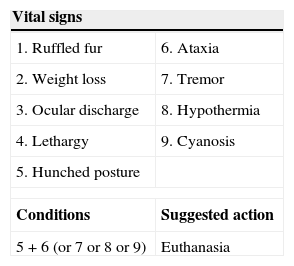
Experimental design. Induction of sepsisFor induction of peritoneal sepsis we used the model referred by Zamora et al.21 Briefly, sepsis was induced by intraperitoneal injection of a mixture 1:1, fecal material (0.65g/kg) and Ringer lactate solution. Fecal material was collected from the caecum of sacrificed donor rats (same strain, weight and age). Post inoculation analgesia consisted of 0.05mg/kg buprenorphine given subcutaneously twice daily (Buprex®, Mundipharm, Limburg, Germany) during the first 3 days post inoculation. After that, animals received food and water ad libitum. In accordance with the recommendations of some authors,22,23 animals were assessed every 12h (from 6h post inoculation) for several vital signs (Table 1). At the end of the trial (10 days post inoculation), survivors were anesthetized with an intraperitoneal combination of diazepam (Valium, Roche, Madrid, Spain) and ketamine (Ketolar 500; Pfizer, Madrid, Spain), at dosage of 5mg and 80mg/kg, respectively, and samples (blood, peritoneal fluid, heart, lung, small intestine and mesenteric lymph nodes) were obtained. Finally, rats were sacrificed by exsanguination via abdominal aorta. If some rats showed hunching plus ataxia, tremor, hypothermia or cyanosis before 10 days from the start of experiment, they were anesthetized, with the previously described protocol. Peritoneal fluid, blood, and biopsies of lung, hearth and jejune were collected and killed humanely immediately.
Scoring of rodent protection test.
| Vital signs | |
|---|---|
| 1. Ruffled fur | 6. Ataxia |
| 2. Weight loss | 7. Tremor |
| 3. Ocular discharge | 8. Hypothermia |
| 4. Lethargy | 9. Cyanosis |
| 5. Hunched posture | |
| Conditions | Suggested action |
| 5+6 (or 7 or 8 or 9) | Euthanasia |
Ozone was administered rectally using a silicone catheter 14G, using an ozone delivery system (Alpha Plus, Dr. Hänster, Nordring, Germany). The ozone concentration was measured using and UV spectrophotometer at 254nm. Each rat was administered once daily a volume of gas (mixture of O3/O2) of 20ml/kg. Increasing concentrations of O3 (from 20 to 50μg/ml) were administered in 10 consecutive days prior to feces inoculation or administration of saline solution (20, 25, 30, 35, 40, 45, 50 … 50μg/ml) to induce a greater tolerance to oxidative stress. Therefore, the total ozone dose administered increased from 0.4 to 1mg/kg in 10 days. Later, during others 10 days of treatment and evaluation post-inoculation (or administration of saline solution), each animal was administered 3 days per week (Monday, Wednesday and Friday) 50μg/ml of O324
Treatment groupsAnimals were randomly allocated to the following groups (n=8): Control animals (C), given a single intraperitoneal dose of 1ml saline. Group (I), rats were infected as described previously. Group (O3), rats (with saline administration) were administered only with ozone therapy as described previously. Group (AMC), rats were infected (as in group I) and treated with amoxicillin/clavulanate (100/10mg/kg) intramuscularly once daily during the first three days post experimental infection. Group (AMC/O3), rats were infected and treated (as in group AMC) but also with ozone therapy as described previously. Group (I/O3), infected rats, (as in group I) were treated only with ozone therapy as described previously. Surviving animals were sacrificed 10 days after saline administration or experimental infection depending on the study groups.
Sampling in survival ratsAfter 10 days post inoculation, blood samples and peritoneal fluid of surviving rats from different study groups were collected. After euthanasia, necropsy was done of such animals and blood, peritoneal and histological samples were taken. Blood samples (0.3ml) were collected from the cranial cave vein of the surviving rats, with intraperitoneal anesthesia combination of diazepam and ketamine. In anesthetized rats a 20 gauge sterile needle was inserted 1–2 inches into the disinfected abdomen. Subsequently, 15ml sterile saline solution was introduced into the abdominal cavity and zone was massaged. Finally, 5ml of fluid were pulled out into sterile syringe. Samples were processed immediately for microbiology studies. After peritoneal fluid and blood sampling, survivor rats were sacrificed by exsanguination. During necropsy, fragments of lower lobe of the left lung, small intestine with Peyer's patches, mesenteric lymph node and left ventricle myocardium were taken for histological studies. Tissues were fixed in formalin for 24h, embedded in paraffin and cut into 4μm sections.
Sampling in dead or euthanized by humanitarian endpoint criteria ratsSamples of peritoneal fluid from dead or euthanized rats were collected as previously described. Animals were euthanized after the experimental period. Necropsies were performed immediately after death in euthanized animals and within 8h after death in those animals that died within the experimental period. In dead animals, half of left ventricle was sampled for microbiological studies, due to the impossibility of collecting fresh blood samples. In the case of euthanized rats, before sacrifice blood was collected for microbiological analysis using the technique described.
LeucogramsIn surviving rats, we obtained 0.3ml blood as described previously. For differential blood count of white blood cells (WBC), which were further differentiated into lymphocytes, monocytes, neutrophils segmented, monocytes, eosinophils and neutrophils, an autoanalyzer (Vet abc_Animal Blood Counter, ABX Diagnostics, Göttingen, Germany) was used being carefully validated for the analysis of rat blood.
Microbiological analysisSamples were processed in the laboratory within 2h of collection. To investigate aerobic microorganisms, 100μl of peritoneal fluid and blood (or left ventricle biopsies) from each animal were cultured on 10ml. Brain Hearth Infusion broth (Difco Laboratories, Detroit, MI) overnight at 37°C. 50μl of these cultures were plated onto CLED (Biomedics, Madrid, Spain), MacConkey (Difco), Mannitol Salt agar (Difco), and Sabouraud Dextrose Agar (Difco) and incubated for 24h at 37°C. The oxidase test was made to colonies of Gram-negative bacteria using Oxichrome reagent (Innovative Diagnostic Systems, Norcross, GA). Oxidase-negative bacteria were identified using the API 20E system (BioMérieux, Marcy L’Etoile, France). To investigate anaerobic microorganisms, 100ml of peritoneal fluid and blood (or left ventricle biopsies) were cultured on 10ml of Fluid Thioglicollate Medium (BBL, Becton Dickinson) and incubated at 37°C during 7 days; if any sign of growth was detected during this time, a subculture was made on Blood, Chocolate and McConkey agar plates which were incubated for 48h at 37°C in 5% CO2 atmosphere and to Brucella Anaerobic agar and BBE agar plates, incubated for 4 days at 37°C in anaerobiosis. Antibiotic sensitivity tests were carried out on Mueller-Hinton agar (Difco) using the disk diffusion method. Fourteen antibiotics from different groups were tested in accordance with The European Committee on Antimicrobial Susceptibility Testing recommendations (EUCAST). Baiquiloprim/sulfadimetoxacin (1.25μg/23.75μg) disks were tested also, because of its specific use in veterinary medicine. The antibiotics used are listed in Table 2.
Antibiotics tested against bacterial isolates.
| Antimicrobial group | Antimicrobial agent |
|---|---|
| Beta lactams | |
| Penicillins | Amoxicillin/clavulanic acid |
| Piperacillin | |
| Ticarcillin | |
| Cephalosporins | Cefotaxime |
| Cefoxitin | |
| Ceftazidime | |
| Carbapenems | Imipenem |
| Aminoglycosides | Amikacin |
| Gentamicin | |
| Netilmicin | |
| Tobramycin | |
| Quinolones | Ciprofloxacin |
| Levofloxacin | |
| Others | Trimethropin/sulfamethoxazol |
| Baiquiloprim/Sulfadimetoxacin | |
Slides were stained with hematoxylin and eosin (H&E) and examined under light microscope. Each slide was evaluated by two expert investigators blinded to the experiment groups. In all organs, the presence of bacteria inside vessels was evaluated (Microscopic observation of blue bacterial plungers inside the vessels, constituted by small rod-shapes). In addition to this, lung injury was determined based on different signs, such as edema, hemorrhage, leucocyte infiltration and alveolar septa thickening and pleuritis. Heart damage was determined if inflammatory response was present in the epicardium and/or myocardium. Finally intestinal damage was evaluated at mucosal and serosal levels with the determination of inflammatory response.
Statistical studyThe study of the quantitative variables was completed with the Kruskal–Wallis and Mann–Whitney tests, for qualitative variables we used Fischer's exact test, and SPSS software, version 15. To verify the normalcy of the continuous variables, the Kolmogorov–Smirnov test was used. The data are presented as mean value±standard deviation, and a P<0.05 was considered statistically significant.
ResultsSurvival and clinical evolutionAmong the infected and treated animals groups, rats belonging to AMC had a higher survival than AMC/O3, with marginally significant differences (P=0.077). In this way, numbers of surviving animal/total, during the 10 days post inoculation (fecal or saline) for the different experimental groups were: C (8/8), I (4/8), O3 (8/8), I/O3 (5/8), AMC (8/8), and AMC/O3 (4/8). In all groups in which deaths occurred, these happened between 24h and 72h post inoculation. No rats died from non-septic groups. Three animals were killed with humanitarian endpoint criteria, two of them belonging to group I (48h post inoculation) and one belonging to group I/O3 (72h post inoculation). They showed lethargy, hunched posture and tremor.
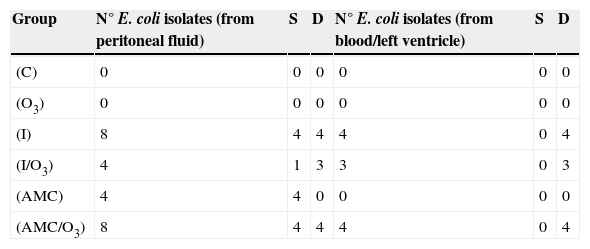
MicrobiologyBacteria isolated from peritoneal fluid or blood were identified as Escherichia coli belonging to two different biotypes. All of them were susceptible to all antibiotics tested. No anaerobic bacteria were detected. E. coli was isolated from peritoneal fluid in rats from all infected groups (I, I/O3, AMC, and AMC/O3). E. coli was isolated from blood only in dead animals and not in the surviving ones (treated with or without AMC and/or O3/O2). The number and origin of isolates obtained from different animals in the study groups are shown in Table 3.
Number of E. coli isolates isolated from peritoneal fluid, blood or left ventricle of surviving (S) and dead (D) rats during 10 days post inoculation in the different study groups.
| Group | N° E. coli isolates (from peritoneal fluid) | S | D | N° E. coli isolates (from blood/left ventricle) | S | D |
|---|---|---|---|---|---|---|
| (C) | 0 | 0 | 0 | 0 | 0 | 0 |
| (O3) | 0 | 0 | 0 | 0 | 0 | 0 |
| (I) | 8 | 4 | 4 | 4 | 0 | 4 |
| (I/O3) | 4 | 1 | 3 | 3 | 0 | 3 |
| (AMC) | 4 | 4 | 0 | 0 | 0 | 0 |
| (AMC/O3) | 8 | 4 | 4 | 4 | 0 | 4 |
C, group control, no infected; I, group infected animals; O3, no infected and treated with ozone; I/O3, infected and treated with ozone; AMC, infected and treated with amoxicillin/clavulanate; AMC/O3, infected and treated with amoxicillin/clavulanate and ozone.
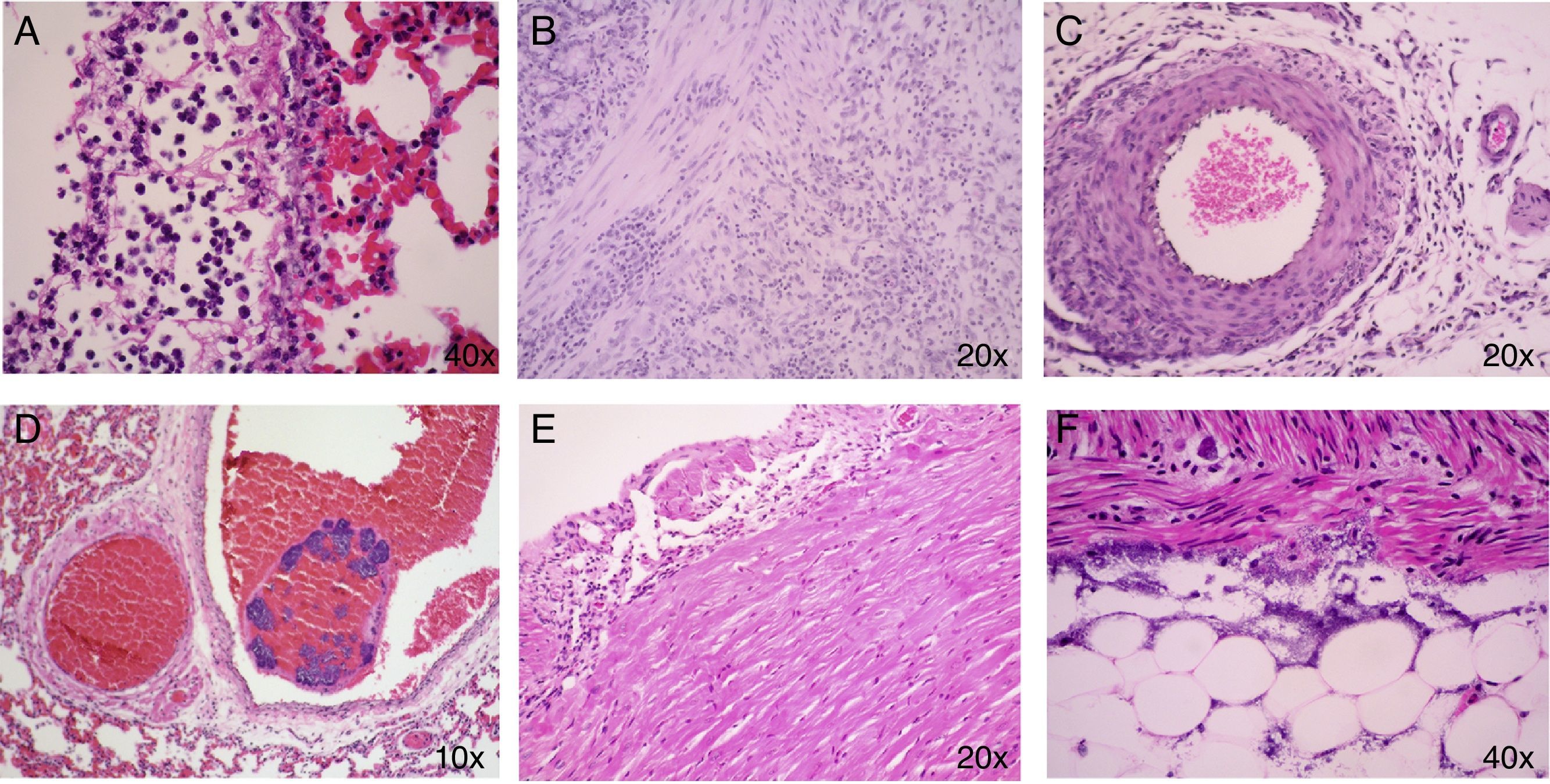
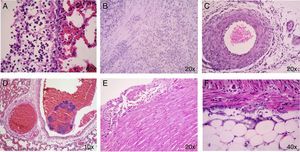
No histological changes were observed in the different tissues from the control animals (C) nor in no infected and treated with ozone animals (O3). In the infected animals groups (I, I/O3, AMC, and AMC/O3), the main histopathological lesions consisted in the presence of bacteria within the blood vessels (bacteriemia) of lungs, heart (epicardium and myocardium) and in the fat tissue of mesenterium. Inflammatory processes associated with bacteria were observed in lungs, and consisted in interstitial pneumonia and fibrino-purulent pleuritis, in the heart, with non-purulent pericarditis, and in the fat tissue of abdominal cavity and serosal surface of intestine, with peritonitis, that included vasculitis and serositits respectively. The intensity of all these inflammatory processes was variable, from mild to moderate (Fig. 1). The number and percentage of the main histological lesions found in both surviving and dead animals are showed in Table 4(a) and (b). In surviving animals from the different study groups, interstitial pneumonia was the predominant lesion, followed by serositis/peritonitis. In contrast, in dead animals, myocarditis/endocarditis were found in all animals.
(A) Lung congestion and fibrino-purulent pleuritis. (B) and (F) Small intestine: The inflammatory infiltrate appears from the serosa of the organ and mesenteric fat to the muscle layer of the intestine. This infiltrate consists of mononuclear cells and some neutrophils. It is also observed proliferation of fibroblasts. (C) Vasculitis in peritoneum. (D) Presence of bacteria within the blood vessels (Bacteriemia) of lungs. (E) Non-purulent pericarditis.
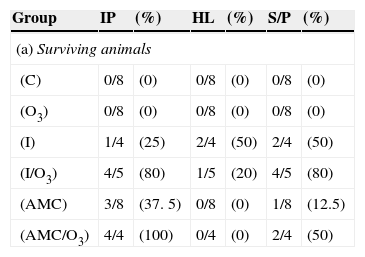
(a) Number of surviving animals after 10 days post inoculation with histological lesions in different organs/total surviving animals in the different study groups. (b) Number of dead animals before 10 days post inoculation with histological lesions in different organs/total dead animals in the different study groups.
| Group | IP | (%) | HL | (%) | S/P | (%) |
|---|---|---|---|---|---|---|
| (a) Surviving animals | ||||||
| (C) | 0/8 | (0) | 0/8 | (0) | 0/8 | (0) |
| (O3) | 0/8 | (0) | 0/8 | (0) | 0/8 | (0) |
| (I) | 1/4 | (25) | 2/4 | (50) | 2/4 | (50) |
| (I/O3) | 4/5 | (80) | 1/5 | (20) | 4/5 | (80) |
| (AMC) | 3/8 | (37. 5) | 0/8 | (0) | 1/8 | (12.5) |
| (AMC/O3) | 4/4 | (100) | 0/4 | (0) | 2/4 | (50) |
| Group | IP | (%) | LPC | (%) | PLR | (%) | HL | (%) | S/P | % |
|---|---|---|---|---|---|---|---|---|---|---|
| (b) Dead animals | ||||||||||
| (I) | 1/4 | (25) | 3/4 | (75) | 2/4 | (50) | 4/4 | (100) | 4/4 | (100) |
| (I/O3) | 2/3 | (66.6) | 3/3 | (100) | 2/3 | (66.6) | 3/3 | (100) | 0/3 | (0) |
| (AMC/O3) | 0/4 | (0) | 4/4 | (100) | 4/4 | (100) | 4/4 | (100) | 3/4 | (75) |
C, group control, no infected; I, group infected animals no treated; O3, no infected and treated with ozone; I/O3, infected and treated with ozone; AMC, infected and treated with amoxicillin/clavulanate; AMC/O3, infected and treated with amoxicillin/clavulanate and ozone.
IP, Interstitial pneumonia; LPC, lung parenchyma congestion with hemorrhages; PLR, fibrino-purulent pleuritis with hyperplasia of mesothelial cells; HL, heart lesions (bacteria in epicardium and myocardium, congestion, hemorrhages or pericarditis; S/P, serositis/peritonitis.
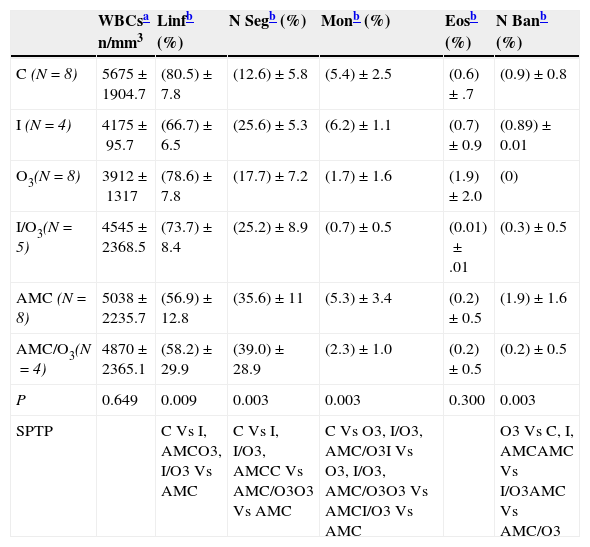
When leucograms of surviving animals were analyzed (Table 5), we didn’t found differences in the total number of leucocytes from different experimental groups. However, there were significant differences in the white blood cells differential count of the different experimental groups. In this way a significant decrease (P=0.003) in the percentage of neutrophils bands from rats belonging to AMC/O3 (0.2±0.5%) vs AMC (1.9±1.6%) and O3 (0%) vs C (0.9±0.8%) was observed. Similar depressor effect was founded when we compared the percentage of monocytes in O3 (1.7±1.6%) vs C (5.4±2.5%). By contrast an increase in the percentage of linfocytes in I/O3 (73.7±8.4%) vs AMC (56.9±12.8%) was noted (P=0.009).
Leucograms in surviving rats.
| WBCsa n/mm3 | Linfb (%) | N Segb (%) | Monb (%) | Eosb (%) | N Banb (%) | |
|---|---|---|---|---|---|---|
| C (N=8) | 5675±1904.7 | (80.5)±7.8 | (12.6)±5.8 | (5.4)±2.5 | (0.6)±.7 | (0.9)±0.8 |
| I (N=4) | 4175±95.7 | (66.7)±6.5 | (25.6)±5.3 | (6.2)±1.1 | (0.7)±0.9 | (0.89)±0.01 |
| O3(N=8) | 3912±1317 | (78.6)±7.8 | (17.7)±7.2 | (1.7)±1.6 | (1.9)±2.0 | (0) |
| I/O3(N=5) | 4545±2368.5 | (73.7)±8.4 | (25.2)±8.9 | (0.7)±0.5 | (0.01)±.01 | (0.3)±0.5 |
| AMC (N=8) | 5038±2235.7 | (56.9)±12.8 | (35.6)±11 | (5.3)±3.4 | (0.2)±0.5 | (1.9)±1.6 |
| AMC/O3(N=4) | 4870±2365.1 | (58.2)±29.9 | (39.0)±28.9 | (2.3)±1.0 | (0.2)±0.5 | (0.2)±0.5 |
| P | 0.649 | 0.009 | 0.003 | 0.003 | 0.300 | 0.003 |
| SPTP | C Vs I, AMCO3, I/O3 Vs AMC | C Vs I, I/O3, AMCC Vs AMC/O3O3 Vs AMC | C Vs O3, I/O3, AMC/O3I Vs O3, I/O3, AMC/O3O3 Vs AMCI/O3 Vs AMC | O3 Vs C, I, AMCAMC Vs I/O3AMC Vs AMC/O3 |
C, group control, no infected. I, group infected animals no treated, O3, no infected and treated with ozone; I/O3, infected and treated with ozone; AMC, infected and treated with amoxicillin/clavulanate; AMC/O3, infected and treated with amoxicillin/clavulanate and ozone. WBCs, white blood cells; Linf, lymphocytes. N Seg, neutrophils segmented. Mon, monocytes; Eos, eosinophils. N Ban, neutrophils bands. P, Kruskal–Wallis test. SPTP, significative pair to pair comparations are showed (Mann–Whitney test)
In our study, an apparent worsening of the clinical condition of the septic animals treated with the combination AMC/O3vs AMC is observed. This is evidenced by increased mortality, increased number of animals with signs of pneumonia (in the group of survivors), a higher percentage of cardiac, pulmonary, and intestinally histopathological lesions (in the group of dead animals) and a significant decrease in the percentage of neutrophils bands from surviving ones belonging to AMC/O3vs AMC.
Reports of the immunological effects of ozone are contradictory, some demonstrating immunosuppression through reduced natural killer cell activity in the lung25 and others showing enhanced inflammatory responses with opposite clinical results according to author and infection disease model.10–12,20,26 In our case, we obtained a similar range of mortality in the infected group, to the found by Zamora et al.21 There are reports, both in animals and humans, which demonstrate beneficial effects of ozone in peritonitis.17,27,28 Our results disagree with these observations since we found that ozone pre-treatment causes a reduction of 50% in survival when comparing infected rats treated with AMC and infected rats treated with AMC/O3. Therefore, our mortality rate in the infected group (I) is comparable to the clinical situation in human patients with septic shock, and it is in accordance too with other authors20 who achieved a survival rate of 50% during the first 120h post infection in a peritonitis rat model with ozonized oxygen pre-treatment using similar doses by intraperitoneal route.
Although intraabdominal infections are polymicrobial29 we isolated only two different biotypes of E. coli (both from peritoneal fluid and blood). Our results were in accordance to those obtained by others authors30 using a similar porcine model of septic peritonitis originated from pig feces. In their work, E. coli was recovered from blood cultures of all septic animals, and also other species such as Enterobacter aerogenes, Aeromonas caviae, Citrobacter diversus and Streptococcus viridians. Further studies are needed to elucidate the role played in pathogenesis, individually or in synergism, of different bacterial species present in fresh fecal inocula used in experimental sepsis models.
We found that only non-surviving animals had positive blood cultures. Although we do not have a clear explanation for this finding, it is plausible that only those animals who survived were able to eliminate bacteria from blood or blocked the translocation from the peritoneal focus into the bloodstream.
When we analyzed the histological findings, respiratory injury was the most evident. Septic animals had histological evidence of pulmonary injury, such as, hemorrhage, congestion, inflammatory infiltrates, pneumonia or pleuritis, which were more numerous in animals pre-treated with O3. The relationship between sepsis and acute lung injury has been previously reported in large-animal or rodent sepsis models30–33 and clinical studies.34 In several of these studies presence of perivascular edema in the septic animal lungs is described, but it has not been evidenced in our work. However, cardiac lesions were found exclusively in dead animals of different groups. These findings are consistent with those obtained by other authors which showed, using experimental models of sepsis and human clinical studies, that blood flow to heart increase with coronary blood flow and coronary conductance indicating vasodilatation of the coronary circulation, congestion, hemorrhages and myocardial dysfunction.30,35,36
Regarding the significant decrease in the number of neutrophils bands from survivors belonging to group AMC/O3vs AMC, because we only have blood samples from animals surviving the experiment, we do not know the differential count in the acute phase of sepsis but it seems obvious to conclude that pre-conditioning with O3 has a modulating effect on white blood cells. Ozone also induces granulocyte recruitment and enhances their function in the lung.26,37 However, granulocyte accumulation can be impaired by an adaptive mechanism after repetitive ozone exposure38 Though taking into account, that ozone formation is an inherent feature of adaptive immunity, triggered by antibodies, we speculate that the chosen dose of ozone, which was recommended by another groups, was still too high to exclude negative immunological effects. In addition, we may have induced ozone adaptation (by giving it on 10 days prior to infection), thereby reducing absolute neutrophil count and function as it was demonstrated by others in the lung.38 Perhaps it was one of the reasons why we have not found neutrophilia in pre-conditioned animals. Another explanation could be related to the overcoming of sepsis and improved clinical status, although it is true that these surviving animals (regardless of treatment group) had injuries associated with focal peritonitis, serositis, pneumonia and/or positive cultures of peritoneal fluid with varying incidence. With these results, we would expect a significant change in the total count of white blood cells and/or increase in neutrophils, an aspect that has not been shown elsewhere. We speculate, like others authors in a similar model that we used,20 that ozone pre-treatment caused a trend to trend aggravate the clinical status, which may be due to an exaggerated pro-inflammatory cytokine release, disturbing the delicate pro- and anti-inflammatory cytokine balance after infection at the site of infection and systemically.
These results may be contradictory with the known bactericidal properties of the ozone, but in our study we did not evaluate the direct bactericidal O3/O2 mixture effect, but rather its possible advantages as oxidative modulator role on the immune system. Thus, in our study, O3/O2 mixture was administered rectally at increasing O3 concentrations as immune pre-conditioner, in which the gas does not act directly on the primary infective focus. In our opinion this aspect has been overlooked by other authors18,19,21 who used the intraperitoneal route to administer O3 directly on the infective inoculums and that might have influenced on the beneficial results that they attributed it.
The present study has some limitations and strengths. First, we have not explored whether the rectal application of ozone at doses below 1mg/kg cause the same negative effect on clinical status observed, when it is combined with AMC or alone. Neither we have studied the unique effect of pre-conditioning with ozone without further treatment with that gas. Perhaps 10 days of treatment after the onset of infection may mask the beneficial effect of prior pre-conditioning with the gas mixture. These should be determined in future although in a model similar to ours,20 wherein the pre-treatment during only 5 days pre infection with similar ozone dose that we used, without post infection treatment, results indicated a worsening of the clinical situation also. Second, we have not serial histological neither blood samples throughout the 10 day study. Samples for the different types of study came from died animals during 10 days or, from surviving animals. It would be interesting to conduct studies that allow us to understand the evolution of lesions and leucograms, throughout the experimental period with shorter frequencies. Third, in our opinion this is the first work that performs an assessment of the possible beneficial effect of ozone pre-conditioning by minimally invasive via and non-toxic doses, combining mortality dates, histological, microbiological, hematological studies and vital signs. Results confirm a worsening of the clinical status of the animals AMC/O3vs AMC with an excellent correlation between the study variables. However, facing the hypothesis that ozone Increased some pro-inflammatory cytokines and chemokines in plasma and in the site of infection, as some authors22 have described, we think that it would be interesting to perform biochemical studies to assess the antioxidant serum state and determination of some cytokines and chemokines such as TNF-α and MIP-2, which for economic reasons, we could not measure at the time, but that will be the subject of future research.
Our results suggest that rectal pre-treatment with ozonized oxygen aggravates clinic status in septic rats treated with amoxicillin/clavulanate. Although we cannot recommend pre-conditioning rectally with ozonized oxygen for patients at risk for peritonitis based on the results of our study, we conclude that O3/O2 doses and application days could be the aims of future studies due to the possible dose-dependent effect. It is our hope that the present study will be able to stimulate more interests and discussion of O3 and its role on the immune system modulation.
Conflict of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
We would like to thank Mr. Miguel Angel Silva, technician at the animal facility of the Research Unit at Hospital Universitario de Gran Canaria Dr. Negrín, Ms. Elena Carretón from Animal Pathology Department, University of Las Palmas de Gran Canaria, Mr. Ramón Saavedra and Mr. Juan Ramirez Verona from the Illustration and Iconography Service at the same hospital, for their technical assistance.