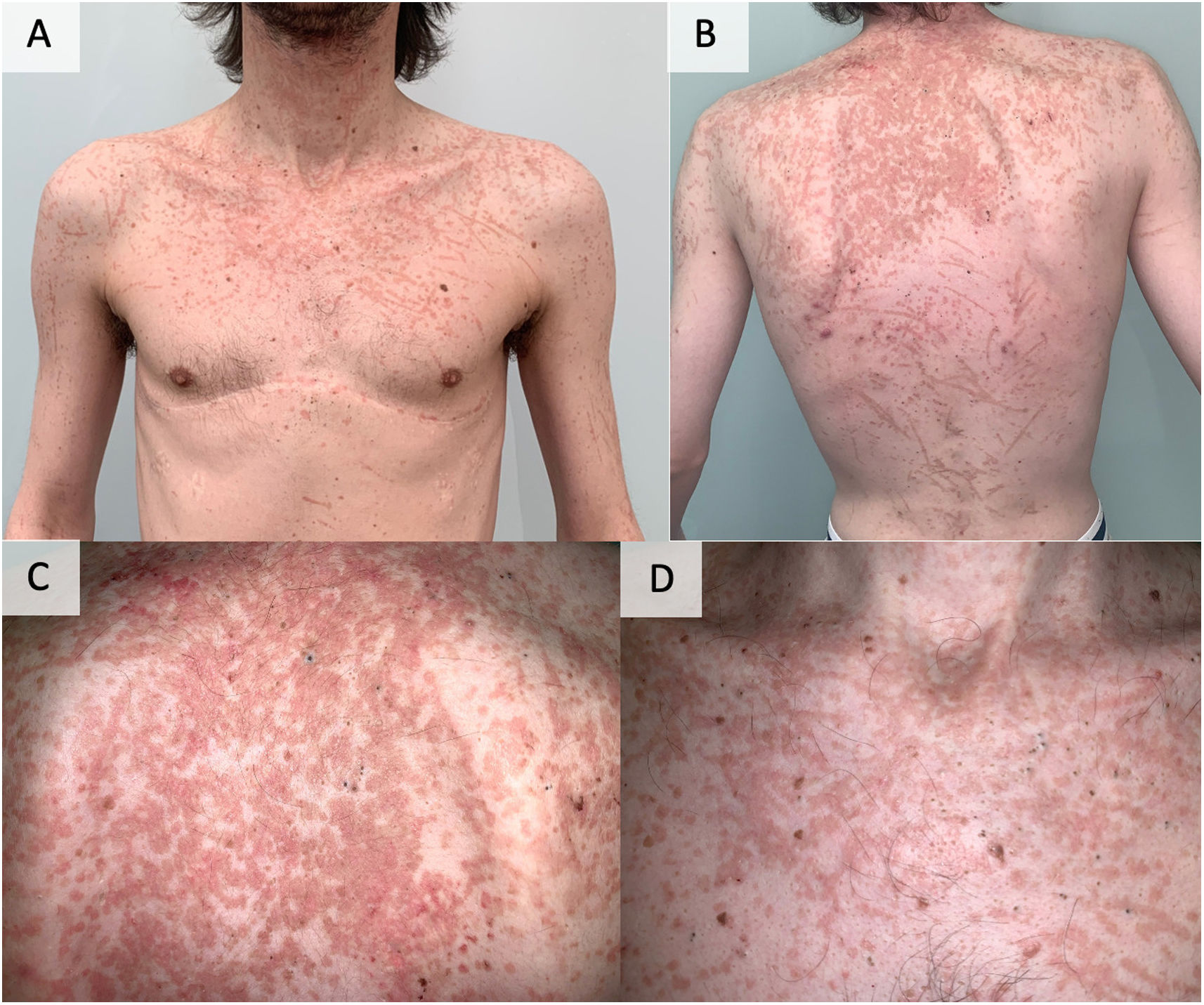
A 31-year-old male, with a relevant history of bilateral lung transplantation due to cystic fibrosis five years prior, presented for evaluation of skin lesions. It was a patient undergoing treatment with 100mg/day of cyclosporine for rejection prevention. There were no previous records of rejection or opportunistic infections. His pulmonary function was stable. The lesions had appeared approximately 3–4 months ago, without any clear triggers. They consisted of papules and plaques, with an orange color and a generalized distribution (Fig. 1). The lesions were more prominent on the trunk, neck, and upper limbs. Often, the lesions tended to be arranged in a geometric configuration, forming clearly linear figures. This pattern reflected a self-induced spread of the lesions, resembling a pseudo-Koebner phenomenon. With our clinical suspicion, a skin biopsy was performed.
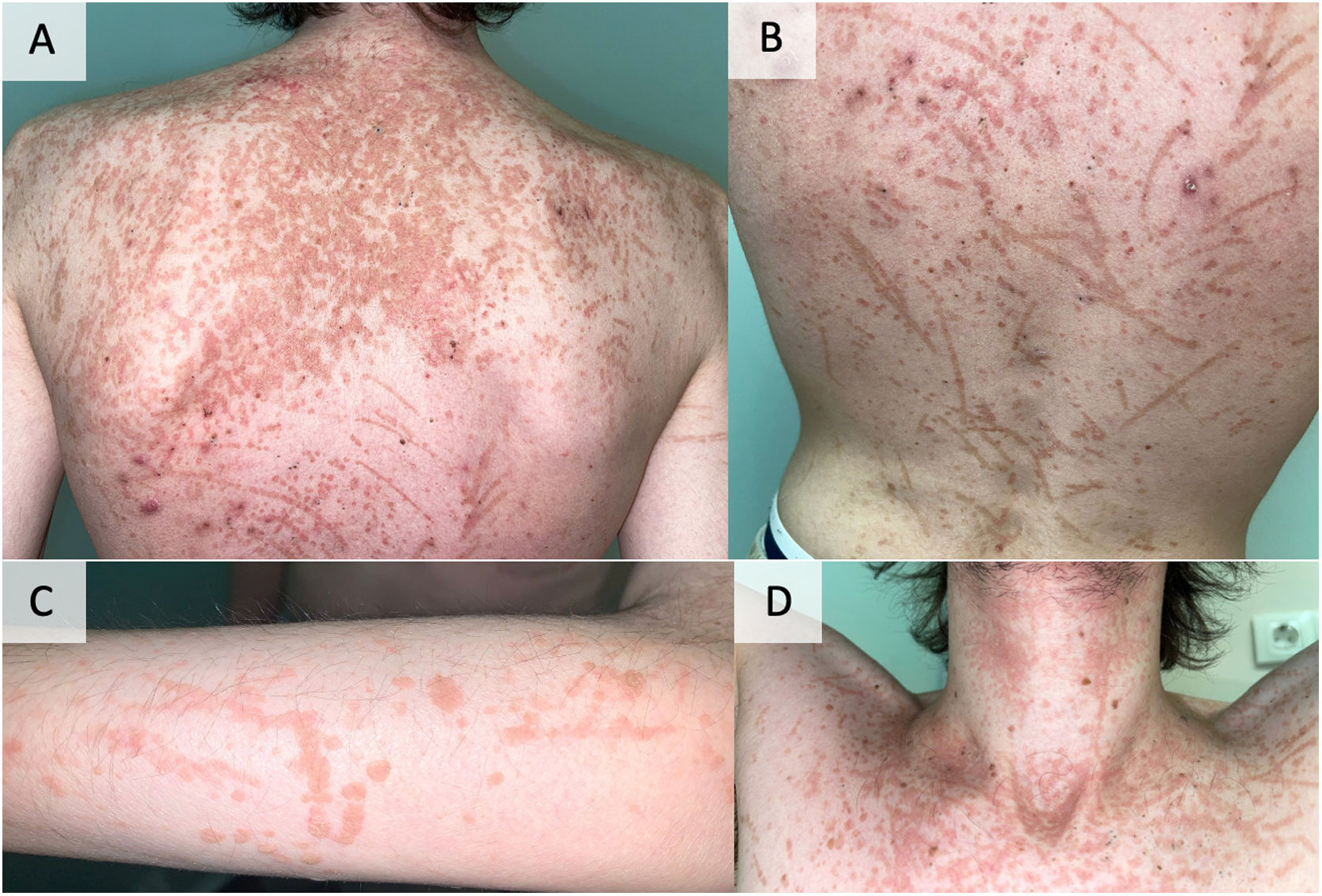
Evolution and diagnosisThe biopsy was consistent with a flat wart, and the human papillomavirus (HPV) sequencing was positive for HPV 5. The lesion pattern of flesh-colored papules with pseudo-Koebner phenomenon (Fig. 2) and the biopsy compatible with viral wart (Fig. 3) confirmed the diagnosis of acquired epidermodysplasia verruciformis (AEV). As part of the therapeutic approach, and after consultation with his pulmonologist, mycophenolate, prednisone, and cyclosporine were discontinued, and sirolimus was added instead. Subsequently, topical treatment with tretinoin was initiated. Due to a partial response, the treatment was changed to isotretinoin 5mg/day, with a very good response and no significant adverse effects. At 9 months of treatment, the patient had minimal residual lesions, and it was decided to reduce the dose to 5mg every other day.
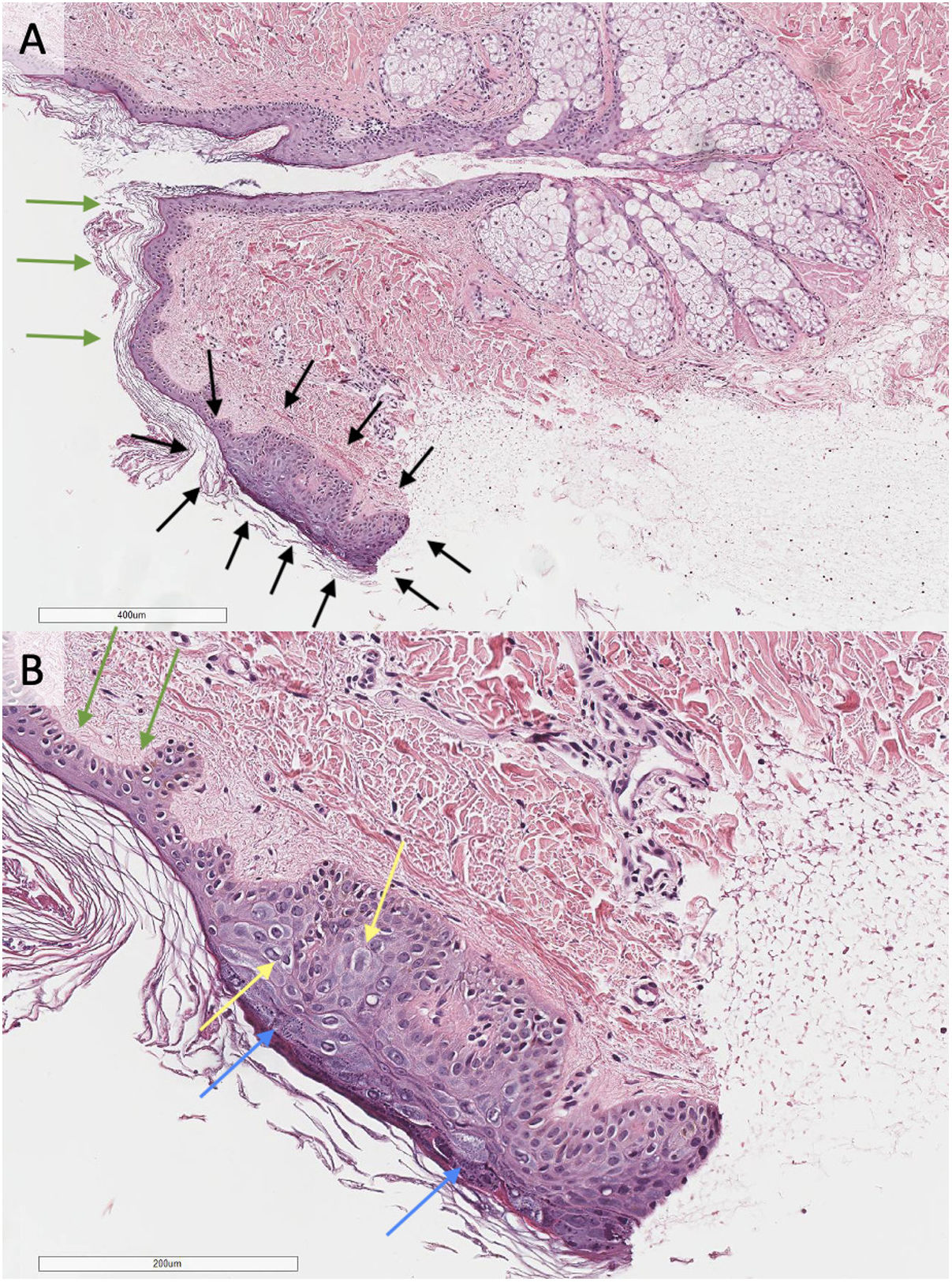
Histological study of a biopsy compatible with flat wart taken from the anterior mid-thoracic region (5mm punch biopsy, hematoxylin–eosin staining, panel A 20×; panel B 40×). In the caudal region of the biopsy (surrounded by black arrows, panel A), a focal area with acanthotic epidermis displaying hypergranulosis (blue arrows, panel B) and vacuolization of keratinocytes (yellow arrows, panel B) in upper layers is observed. The adjacent epidermis shows no abnormalities (green arrows, panels A and B).
Epidermodysplasia verruciformis (EV) is a rare genodermatosis characterized by chronic infection with the HPV leading to the development of polymorphic skin lesions and a high risk of skin cancer. It is generally hereditary due to mutations in the EVER1 and EVER2 genes. However, cases of an acquired form with clinical features identical to the hereditary form have been described. This form is called acquired AEV and is associated with acquired immunodeficiency conditions.
EV was primarily described in 1922 by Lutz and Lewandowsky. Its etiopathogenesis, caused by mutations in the EVER1 and EVER2 genes, was firstly described in 2002.1 They are associated with a specific zinc transporter. The mutation resulted in a particular susceptibility to HPV infection, especially HPV 5 and HPV 8. Additionally, between 30% and 60% of patients presented with cutaneous squamous cell carcinoma (cSCC) as a secondary complication.1 Later, in 2009, Rogers et al. coined the term “acquired epidermodysplasia verruciformis” (AEV) following multiple case reports of EV in patients without inherited mutations. AEV was clinically indistinguishable from the hereditary form and was typically observed in immunocompromised patients, especially those with poorly controlled human immunodeficiency virus (HIV) and solid organ transplant recipients with chronic immunosuppression.2 However, subsequently, there have been reports of cases with other types of immunosuppression, such as lupus erythematosus, Hodgkin's lymphoma or atopic dermatitis. Although it has been hypothesized that these patients may have a genetic susceptibility to develop this disease, a genetic substrate for AEV has not been identified yet.2 Though, there is an equal predisposition to HPV infection, with HPV 5 and 8 being the most common types, although more than 20 genotypes have been described.3 Both in EV and AEV, the clinical presentation includes flat, wart-like papules resembling tinea versicolor. There are forms that present as verrucous lesions on sun-exposed surfaces especially associated with cSCC. Regarding the risk of malignancy in AEV, a recent series found a malignancy rate of 28% with a median follow-up of 5.5 years.2,3 EV and AEV do not have a curative treatment. Multiple treatments have been used, both topical (cryotherapy, keratolytics, retinoids) and systemic (interferon-alpha, isotretinoin), among others. In the same way, it is important to minimize the degree of immunosuppression as much as possible and the control of HIV, if present. Traditionally, cyclosporine or tacrolimus prophylaxis has been employed in the prevention of lung rejection. However, in recent years, mTOR inhibitors such as sirolimus or everolimus have become a new therapeutic tool for rejection prophylaxis. They represent an effective therapeutic option with a better safety profile regarding malignant skin neoplasms and viral infections compared to cyclosporine and tacrolimus. Despite being an incurable condition, with the combination of adjustments to immunosuppressive prophylaxis and the application of topical or systemic treatments, it can be a manageable disease. Finally, in case of development, the treatment of cSCC does not differ from sporadic cases.3 With the increasing number of immunosuppressed patients, it is essential for every physician to have knowledge of AEV.
Ethical approvalProcedures followed here were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. We have not use patients’ names, initials or hospital numbers.
Authors’ contributions- -
Miguel Mansilla-Polo and Begoña Escutia-Muñoz managed clinical treatment and procedures, contributing to the development of this paper.
- -
Margarita Llavador-Ros directed the pathological study.
- -
Rafael Botella-Estrada supervised the work.
- -
All authors had access to the data and played a role in writing this manuscript.
This article has no funding source.
Conflicts of interestThe authors have declared no conflicts of interest.