An increasing number of treatment-experienced perinatally HIV-infected patients are being transitioned from pediatric centers to adult HIV-care.1 Most of them had complex antiretroviral treatment history including mono- or bi therapy.2 In addition, adolescent adherence is particularly complex because of orphanhood, neurocognitive deficits, severe HIV infection, and stigma and discrimination.3–5 Unfortunately, few data of the efficacy and safety of antiretroviral-treatment (ART) in these patients are available.
The aim of this study was to evaluate the efficacy and the safety of ART initiated for virologic failure in a cohort of perinatally HIV- infected adolescents after being transitioned to an adult HIV-care. We performed a single-center retrospective study of treatment-experienced HIV-1 perinatally infected adolescent patients with a virologic failure who started a new ART at the adult HIV-care center, ‘Cosme Argerich’ Hospital, Buenos Aires, Argentina (2005–2011). Immunological, virological and clinical data was assessed at baseline and after 48 weeks of ART initiation. Only patients with an ongoing virologic failure and a baseline genotypic-test performed were included. Patients with missing data at 48 weeks were excluded. Baseline genotypic-tests were interpreted using the Stanford genotypic-algorithm and the Genotypic sensitivity score (GSS) was calculated for each new ART. Adherence was measure according to physician's evaluation.
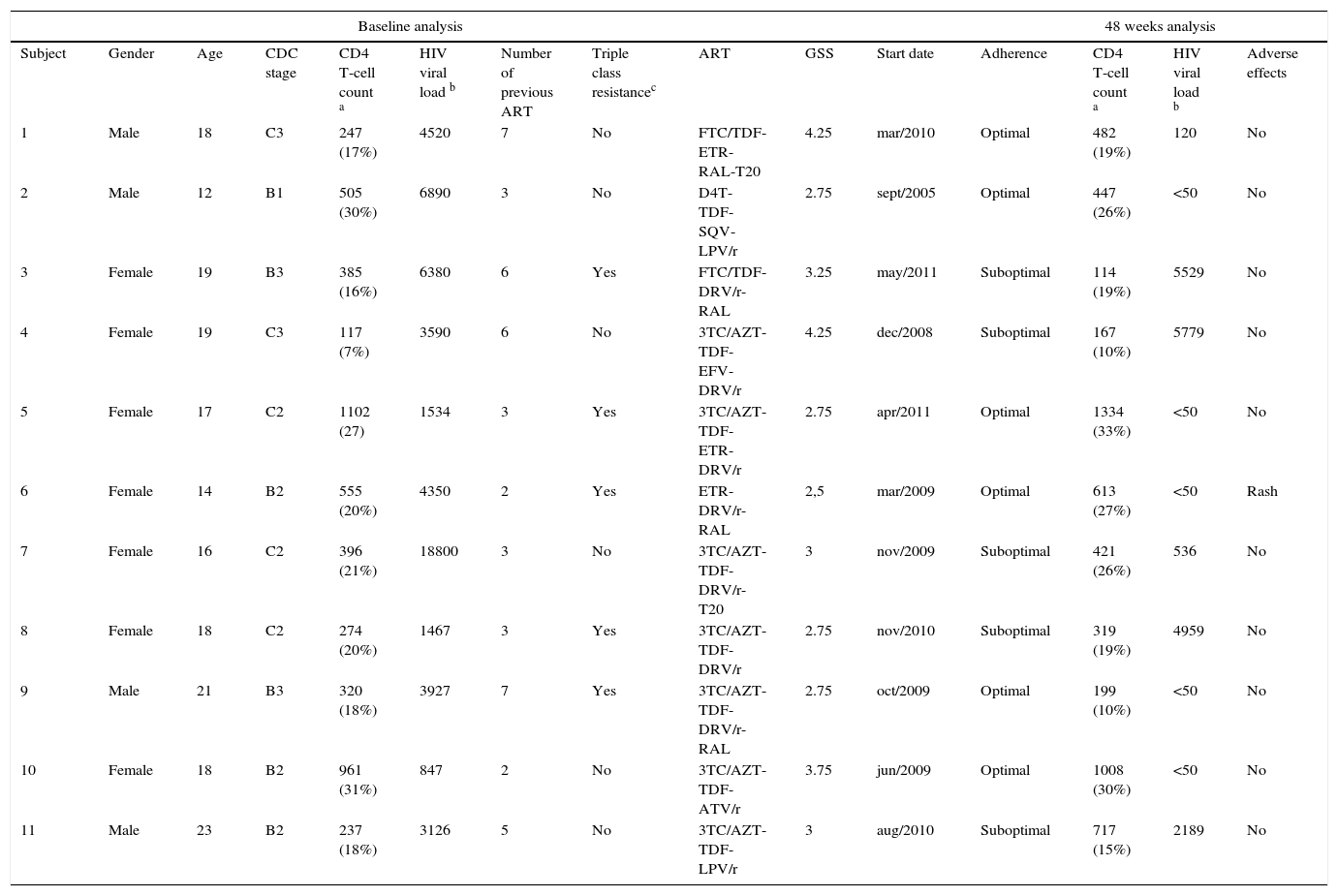
During the study period a total of 37 perinatally HIV-infected adolescents were transitioned to our institution, of whom 23 (62%) had an ongoing virologic failure. Of these 23 patients with virologic failure, 11 patients were included to the study. Twelve patients were excluded due to lost to follow-up or missing data at 48 weeks. Male sex was observed in 4 (36%) subjects. The median age was 18 years (IQR 16-19). CDC-stage C was observed in 5 (45%) subjects. Median CD4 T-cell count (IQR): 385 cells/mL (247-555). Median HIV-1 RNA viral load (IQR): 3927 copies/mL (1534-6380). Four patients had no history of undetectable viral load in their lives. The median of previous ART regimens was 3 (IQR 3-6) and the total duration under ART was 15 (IQR 12-16) years. Triple-class experienced-patient was observed in ten (91%) subjects. The median of NNRTIs, NNRTIs and PIs drugs previously used (IQR) was 5,4–6 1 (1-1) and 21–3 respectively.
The most frequent HIV resistance associated mutations (RAM) observed at baseline genotypic-tests were: 1)NRTI-RAMs: D67N (64%), M184V/I (55%), M41L (45%), L210W (45%), T215Y (45%); 2)NNRTI-RAMs: K103N/S (55%), L100I (27%), Y181C (9%); 3)PI-RAMs: V82A (45%), L90M (36%), V32I (18%), M46I (18%), I47V (18%), I54L (18%).Triple-class resistance was observed in five (45%) of the patients. In only two (18%) patients genotypic-tests performed for virologic failure previous to the transition (while on pediatric care) were available.
New ART based on genotypic-test including etravirine, darunavir/ritonavir, raltegravir or enfuvirtide was initiated in eight (73%) patients. GSS ≥2 and 3≥ of the new ART was achieved in eleven (100%) and six (55%) patients respectively. Three out of seven women initiated ART during pregnancy.
At week 48, the median CD4-T cell count (IQR) was 447 cells/mL (261-1121) and a viral load <50 copies/mL was observed in 5 (45%) patients. All except one subject with virologic failure had suboptimal adherence during the study period. Regarding adverse effects, only one episode of rash (grade 2) was observed, whereas no grade 4 or AIDS-related events were observed during the study period. Table 1 summarizes demographic, clinical characteristics and ART outcomes of the 11 perinatally HIV-infected adolescents.
Demographic, clinical characteristics and antiretroviral therapy efficacy of 11 perinatally HIV-1 infected patients transitioned to an adult HIV-care hospital with virologic failure.
| Baseline analysis | 48 weeks analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Gender | Age | CDC stage | CD4 T-cell count a | HIV viral load b | Number of previous ART | Triple class resistancec | ART | GSS | Start date | Adherence | CD4 T-cell count a | HIV viral load b | Adverse effects |
| 1 | Male | 18 | C3 | 247 (17%) | 4520 | 7 | No | FTC/TDF-ETR-RAL-T20 | 4.25 | mar/2010 | Optimal | 482 (19%) | 120 | No |
| 2 | Male | 12 | B1 | 505 (30%) | 6890 | 3 | No | D4T-TDF-SQV-LPV/r | 2.75 | sept/2005 | Optimal | 447 (26%) | <50 | No |
| 3 | Female | 19 | B3 | 385 (16%) | 6380 | 6 | Yes | FTC/TDF-DRV/r-RAL | 3.25 | may/2011 | Suboptimal | 114 (19%) | 5529 | No |
| 4 | Female | 19 | C3 | 117 (7%) | 3590 | 6 | No | 3TC/AZT-TDF-EFV-DRV/r | 4.25 | dec/2008 | Suboptimal | 167 (10%) | 5779 | No |
| 5 | Female | 17 | C2 | 1102 (27) | 1534 | 3 | Yes | 3TC/AZT-TDF-ETR-DRV/r | 2.75 | apr/2011 | Optimal | 1334 (33%) | <50 | No |
| 6 | Female | 14 | B2 | 555 (20%) | 4350 | 2 | Yes | ETR-DRV/r-RAL | 2,5 | mar/2009 | Optimal | 613 (27%) | <50 | Rash |
| 7 | Female | 16 | C2 | 396 (21%) | 18800 | 3 | No | 3TC/AZT-TDF-DRV/r-T20 | 3 | nov/2009 | Suboptimal | 421 (26%) | 536 | No |
| 8 | Female | 18 | C2 | 274 (20%) | 1467 | 3 | Yes | 3TC/AZT-TDF-DRV/r | 2.75 | nov/2010 | Suboptimal | 319 (19%) | 4959 | No |
| 9 | Male | 21 | B3 | 320 (18%) | 3927 | 7 | Yes | 3TC/AZT-TDF-DRV/r-RAL | 2.75 | oct/2009 | Optimal | 199 (10%) | <50 | No |
| 10 | Female | 18 | B2 | 961 (31%) | 847 | 2 | No | 3TC/AZT-TDF-ATV/r | 3.75 | jun/2009 | Optimal | 1008 (30%) | <50 | No |
| 11 | Male | 23 | B2 | 237 (18%) | 3126 | 5 | No | 3TC/AZT-TDF-LPV/r | 3 | aug/2010 | Suboptimal | 717 (15%) | 2189 | No |
ART: antiretroviral treatment.; ATV/r: atazanavir/ritonavir; AZT: zidovudine; D4T: stavudine; DRV/r: darunavir/ritonavir; EFV: efavrienz; ETR: etravirine; FTC: emtricitabine; GSS: genotypic sensitivity score; LPV/r: lopinavir/ritonavir; RAL: raltegravir; SQV: saquinavir; T20: enfuvirtide; TDF: tenofovir;3TC: lamivudine. a cells/mL.b copies/mLc based on baseline genotypic test
Despite the limited number of patients, these preliminary data show a high prevalence of heavy treatment-experience patients with highly drug-resistance viruses. Although all the patients had at least 2 active drugs in their ART a high virologic failure rate was observed at week 48. These findings are similar to other studies that reported lower rates of HIV-1 virologic suppression and higher rates of loss to follow-up in HIV-infected adolescents and young adults compared with adults. However, the majority of these studies excluded or included a small number of subjects with perinatally acquired HIV infection.6–10 For example, a recently published study reported a 58.7% of virologic suppression at 6-months in 46 HIV-infected adolescents and young adults, but only 7 perinatally HIV-infected patients were included.10
The high rate of virologic failure observed in our study could be attributed to the suboptimal adherence observed in almost all patients of the study. In the same way, suboptimal adherence to ART has been reported in the majority of the perinatally HIV infected adolescents cohort studies from both resource-rich and limited settings.9
A high proportion of the women initiated ART during pregnancy what highlights the need of sexual and family planning education in this population.
The management of perinatally HIV infected adolescents remains a challenge for adult HIV clinics. Thus, a multidisciplinary approach is necessary to maximize the likelihood of a successful treatment in this population.