Bacterial/fungal coinfection and superinfections contribute to the increased morbi-mortality of viral respiratory infections (RIs). The main objective of this study was to determine the incidence of these infections in hospitalized patients with COVID-19.
MethodRetrospective observational study of all patients admitted for COVID-19 and bacterial/fungal infections at the Hospital Clínico Universitario of Valladolid, Spain (March 1–May 31, 2020). Demographic, clinical and microbiological data were compared based on Intensive Care Unit (ICU) admission and predictors of mortality by were identified using multivariate logistic regression analyses.
ResultsOf the 712 COVID-19 patients, 113 (16%) presented bacterial/fungal coinfections or superinfections. Their median age was 73 years (IQR 57−89) and 59% were men. The profiles of ICU patients (44%) included male, SARS-CoV-2 pneumonia, leukocytosis, elevated inteleukin-6, with interferon β-1b and tocilizumab and superinfection (p < 0.05). Coinfections were diagnosed in 5% (39/712) patients. Most common pathogens of respiratory coinfection (18) were Streptococcus pneumoniae (6) and Staphylococcus aureus (6). Superinfections were detected in 11% (80/712) patients. Urinary (53) and RI (39) constituted the majority of superinfections Acinetobacter baumannii multidrug-resistant was the main agent of IR and bacteremia. An outbreak of A. baumannii contributed to this result. Three patients were considered to have probable pulmonary aspergillosis. Mortality was higher in UCI patients (50% vs. 29%, p = 0.028). The predictive factors of mortality included being a male with various comorbidities, SARS-CoV-2 pneumonia, bacteremia and superinfections from A. baumannii.
ConclusionThe outbreak of A. baumannii was a determining factor in the increases of the incidence of infection and the morbi-mortality of ICU patients.
La coinfección/sobreinfección bacteriana/fúngica contribuye al incremento de la morbi-mortalidad de las infecciones respiratorias (IRs) virales. El objetivo de este trabajo es conocer la incidencia de estas infecciones en los pacientes hospitalizados por COVID-19.
MétodoEstudio retrospectivo observacional de todos los pacientes ingresados por COVID-19 e infección bacteriana/fúngica en el Hospital Clínico de Valladolid (1 marzo–31 mayo, 2020). Comparación de datos demográficos, clínicos y microbiológicos en función del ingreso en UCI e identificación de los factores predictores de mortalidad mediante regresión logística multivariante.
ResultadosDe 712 pacientes con COVID-19, 113 (16%) presentaron coinfección/sobreinfección bacteriana/fúngica. Mediana de edad 73 años (RIQ 57−89) y 59% de hombres. Perfil del paciente de UCI (44%): hombre con neumonía por SARS-CoV-2, leucocitosis, inteleuquina-6 elevada, con interferónβ-1b y tocilizumab y sobreinfección (p < 0,05). El 5% (39/712) de los pacientes presentaron una coinfección. Streptococcus pneumoniae (6) y Staphylococcus aureus (6) fueron los principales patógenos de las coinfecciones respiratorias (18) El 11% (80/712) se sobreinfectaron. Las infecciones más frecuentes fueron las urinarias (53) e IR (39). Acinetobacter baumannii multirresistente fue el principal agente de las IR y bacteriemias. Un brote por A. baumannii contribuyó a este resultado. Tres pacientes se diagnosticaron como probable aspergilosis pulmonar. La mortalidad fue superior en los pacientes de UCI (50% vs 29%, p = 0,028). Factores predictores de mortalidad: hombre con varias comorbilidades, neumonía por SARS-CoV-2, bacteriemia y sobreinfectado por A. baumannii.
ConclusiónEl brote por A. baumannii fue determinante en la incidencia de la infección y en la morbi-mortalidad de los pacientes de UCI.
The COVID-19 pandemic constitutes an unprecedented challenge in healthcare worldwide, with 45,942,902 confirmed cases and 1,192,644 deaths, as of 1 November 2020 (https://covid19.who.int/). Several factors are decisive in the prognosis of COVID-19: age,1–3 risk of multiple organ failure,1 comorbidities (hypertension, dyslipidaemia, cardiovascular disease, chronic obstructive pulmonary disease [COPD], etc.),1–4 and D-dimer1,5 and C-reactive protein2 values. Bacterial and fungal coinfection and superinfection could be another marker of the progression of COVID-19,1,4–6 due to its similarity to other viral respiratory processes. Thus, in the SARS-CoV-1 virus pandemic, bacterial coinfection was 22%7 and in influenza virus pandemics, bacterial coinfection ranged between 2% and 65%8,9 and fungal coinfection between 15–25%.10 In patients admitted to hospital for COVID-19, coinfection and superinfection vary widely depending on the population studied: 2–27% in hospitalised adult patients,4,11,12 14–58% in severe or critical patients5,12–14 and 50% in deaths from COVID-19.1
In patients admitted for COVID-19, there are several factors, not mutually exclusive, that predispose them to bacterial and fungal infection. First, the action of the SARS-CoV-2 virus: tissue destruction,6 infection of the enterocytes and alteration of intestinal haemostasis.15 Second, the high release of cytokines and dysregulation of the immune system.1,2,6 Third, the characteristics of the patient1–3,6,16 and their comorbidities (COPD, diabetes, chronic renal failure [CRF], immunosuppression), invasive medical devices, prolonged stays, etc. Added to all this is the emergency situation caused by the pandemic: overwhelmed medical services, lack of trained personnel, work stress, etc., which make it difficult to apply pre-pandemic infection control measures and favour the appearance of nosocomial outbreaks.
In the province of Valladolid (Spain), in the first wave of the pandemic, the cumulative incidence of COVID-19 was 780 cases/100,000 inhabitants with an overall lethality of 10.42%, and of 22.21% in patients older than 80.17 The Hospital Clínico Universitario de Valladolid (HCUV) cares for one of the oldest populations in Spain. The objectives of this study were: to know the incidence of bacterial/fungal coinfection/superinfection in patients with COVID-19 admitted to the HCUV, to analyse the demographic, clinical and microbiological characteristics of these patients based on their admission to the intensive care unit (ICU), and to identify predictors of mortality.
Material and methodsStudy designAn observational retrospective study of all patients admitted to the HCUV for COVID-19 and bacterial or fungal coinfection/superinfection between 8 March and 31 May 2020. Patient information was obtained from the minimum data set (MDS) of the hospital discharge report, according to the ICD-10 international classification of diseases and the Microbiology Information System. The HCUV is a university hospital with 777 beds that serves a population of 235,000 inhabitants.
The study was approved by the HCUV Ethics Committee under number PI 20-1806. Patient identification remained anonymous and the need for informed consent was waived due to the observational nature of the non-interventional study.
Laboratory proceduresConfirmation of COVID-19 was made by detecting SARS-CoV-2 virus RNA by real-time reverse transcription polymerase chain reaction (RT-PCR) testing on respiratory samples or by detecting IgG and IgM antibodies (Elecsys Anti-SARS-CoV-2®, Roche®, Mannheim, USA) in patients with clinical criteria for COVID-19.18
The diagnosis of bacterial or fungal infection was made following standard laboratory procedures.19 Microorganisms were identified by MALDI-TOF® mass spectrometry (Bruker Daltonik®, Bremen, Germany). An antibiotic sensitivity study was performed using the PHOENIX M50® system (Becton-Dickinson®, Sparks, USA) and/or E-test gradient diffusion strips (BioMérieux®, Marcy ĹEtoile, France). A yeast susceptibility test was performed by microdilution using a Sensititre YO10® plate (ThermoFisher Scientific®, Altrincham, United Kingdom). Antibiotic susceptibility was interpreted according to the criteria of the European Committee on Antimicrobial Susceptibility Testing v.10.0.20
Data collectionThe data was anonymised and collected in a database designed for this study. The variables were grouped into: demographic data, comorbidities, risk factors, number and type of infection, microbiological and analytical data, diagnostic imaging tests, antibiotic treatment (empirical and targeted) and progression during hospital admission. The variables were obtained from the patient's clinical history. Leukocyte, procalcitonin and C-reactive protein values were collected for each infectious process. In patients with multiple infections, interleukin-6, ferritin and D-dimer values recorded were those of the first infectious process diagnosed.
The patients were grouped into two categories based on their admission to the ICU (normal or extended). Patients with microbiological isolates considered contaminants were excluded from the study.
DefinitionsThe diagnosis of infection was based on clinical symptoms, the isolation of an aetiological agent and the assessment of the group of clinicians responsible for the patient. Bacteraemia/fungaemia was defined as the isolation of a pathogen in one or more blood cultures, and for microorganisms from the skin flora, its growth was assessed in two or more blood cultures extracted by different routes. For catheter-associated bacteraemia (CAB), the isolation of the same microorganism in blood and in the catheter tip (count ≥103 CFU) of samples obtained in parallel or in two blood cultures extracted simultaneously from the catheter and by venipuncture. Respiratory infection (RI) was considered the significant isolation of a potentially pathogenic microorganism in a bronchoalveolar lavage (BAL), in a bronchial aspirate (BA) or in evaluable sputum (>25 PMN and <10 epithelial cells × 100) or the detection of the Streptococcus pneumoniae antigen in urine by immunochromatography using a BinaxNOW® test (Abbot Diagnostics Scarbourg®, Maine, USA). For the diagnosis of invasive pulmonary aspergillosis, the Armstrong-James et al. algorithm21 was followed. Urinary tract infection (UTI) was defined as the significant isolation19 of a microorganism in the urine sample of a patient with signs and/or symptoms of infection. Diagnosis of skin and soft tissue infection (SSTI) was based on Gram stain (PMN and presence of bacteria or fungi) and agreement with the culture results.
Infections were classified as coinfections or superinfections. Coinfection was defined as a community-acquired infection diagnosed within the first 48 h of hospital admission for COVID-19. Superinfection was considered to be an infection acquired from 48 h after hospital admission.
Statistical analysisThe results were analysed using SPSS version 20.0® (SPSS®, Chicago, USA). Qualitative variables were compared using the χ2 test and quantitative variables using the Student’s t-test. Differences were considered significant if p < 0.05. The variables that showed a significant result in a univariate way were included in the backward stepwise multivariate logistic regression model to determine which of them were independently related to ICU admission and mortality.
ResultsDuring the study period, 712 patients were admitted to the HCUV for COVID-19 with a mortality during hospitalisation of 25% (178/712). Of the total number of patients, 113 (16%) presented bacterial or fungal infection and 17 (2%) were discarded because they did not meet the infection criteria. COVID-19 was confirmed by PCR in 108 patients and by detection of IgG and IgM antibodies in five patients.
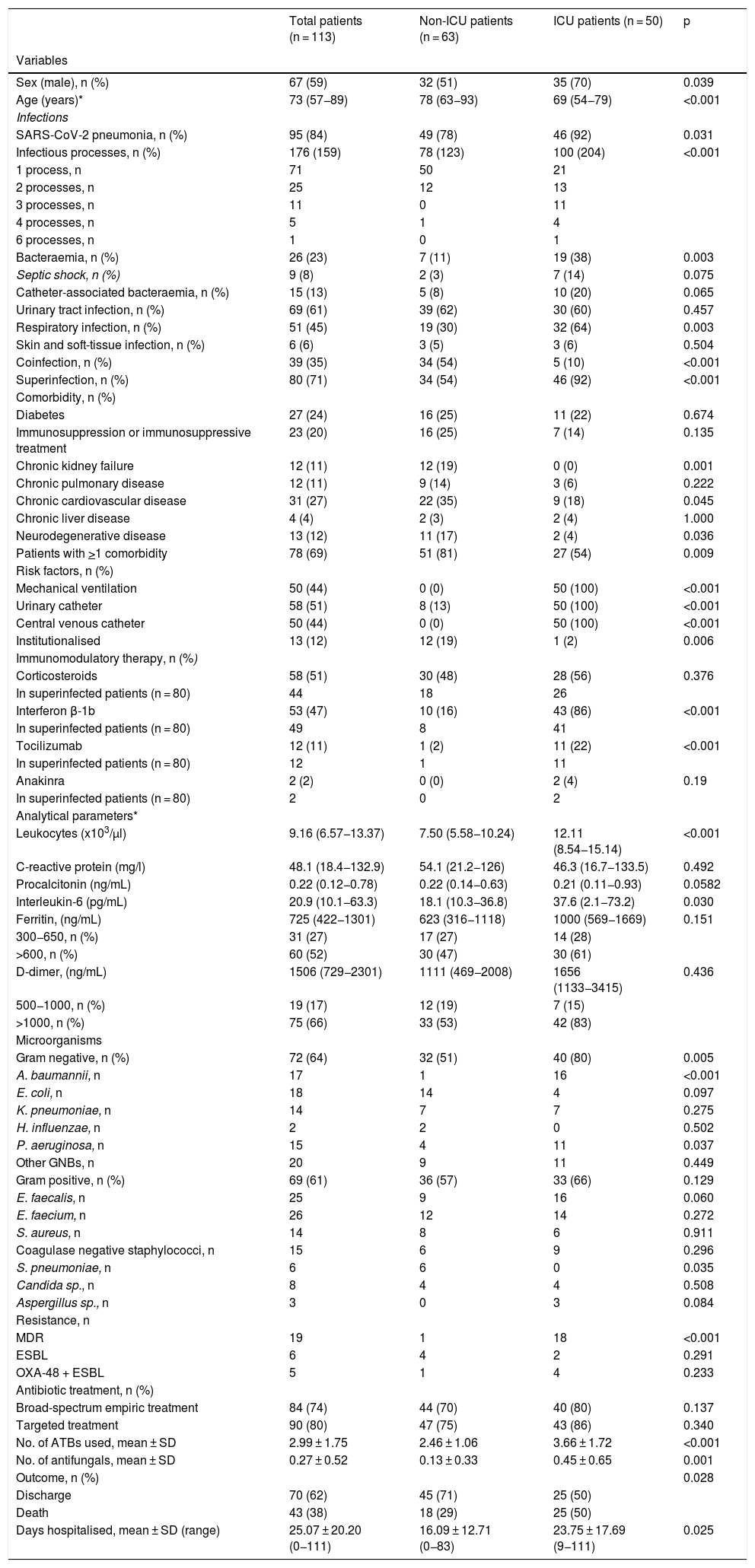
The main demographic, clinical and microbiological characteristics and statistical significance of the univariate analysis are shown in Table 1. The median age was 73 years (interquartile range [IQR] 57−89) and 59% were men. Fifty patients (44%) required admission to the ICU, with a median stay of 20 days (IQR 1–41). In 95 patients (84%), the main diagnosis was SARS-CoV-2 pneumonia, significantly higher in ICU patients (92% vs. 78%; p = 0.031). The ICU patients presented more infectious processes, mostly superinfections and a higher proportion of bacteraemia and RIs (p < 0.05). Overall, 69% of the patients had one or more comorbidities, with chronic heart disease (27%) and diabetes (24%) being the most frequent. The main risk factors for infection in patients admitted to the ICU were mechanical ventilation, central venous catheter, urinary catheter and the immunomodulators interferon beta-1b (IFN-β) and tocilizumab (p < 0.05). In total, 51% of patients with superinfection had received one or more immunomodulatory treatments.
Demographic, clinical and microbiological characteristics of patients with COVID-19 and bacterial or fungal infection.
| Total patients (n = 113) | Non-ICU patients (n = 63) | ICU patients (n = 50) | p | |
|---|---|---|---|---|
| Variables | ||||
| Sex (male), n (%) | 67 (59) | 32 (51) | 35 (70) | 0.039 |
| Age (years)* | 73 (57−89) | 78 (63−93) | 69 (54−79) | <0.001 |
| Infections | ||||
| SARS-CoV-2 pneumonia, n (%) | 95 (84) | 49 (78) | 46 (92) | 0.031 |
| Infectious processes, n (%) | 176 (159) | 78 (123) | 100 (204) | <0.001 |
| 1 process, n | 71 | 50 | 21 | |
| 2 processes, n | 25 | 12 | 13 | |
| 3 processes, n | 11 | 0 | 11 | |
| 4 processes, n | 5 | 1 | 4 | |
| 6 processes, n | 1 | 0 | 1 | |
| Bacteraemia, n (%) | 26 (23) | 7 (11) | 19 (38) | 0.003 |
| Septic shock, n (%) | 9 (8) | 2 (3) | 7 (14) | 0.075 |
| Catheter-associated bacteraemia, n (%) | 15 (13) | 5 (8) | 10 (20) | 0.065 |
| Urinary tract infection, n (%) | 69 (61) | 39 (62) | 30 (60) | 0.457 |
| Respiratory infection, n (%) | 51 (45) | 19 (30) | 32 (64) | 0.003 |
| Skin and soft-tissue infection, n (%) | 6 (6) | 3 (5) | 3 (6) | 0.504 |
| Coinfection, n (%) | 39 (35) | 34 (54) | 5 (10) | <0.001 |
| Superinfection, n (%) | 80 (71) | 34 (54) | 46 (92) | <0.001 |
| Comorbidity, n (%) | ||||
| Diabetes | 27 (24) | 16 (25) | 11 (22) | 0.674 |
| Immunosuppression or immunosuppressive treatment | 23 (20) | 16 (25) | 7 (14) | 0.135 |
| Chronic kidney failure | 12 (11) | 12 (19) | 0 (0) | 0.001 |
| Chronic pulmonary disease | 12 (11) | 9 (14) | 3 (6) | 0.222 |
| Chronic cardiovascular disease | 31 (27) | 22 (35) | 9 (18) | 0.045 |
| Chronic liver disease | 4 (4) | 2 (3) | 2 (4) | 1.000 |
| Neurodegenerative disease | 13 (12) | 11 (17) | 2 (4) | 0.036 |
| Patients with >1 comorbidity | 78 (69) | 51 (81) | 27 (54) | 0.009 |
| Risk factors, n (%) | ||||
| Mechanical ventilation | 50 (44) | 0 (0) | 50 (100) | <0.001 |
| Urinary catheter | 58 (51) | 8 (13) | 50 (100) | <0.001 |
| Central venous catheter | 50 (44) | 0 (0) | 50 (100) | <0.001 |
| Institutionalised | 13 (12) | 12 (19) | 1 (2) | 0.006 |
| Immunomodulatory therapy, n (%) | ||||
| Corticosteroids | 58 (51) | 30 (48) | 28 (56) | 0.376 |
| In superinfected patients (n = 80) | 44 | 18 | 26 | |
| Interferon β-1b | 53 (47) | 10 (16) | 43 (86) | <0.001 |
| In superinfected patients (n = 80) | 49 | 8 | 41 | |
| Tocilizumab | 12 (11) | 1 (2) | 11 (22) | <0.001 |
| In superinfected patients (n = 80) | 12 | 1 | 11 | |
| Anakinra | 2 (2) | 0 (0) | 2 (4) | 0.19 |
| In superinfected patients (n = 80) | 2 | 0 | 2 | |
| Analytical parameters* | ||||
| Leukocytes (x103/μl) | 9.16 (6.57−13.37) | 7.50 (5.58−10.24) | 12.11 (8.54−15.14) | <0.001 |
| C-reactive protein (mg/l) | 48.1 (18.4−132.9) | 54.1 (21.2−126) | 46.3 (16.7−133.5) | 0.492 |
| Procalcitonin (ng/mL) | 0.22 (0.12−0.78) | 0.22 (0.14−0.63) | 0.21 (0.11−0.93) | 0.0582 |
| Interleukin-6 (pg/mL) | 20.9 (10.1−63.3) | 18.1 (10.3−36.8) | 37.6 (2.1−73.2) | 0.030 |
| Ferritin, (ng/mL) | 725 (422−1301) | 623 (316−1118) | 1000 (569−1669) | 0.151 |
| 300−650, n (%) | 31 (27) | 17 (27) | 14 (28) | |
| >600, n (%) | 60 (52) | 30 (47) | 30 (61) | |
| D-dimer, (ng/mL) | 1506 (729−2301) | 1111 (469−2008) | 1656 (1133−3415) | 0.436 |
| 500−1000, n (%) | 19 (17) | 12 (19) | 7 (15) | |
| >1000, n (%) | 75 (66) | 33 (53) | 42 (83) | |
| Microorganisms | ||||
| Gram negative, n (%) | 72 (64) | 32 (51) | 40 (80) | 0.005 |
| A. baumannii, n | 17 | 1 | 16 | <0.001 |
| E. coli, n | 18 | 14 | 4 | 0.097 |
| K. pneumoniae, n | 14 | 7 | 7 | 0.275 |
| H. influenzae, n | 2 | 2 | 0 | 0.502 |
| P. aeruginosa, n | 15 | 4 | 11 | 0.037 |
| Other GNBs, n | 20 | 9 | 11 | 0.449 |
| Gram positive, n (%) | 69 (61) | 36 (57) | 33 (66) | 0.129 |
| E. faecalis, n | 25 | 9 | 16 | 0.060 |
| E. faecium, n | 26 | 12 | 14 | 0.272 |
| S. aureus, n | 14 | 8 | 6 | 0.911 |
| Coagulase negative staphylococci, n | 15 | 6 | 9 | 0.296 |
| S. pneumoniae, n | 6 | 6 | 0 | 0.035 |
| Candida sp., n | 8 | 4 | 4 | 0.508 |
| Aspergillus sp., n | 3 | 0 | 3 | 0.084 |
| Resistance, n | ||||
| MDR | 19 | 1 | 18 | <0.001 |
| ESBL | 6 | 4 | 2 | 0.291 |
| OXA-48 + ESBL | 5 | 1 | 4 | 0.233 |
| Antibiotic treatment, n (%) | ||||
| Broad-spectrum empiric treatment | 84 (74) | 44 (70) | 40 (80) | 0.137 |
| Targeted treatment | 90 (80) | 47 (75) | 43 (86) | 0.340 |
| No. of ATBs used, mean ± SD | 2.99 ± 1.75 | 2.46 ± 1.06 | 3.66 ± 1.72 | <0.001 |
| No. of antifungals, mean ± SD | 0.27 ± 0.52 | 0.13 ± 0.33 | 0.45 ± 0.65 | 0.001 |
| Outcome, n (%) | 0.028 | |||
| Discharge | 70 (62) | 45 (71) | 25 (50) | |
| Death | 43 (38) | 18 (29) | 25 (50) | |
| Days hospitalised, mean ± SD (range) | 25.07 ± 20.20 (0−111) | 16.09 ± 12.71 (0−83) | 23.75 ± 17.69 (9−111) | 0.025 |
The median and IQR of the inflammatory markers interleukin-6, ferritin and C-reactive protein were elevated in all patients, although only interleukin-6 was significantly higher in ICU patients.
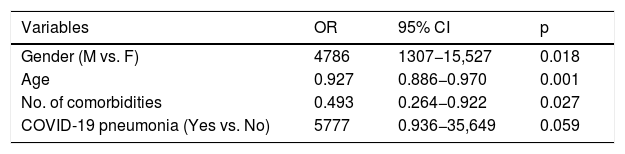
The predictive factors for admission to the ICU were: male gender (OR 4.32; 95% CI 0.96–19.48) and SARS-CoV-2 pneumonia (OR 5.77; 95% CI 0.936–35.649) (Table 2).
Predictive factors for admission to the ICU of patients with COVID-19 and bacterial/fungal infection. Multivariate regression model.
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Gender (M vs. F) | 4786 | 1307−15,527 | 0.018 |
| Age | 0.927 | 0.886−0.970 | 0.001 |
| No. of comorbidities | 0.493 | 0.264−0.922 | 0.027 |
| COVID-19 pneumonia (Yes vs. No) | 5777 | 0.936−35,649 | 0.059 |
Variables specified in step 1: sex, age, catheter-associated bacteraemia, septic shock, respiratory infection, no. of comorbidities, institutionalised, COVID-19 pneumonia, no. of antibiotics.
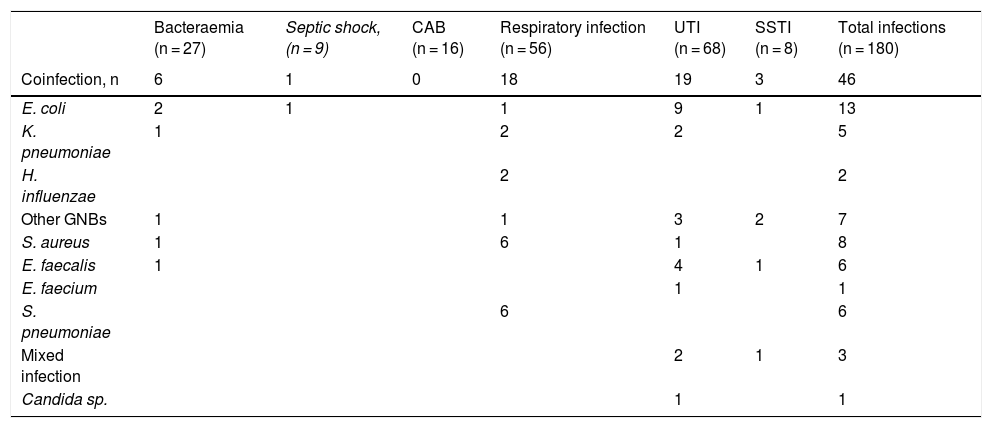
Overall, 5% of patients (39/712) had a bacterial/fungal coinfection. In total, 46 coinfections were documented, predominantly UTIs (19) and RIs (18). The main aetiologic agents were gram-negative bacilli (GNB) (27/46, 59%). E. coli (9/19, 47%) and E. faecalis (4/19, 21%) were the main aetiologic agents of the UTIs, and S. pneumoniae (6/18, 33%) and S. aureus (6/18, 33%) of the RIs. Five GNBs from these coinfections were extended spectrum beta-lactamase (ESBL) producers.
SuperinfectionsIn all, 11% (80/712) of the patients were superinfected, with 134 infections diagnosed: 94% bacterial and 6% fungal (Table 3). UTIs (49) predominated, followed by RIs (39) and bacteraemias (21). Eight patients had septic shock (7%). Of the total superinfections, 54% were due to GNB. The most frequent pathogens were E. faecium and A. baumannii. There was only one case of candidaemia due to C. glabrata. All the A. baumannii strains were multidrug resistant (MDR), and only sensitive to colistin. In 34 GNBs, one or several resistance mechanisms of special epidemiological interest were detected.
Aetiological agents, resistance mechanisms and analytical values in bacterial or fungal coinfections and superinfections in patients with COVID-19.
| Bacteraemia (n = 27) | Septic shock, (n = 9) | CAB (n = 16) | Respiratory infection (n = 56) | UTI (n = 68) | SSTI (n = 8) | Total infections (n = 180) | |
|---|---|---|---|---|---|---|---|
| Coinfection, n | 6 | 1 | 0 | 18 | 19 | 3 | 46 |
| E. coli | 2 | 1 | 1 | 9 | 1 | 13 | |
| K. pneumoniae | 1 | 2 | 2 | 5 | |||
| H. influenzae | 2 | 2 | |||||
| Other GNBs | 1 | 1 | 3 | 2 | 7 | ||
| S. aureus | 1 | 6 | 1 | 8 | |||
| E. faecalis | 1 | 4 | 1 | 6 | |||
| E. faecium | 1 | 1 | |||||
| S. pneumoniae | 6 | 6 | |||||
| Mixed infection | 2 | 1 | 3 | ||||
| Candida sp. | 1 | 1 |
| Superinfection, n | 21 | 8 | 16 | 39 | 53 | 5 | 134 |
|---|---|---|---|---|---|---|---|
| E. coli | 0 | 1 | 1 | 6 | 0 | 7 | |
| K. pneumoniae | 3 | 1 | 2 | 4 | 1 | 9 | |
| A. baumannii | 6 | 3 | 1 | 13 | 2 | 22 | |
| P. aeruginosa | 1 | 1 | 10 | 5 | 1 | 17 | |
| S. maltophilia | 1 | 1 | 2 | ||||
| Other GNBs | 3 | 8 | 3 | 1 | 15 | ||
| S. aureus | 1 | 2 | 1 | 5 | 1 | 9 | |
| E. faecalis | 3 | 13 | 2 | 19 | |||
| E. faecium | 6 | 16 | 1 | 24 | |||
| Coagulase negative staphylococci | 14 | 14 | |||||
| Mixed infection | 4 | 4 | 3 | 2 | 21 | ||
| Aspergillus sp. | 3 | 3 | |||||
| Candida sp. | 1 | 6 | 1 | 8 |
| Resistance mechanism, n | |||||
|---|---|---|---|---|---|
| ESBL | 2 | 1 | 5 | 1 | 9 |
| OXA-48 + ESBL | 2 | 2 | 2 | 6 | |
| MDR | 7 | 15 | 2 | 24 | |
| MRSA | 2 | 2 |
| Analytical data* | ||||||
|---|---|---|---|---|---|---|
| Leukocytes (x109/l) | 12.63 (8.35−15.10) | 8.300 (3.77−17.46) | 9.72 (6.5−12.21) | 13.4 ± 7.9 (1.9−15.66) | 8.46 (6.85−10.71) | 8.44 (5.72−10.74) |
| Procalcitonin (ng/mL) | 0.78 (0.24−1.11) | 0.78 (0.5−1.08) | 0.16 (0.11−1.43) | 0.35 (0.12−18.6) | 0.18 (0.11−0.35) | 0.15 (0.14−0.61) |
| CRP (μg/mL) | 91.8 (26.5−188.2) | 187.5 (92.3−330) | 76.6 (38.8−134.3) | 67.4 (32.1−173.1) | 50.1 (15.5−112.5) | 25.8 (5.7−168.5) |
CAB: catheter-associated bacteraemia; ESBL: extended spectrum beta-lactamase; SSTI: skin and soft tissue infection; UTI: urinary tract infection; OXA-48: OXA-48 class D carbapenemase; MDR: multidrug resistant; MRSA: methicillin-resistant S. aureus.
Only three patients admitted to the ICU met the criteria for probable invasive aspergillosis. All had comorbidities (stage IV non-Hodgkin lymphoma, obesity, hypertension, heart disease, chronic ischaemia and dyslipidaemia, multiple sclerosis), had received corticosteroid treatment, and had been on mechanical ventilation for more than 10 days. Given the clinical and radiological worsening, a fungal study was requested. In two of the patients Aspergillus fumigatus was isolated in repeated BAs and Aspergillus niger in the third one. The first patient was treated with voriconazole, which was replaced by isavuconazole due to liver disease, and he died 28 days after admission. The second received treatment with voriconazole and was discharged after 42 days of hospitalisation, whilst the third received isavuconazole, but died after 10 days.
The median values for leukocytes, C-reactive protein and procalcitonin were elevated in bacteraemia, septic shock and respiratory infection, although the results were widely dispersed (Table 3).
Antibiotic treatment and progressionIn all, 75% of the patients received broad-spectrum empirical antibiotic treatment on admission, mainly ceftriaxone, and 80% received targeted treatment (Table 1). The most used antibiotics were piperacillin/tazobactam (26), carbapenems (21), linezolid (20), and levofloxacin (15). All patients were treated on admission with hydroxychloroquine, azithromycin, ritonavir/lopinavir. No diarrhoea due to Clostridium difficile was found.
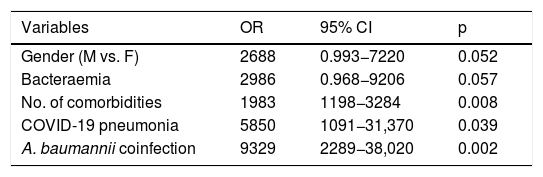
The mean stay was significantly longer in patients admitted to the ICU (23.75 ± 17.7 vs. 16.09 ± 12.71; p = 0.025). Overall mortality was 37%, significantly higher in critical patients (50% vs. 29%; p = 0.028). The independent predictors of mortality were: being male, with bacteraemia, various comorbidities, SARS-CoV-2 pneumonia and superinfection with A. baumannii (Table 4).
Predictors of mortality in patients with COVID-19 and bacterial/fungal infection. Multivariate regression model.
| Variables | OR | 95% CI | p |
|---|---|---|---|
| Gender (M vs. F) | 2688 | 0.993−7220 | 0.052 |
| Bacteraemia | 2986 | 0.968−9206 | 0.057 |
| No. of comorbidities | 1983 | 1198−3284 | 0.008 |
| COVID-19 pneumonia | 5850 | 1091−31,370 | 0.039 |
| A. baumannii coinfection | 9329 | 2289−38,020 | 0.002 |
Variables specified in step 1: sex, bacteraemia, septic shock, urinary tract infection, number of comorbidities, mechanical ventilation, urinary catheter, central venous catheter, orotracheal intubation, COVID-19 pneumonia, A. baumannii, treatment with interferon β-1b.
The incidence of bacterial/fungal coinfection and/or superinfection in patients with COVID-19 at the HCUV was 16%, similar to that of hospitals in Wuhan (China) (15%1–16%13) and higher than that of the Hospital Clínico in Barcelona (Spain) (7.3%)4 or to that reflected in the meta-analyses by Rawson et al. (8%)11 and by Lansbury et al. (7%, 95% CI 3–12)12. A differentiating fact with respect to these studies was the detection of an outbreak of MDR A. baumannii in the ICU, contributing to a 2% increase in incidence. However, it is difficult to make a comparison between the different studies due to the heterogeneity of the populations studied and the fact that several authors only analysed respiratory coinfection.
In line with other studies,1,2,5,16,22 men with COVID-19 and bacterial infection presented greater severity and mortality. The lower susceptibility of women to the SARS-CoV-2 virus could be due to the role of the X chromosome and sex hormones in innate and adaptive immunity.23 However, our work disagrees with other studies regarding age and comorbidity as predictors of severity,1,2,13 as patients admitted to the ICU were on average 10 years younger than ward patients and had less comorbidity. The characteristics of the population studied, with a median age higher than most series (median 56–70 years),1,2,4,22 as well as differences in criteria for hospital and ICU admission (overwhelmed services) could have influenced these results.
As in previous studies,4,11,12 the incidence of coinfection in our population was low (5%) and would not justify the high number of broad-spectrum antibiotics used empirically on hospital admission. In the meta-analysis by Rawson et al.,11 72% of COVID-19 patients were treated with antibiotics, rising to 80–100% in critically ill patients in ICUs.1,5,13 Given these data, several authors4,6,24 advocated implementing antibiotic treatment protocols based on scientific evidence, making rational use of them, weighing the risk/benefit based on the severity and comorbidity of the patient, and adapting the treatment to the epidemiology and antibiotic sensitivity of each health area.
Bacterial/fungal superinfection (11%) predominated in ICU patients, a result similar to that of Lansbury et al.12 (14%; 95% CI 5–26 vs. 4%, 95% CI 1–9) and Ripa et al.22 (9.3%). The high use of invasive medical devices in ICU patients predisposes to a higher rate of nosocomial infections, mainly respiratory, urinary and CAB.22 Another factor that could have influenced superinfection would be the rate of immunomodulators used, higher than that of patients with COVID-19 hospitalised in Spain.2,3 However, in the study by Ripa et al.22, patients with superinfections had received more treatment with biological immunomodulators (p = 0.045), but they did not find that it was a predictor of superinfection. The epidemiology of these infections is closely related to the predominant hospital flora. However, the outbreak caused by an unusual microorganism in our hospital made A. baumannii the first agent of respiratory superinfections and the second of bacteraemia. The COVID-19 isolation measures implemented to prevent horizontal transmission were not enough to contain the outbreak. The shortage of personal protective equipment and experienced personnel, together with stress, could have contributed to the appearance and extension of the outbreak.
The rates of bacteraemia and septic shock reported in the literature range from 1% to 36%4,25–27 and from 4% to 33%,1,27 respectively. Our septic shock data (8%) are similar to these studies, although the figures for total bacteraemia (23%) and in critical patients (38%) are above the average. Respiratory coinfection/superinfection data are the most studied by all authors, also yielding highly variable figures between 5% and 29%.5,24,27 Our figures, much higher globally (51/113, 45%) and in the ICU (32/50, 64%), are most likely influenced by the MDR A. baumannii outbreak, together with the complexity of the patients, some of whom were referred to the ICU from other hospitals in the region.
The most frequent pathogens of respiratory coinfection were S. pneumoniae and S. aureus, similar to other series4 and to the majority of coinfected viral pneumonias.8 There is little documentation in the literature on UTIs in patients with COVID-19, with the exception of the García-Vidal et al. cohort4, with 23.3% coinfections and 27.3% superinfections and a predominance of GNBs in both infections. Unlike in that study, gram-positive cocci predominated in our series.
Overall, 45% of the GNBs presented resistance of special epidemiological interest (ESBL and/or OXA 48 or MDR), reflecting a selection of the flora due to antibiotic pressure. In most of the series, GNBs basically predominate and it is common to find fungi, especially of the genera Aspergillus spp. and Candida spp.5,11,12,21,26 The role played by these isolates is often doubtful since, even following diagnostic guidelines supported by imaging techniques and other microbiological markers, it is difficult to establish whether it is a mere colonisation or a true infection. In our series, only three patients met the criteria for probable invasive aspergillosis. All three were treated with antifungal drugs and two died.
Inflammatory serological markers that are generally elevated in bacterial infection, such as procalcitonin and C-reactive protein, have low sensitivity and specificity,28–30 and can appear in patients with COVID-19 without causing a bacterial coinfection31. Although for Lv et al.5 they did prove to be useful. In this study, the mean value of C-reactive protein and procalcitonin were elevated in all infectious processes, but their great variability does not make them a reliable marker. In our opinion, more studies are needed to know their true usefulness in the diagnosis of coinfection, as well as research to find new biomarkers.
The mortality of patients admitted to our hospital's ICU with COVID-19 and bacterial coinfection or superinfection was extremely high (50%) compared to the mortality of patients admitted for COVID-19 in several other hospitals in Spain (21%3–28%2) or of patients admitted to ICUs in the United Kingdom (32%)16. And as in other influenza pandemics, coinfection/superinfection contributed to worsening the prognosis of the disease.8,9
This retrospective study has several limiting factors. Firstly, whilst clinically significant infections with microbiological documentation are reported, it is not always easy to distinguish between infection and colonisation, especially in catheterised and intubated patients, so UTI and RI may be overestimated. Secondly, samples were not systematically collected for microbiological study in patients with suspected infection, both due to the lack of invasive sampling (either out of fear or because services were overwhelmed) and due to the widespread use of broad-spectrum antibiotics at admission, so there could be some underdiagnosis.
ConclusionBacterial/fungal coinfection and superinfection in COVID-19 patients is lower than that of other respiratory virus infections, but it significantly increases the severity and mortality of these patients. The A. baumannii outbreak was one of the main determinants of severity and mortality in ICU patients. Diagnosing coinfection is complex but recognising it is vital. Diagnostic algorithms are needed for early and adequate detection and treatment of bacterial complications. A permanent review of hospital protocols will help to ensure that, despite the urgency of the work, the difficulty added by the use of personal protection equipment, the possibility of staff being poorly trained, and other unfavourable factors have as little influence as possible on the appearance of nosocomial outbreaks. Likewise, attention should be paid to later coinfections that may develop derived from the use of corticosteroids and other immunomodulators.
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
To Dr Isabel Antolín Ayala and Dr Milagros Cuervo Abarquero for their collaboration in the microbiological study of these patients. To María Fe Muñoz Moreno for carrying out the statistical study. To the staff of all departments at the HCUV, especially Internal Medicine and the Intensive Care Unit, for their great and intense work caring for patients with COVID-19.
Please cite this article as: Nebreda-Mayoral T, Miguel-Gómez MA, March-Rosselló GA, Puente-Fuertes L, Cantón-Benito E, Martínez-García AM, et al. Infección bacteriana/fúngica en pacientes con COVID-19 ingresados en un hospital de tercer nivel de Castilla y León, España. Enferm Infecc Microbiol Clin. 2022;40:158–165.