Biofilm production is considered a potential virulence factor of some Candida species. For this reason, an understanding of biofilm behavior of Candida albicans and its closely related species Candida dubliniensis is key to the development of effective preventive measures for invasive and oral candidiasis. The aim of this study was to compare the capacity of biofilm production by blood and oral isolates of C. albicans and C. dubliniensis using polystyrene, flat-bottomed 100-well microtiter plates.
MethodsA total of 47 isolates, consisting of 28 C. albicans (16 oral and 12 blood isolates) and 19 C. dubliniensis (11 oral and 8 blood isolates) were compared for their biofilm forming ability under aerobic and static conditions. XTT reduction assay was used to quantify the sessile growth.
ResultsAll tested isolates produced biofilm, measured as XTT metabolic activity. Biofilm formation by C. albicans isolates was statistically significantly higher than biofilm formation by C. dubliniensis isolates at 24h (P=0.03) and 48h (P=0.0001). There was a higher percentage (41.7%) of high producers of biofilms among C. albicans blood isolates than among oral isolates (31.3%), without statistically significant differences.
ConclusionsThis capability may allow C. albicans and C. dubliniensis to maintain their oral ecological niches as commensal or pathogenic microorganisms and can be a major virulence factor during invasive candidiasis. However, the differences in biofilm production among isolates should be taken into account when the anti-biofilm activity of antifungal agents or other virulence factors are tested in vitro.
El desarrollo de biopelículas o biocapas se considera un factor de virulencia potencial de algunas especies de Candida. Una comprensión mejor del comportamiento de las biopelículas de Candida albicans y de la especie cercana Candida dubliniensis es clave para desarrollar medida preventyivas eficaces de las candidiasis superficiales (orales) e invasoras. El objetivo de este trabajo ha sido comparar la capacidad de producir biopelícula por parte de los aislamientos orales y de sangre de C. albicans y C. dubliniensis en placas de microtitulación de poliestireno de 100 pocillos con fondo plano.
MétodosSe estudiaron 47 aislamientos: 28 C. albicans (16 orales y 12 hemáticos) y 19 C. dubliniensis (11 orales y 8 hemáticos). Se empleó una prueba de cuantificación de la actividad metabólica de las biopelículas (reducción de la sal de tetrazolio denominada XTT).
ResultadosTodos los aislamientos mostraron actividad metabólica pero la formación de biopelícula por los aislamientos de C. albicans era significativamente mayor que por los de C. dubliniensis a 24h (P=0,03) y 48h (P=0,0001). Eran más numerosos, los aislamientos de C. albicans muy productores de biopelícula procedentes de sangre (41.7%) que de boca (31.3%) pero las diferencias no eran significativas.
ConclusionesLa capacidad de desarrollar biopelículas, podría permitir a C. albicans y C. dubliniensis mantenerse en el nicho oral como comensales o patógenos y ser un importante factor de virulencia en las candidiasis invasoras. Sin embargo, las diferencias encontradas entre los aislamientos productores de biopelícula deben tenerse en cuenta, sobre todo en los estudios in vitro de virulencia o de la acción anti-biopelícula de los fármacos antifúngicos.
Invasive candidiasis represents about 10% of nosocomial invasive infections. Although other species, such as Candida parapsilosis, Candida glabrata or Candida tropicalis, are being isolated with increasing frequency; Candida albicans is the most frequent aetiological agent of candidiasis.1,2 Many candidiasis are associated with prostheses, catheters and other indwelling medical devices, where these microorganisms develop biofilms. The presence of extracellular polymers and a different cellular phenotype, called sessile is an important feature in the structure of these microbial comunities. Biofilms impede the actions of the immune system and other defence mechanisms and become recalcitrant to current antifungal treatment.3–5C. albicans is also an important pathogen of the oral cavity and other mucosae. This yeast has the ability to grow under diverse oral environmental conditions (e.g. unhygienic dentures, xerostomia) and/or systemic factors, such as diabetes and immunodeficiency. The presence of dentures that are overlaid with proteins and other oral components encourages the development of Candida biofilms and denture stomatitis.4
Candida dubliniensis is an emerging species associated to oral candidiasis, mainly in HIV-infected patients.6–8 The frequence of deep-seated infections caused by C. dubliniensis is low but probably understimated because C. dubliniensis is closely related to C. albicans and many laboratories are not capable of differentiating between both species.1,9–13C. dubliniensis shares many properties with C. albicans and, in addition, shows an important capacity to develop resistance to fluconazole and other antifungal agents under repeated exposure.7,14–16
The aim of the current study has been to compare the capacity of biofilm production by blood and oral isolates of C. albicans and C. dubliniensis from infected patients.
MethodsMicroorganismsA total of 28 C. albicans (16 oral isolates and 12 blood isolates) and 19 C. dubliniensis (11 oral isolates and 8 blood isolates) from the Laboratorio de Micología Médica at the University of the Basque Country were studied. The clinical origin of the isolates and the infections caused have been described previously.8–10,17 Two strains from the National Collection of Pathogenic Fungi (NCPF), C. albicans NCPF 3153 and C. dubliniensis NCPF 3949, as well as C. albicans NCPF 3153 hypha-defective mutant CA-2 (kindly donated by Professor Antonio Cassone, Istituto Superiore di Sanità, Rome, Italy) were included as reference strains. The identity of all isolates was confirmed by conventional mycology methods, such as the germ tube test in serum, microscopic morphology, chlamydoconidia production in corn meal agar with Tween 80, and carbon source assimilation with the commercial kit ID 32 C (bioMérieux, France).18 All isolates were screened for their ability to grow on Sabouraud dextrose agar at 45°C for 48h, and in the chromogenic media CHROM-Pal's medium19 and ChromID Candida.20 The reactivity with a specific polyclonal anti-C. dubliniensis antibody by an immunofluorescence assay21 and by the Bichro-Dubli latex agglutination test (Fumouze Diagnostics, France)22 were also tested. The identities of those isolates classified as C. dubliniensis were confirmed by means of a polymerase chain reaction (PCR) using the specific primers CDBF28-f (5′-AAATGGGTTTGGTGCCAAATTA-3′), and CDBR110-r (5′-GTTGGCATTGGCAATAGCTCTA-3′) described by Kanbe et al.23 which amplify topoisomerase II gene, giving a DNA product size of 816bp.
Preparation of Candida suspensionsPrior to each experiment, yeast isolates were aerobically cultured at 37°C for 24h on Sabouraud dextrose agar (Difco Laboratories, USA) and a loopful of growth was inoculated in YPD broth (yeast extract 10g, peptone 20g, glucose 20g, distilled water 1l). After 24h, the yeasts were harvested, washed twice with phosphate buffered saline (PBS, pH 7.2) and suspended to a concentration of 107 cells/ml. This cell concentration was selected because previous workers have demonstrated an optimal degree of biofilm formation of C. albicans and C. dubliniensis at this particular concentration.24
Production and quantification of Candida biofilmsBiofilm formation was measured using a colorimetric method based on the XTT reduction according to Ramage et al.25,26 Briefly, C. albicans and C. dubliniensis isolates were suspended in RPMI 1640 broth supplemented with L-glutamine and buffered with MOPS, and 100μl of standard cell suspensions of yeasts (107 cells/ml), prepared as above, were transferred into each well of a flat-bottomed 100-well microtitre plate (BioScreen, Finland). XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) (Sigma, USA) was prepared as a saturated solution in 0.5g/l in Ringer's lactate and the solution was filter sterilised using a 0.22-μm-pore-size filter (Sarstedt, Germany), aliquoted and then stored at −70°C until required. Before each assay, an aliquot of stock XTT was thawed and the electron-coupling agent menadione, 10mM prepared in acetone (Sigma) was added to a final concentration of 1μM. The biofilms were washed 3 times with 100μl of PBS to remove loosely adherent cells. Afterwards, 100μl of the XTT–menadione solution was added to each biofilm and control wells to measure background XTT reduction levels, and the plates were incubated in the dark for 1h at 37°C. The colour changes in the XTT reduction assay (which directly correlate with the metabolic activity of the cells within the biofilm) were then measured using a BioScreen C plate incubator (BioScreen) at 492nm. Measurements were repeated at least six times each on different days. The optical densities (OD) corresponding to the biofilm metabolic activities of both Candida species were compared calculating the Student's t-test using the program SPSS (IBM, USA). Biofilm production was scored and divided into 6 categories, according to absorbance at 492nm (A)/transmittance (T), as 6+ (A>1.30/T≤5%), 5+ (A=1–1.29/T=6–10%), 4+ (A=0.70–0.99/T=11–20%), 3+ (A=0.40–0.69/T=21–40%), 2+ (A=0.20–0.39/T=41–60%), and 1+ (A<0.20/T>60%).2,27 Categories 6+ and 5+ included those high producers of biofilm isolates and categories 2+ and 1+ included poor or non-producers of biofilm. Categories were compared using the Chi-squared test with Yates’ correction when necessary. Differences between the species were considered to be statistically significant at P<0.05.
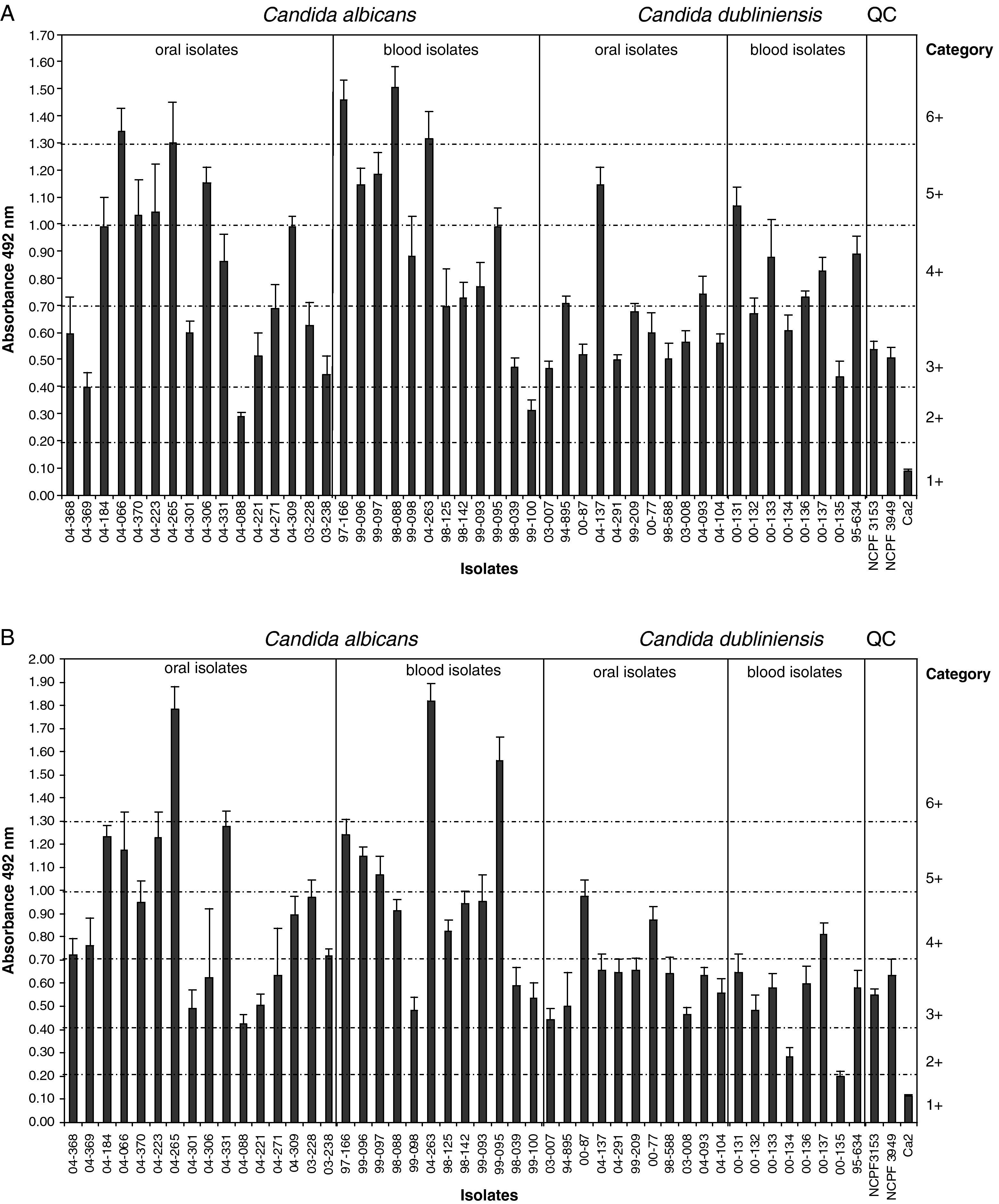
ResultsAll tested isolates of C. albicans and C. dubliniensis produced biofilm on polystyrene (Tables 1 and 2 and Fig. 1). However, a great variability in biofilm production was observed in both species. The biofilm metabolic activities (A492nm) of C. albicans isolates at 24h ranged between 0.291 and 1.506 (mean 0.869±0.352) and those of C. dubliniensis isolates ranged between 0.438 and 1.145 (mean 0.688±0.199) (P=0.03) (Fig. 1A). C. albicans blood isolates showed a higher metabolic activity in 24h biofilms than in oral isolates (0.955±0.379 vs. 0.805±0.328, respectively, P=0.28). A similar pattern was observed for C. dubliniensis (0.763±0.195 vs. 0.634±0.192, respectively, P=0.17).
Metabolic activity (XTT) of biofilms produced by Candida albicans and Candida dubliniensis clinical isolates at 48h according to their clinical origin.
| Species | Biofilm production (XTT metabolic production) | |
| Blood | Oral | |
| C. albicans | 1.007±0.401 | 0.900±0.366 |
| C. dubliniensis | 0.522±0.198 | 0.640±0.163 |
| Reference strains | ||
| C. albicans NCPF 3153 | 0.550±0.024 | |
| C. dubliniensis NCPF 3940 | 0.634±0.069 | |
| C. albicans CA-2 | 0.112±0.008 | |
Distribution of clinical isolates of C. albicans and C. dubliniensis on the basis of their capability for producing biofilm at 48h.
| Category | Biofilm | Species (no. of isolates included and percentage) | ||||
| C. albicans | C. dubliniensis | Total | ||||
| Blood | Oral | Blood | Oral | |||
| 48h | 48h | 48h | 48h | 48h | ||
| High producers of biofilm | 6+ | 2 (16.7%) | 1 (6.3%) | 0 (0%) | 0 (0%) | 3 (6.4%) |
| 5+ | 3 (25%) | 4 (25%) | 0 (0%) | 0 (0%) | 7 (14.9%) | |
| Producers of biofilm | 4+ | 4 (33.3%) | 6 (37.5%) | 1 (12.5%) | 2 (18.2%) | 13 (27.7%) |
| 3+ | 3 (25%) | 5 (31.3%) | 5 (62.5%) | 9 (81.8%) | 22 (46.8%) | |
| Low producers and non-producers of biofilm | 2+ | 0 (0%) | 0 (0%) | 2 (25%) | 0 (0%) | 2 (4.3%) |
| 1+ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Total | 12 (100%) | 16 (100%) | 8 (100%) | 11 (100%) | 47 (100%) | |
Biofilm metabolic activities of C. albicans isolates at 48h were higher than at 24h (0.945±0.378 vs. 0.869±0.352, respectively, P=0.44) with independence of the origin of these isolates (blood vs. oral isolates: 1.007±0.401 vs. 0.900±0.366, respectively, P=0.48). However, at 48h the biofilms of oral isolates of C. dubliniensis showed more metabolic activity (0.640±0.163) than the biofilms of blood isolates (0.522±0.198) (P=0.19) (Table 2 and Fig. 1B). Most C. dubliniensis isolates belonged to genotype I, except isolates 00-133 and 00-135, which were from the genotype II (Fig. 1). There were no statistically significant differences in biofilm production between both genotypes.
C. albicans included a non-statistically significant higher number of isolates classified as high producers of biofilm than C. dubliniensis (P=0.86) at 48h. Ten out of 28 C. albicans isolates (35.7%) were classified as high producers of biofilm at 24h. Of these, 5 were from blood (41.7% of the blood isolates) and 5 from the oral cavity (31.3% of the oral isolates) (P=0.86). This category of high producers of biofilm only grouped 2 out of 19 C. dubliniensis isolates (10.5%) at 24h, but this difference between isolates of both species was not statistically significant (P=0.109). However, at 48h, 10 out of 28 C. albicans isolates (35.7%) and 0 out of 19 C. dubliniensis isolates were classified as high producers of biofilm (P=0.01). At this incubation time, 5 out of 12 (41.7%) blood isolates of C. albicans were high producers of biofilm in comparison to 5 out of 16 oral isolates (31.3%) (P=0.86).
DiscussionThe phenomenon of biofilm formation by microbes on inert surfaces has been extensively studied in bacteria and to a lesser extent in fungi, and there appears to be a direct relationship between the capability of the organisms to form a biofilm and their pathogenicity. However, few studies have compared biofilm production among C. albicans bloodstream and oral isolates28 and much less, between oral and blood isolates of C. dubliniensis. The current results confirm that most C. albicans and C. dubliniensis oral and blood isolates develop biofilms on polystyrene plates. We used the colorimetric method based on the XTT reduction in the current study because it correlates well with other quantitative methods, such as ATP or CFU assays used in cross-comparison of species variation in biofilm production.29 The XTT method is more versatile and less-time consuming, and is also particularly suited for assays using microtitre plates that allow the screening a large number of isolates.
Shin et al.28 observed that a very low percentage of isolates of C. albicans produced biofilm, regardless of the invasive (bloodstream) or non-invasive origin of the isolates (8% vs. 7%). Conversely, the biofilm production was high when isolates from other species were tested, and those from blood and deep tissues were statistically significant higher producers than those isolates from other clinical specimens. Kumar and Menon,30 using the same medium and method for biofilm production, reported that 11 out of 18 C. albicans isolates (61%) were biofilm producers. Three out of 7 bloodstream isolates (43%) produced biofilm. Non-C. albicans isolates were higher producers of biofilm than those of C. albicans. These authors included 5 reference strains of C. dubliniensis, observing that all of them produced biofilm. In contrast, other authors have reported that isolates of C. albicans produced statistically significantly more biofilm than other Candida species.27,29,31–34 All isolates in our study showed a variable capability to produce biofilm, being distributed into 3 categories (from high to low producers of biofilm) considering their metabolic activities, and those isolated from the bloodstream were non-statistically significantly higher producers of biofilm than oral ones.
These discrepancies could be associated with differences in the methodology used, to the potential variability associated to the geographic origin of the isolates, or to other isolate-associated factors. Shin et al.28 and Kumar and Menon30 used a Sabouraud dextrose broth, and a RPMI-based broth was used in our comparison. Sabouraud dextrose broth with a high content of glucose (8%) has been claimed to simulate the hyperglycaemic milieu found in those patients receiving total parenteral nutrition.28,30 However, RPMI-based broth is a better promoter of filamentation than Sabouraud dextrose broth,35 a fact that is essential for the first step (adhesion) of biofilm production and can be influenced by different cultural and host factors.36–38 This influence of the RPMI in germ tube and hypha development could explain the production of biofilm by all the C. albicans and C. dubliniensis isolates tested in the current study.
The geographical origin of isolates in the different studies can influence the biofilm production capability, as has been observed for other phenotypic traits of Candida isolates, such as virulence factors or in vitro antifungal susceptibilities.29,39 This can be another potential explanation of the differences encountered, as Shin et al.28 studied isolates from Korea, and Kumar and Menon30 from India, while in the current study all C. albicans isolates were from Spain. Li et al.,40 studying Canadian isolates of C. albicans, reported that natural clones and clonal lineages of C. albicans exhibited extensive quantitative variations in biofilm formation. Something similar was described by Borecka-Melkusova and Bujdakova41 in C. albicans and C. dubliniensis isolates from Slovakia. Thein et al.29 also reported that growth and virulence of the different species of Candida were highly dependent on the isolates chosen for each study, hence it is important to use multiple clinical isolates and strains for the same species to draw firm conclusions in this regard.
There was a high variability in the biofilm production among C. albicans isolates when the interspecies variation of C. albicans and C. dubliniensis isolates was studied. This variability was lower among C. dubliniensis isolates. Kuhn et al.42 observed that XTT results can vary in accordance with the different sensitivity of C. albicans and Candida parapsilosis to tetrazolium salts. C. dubliniensis is more closely related to C. albicans than Candida parapsilosis and probably has a similar sensitivity to XTT than C. albicans. Nevertheless, we found that both Candida species, C. albicans and C. dubliniensis, exhibited good biofilm forming capability on polystyrene surfaces under aerobic conditions. These findings are in agreement with those of Ramage et al.24 who also reported that C. dubliniensis exhibited good biofilm growth on the surface of polystyrene plates.
One of the key morphogenesis factors for the phase transition in these dimorphic fungi is the nature of the environment, as the hypha growth is promoted by low oxygen and, nutrient starvation, as occurs in this aerobic and static conditions assay.29 Such a phase transition is dependent upon strain as well as species characteristics, as shown here by the CA-2 hypha-deficient strain of C. albicans, which always exists in the yeast phase, irrespective of its environmental milieu. Both species formed heterogeneous biofilms with strain variations in their morphology and XTT metabolic activity, as has been described previously by Henriques et al.32
In conclusion, there are important differences in biofilm production by C. albicans and C. dubliniensis isolates. These differences should be taken into account when the anti-biofilm activity of antifungal agents or other virulence factors are tested in vitro. This capability for biofilm development may enable C. albicans and C. dubliniensis to maintain their oral ecological niches as commensal microorganisms and can be a major virulence factor during invasive candidiasis with important clinical repercussions.
Conflict of interestsThe authors have no conflict of interest to declare.
María Villar-Vidal and Cristina Marcos-Arias had scholarships from the Universidad del País Vasco-Euskal Herriko Unibertsitatea. We thank José Manuel Aguirre-Urizar, Antonio Cassone, Carmen Rubio, Ricardo Salesa, and Derek Sullivan for kindly providing some of the C. albicans and C. dubliniensis isolates and strains used in this study. This work has been funded in part by projects GIC07 123-IT-222–07 (Departamento de Educación, Universidades e Investigación, Gobierno Vasco), S-PE08UN35 and S-PR09UN01 (Saiotek 2008 and 2009, Departamento de Industria, Comercio y Turismo, Gobierno Vasco) and PI061895/2006 (Fondo de Investigación Sanitaria del Ministerio de Sanidad y Consumo de España).