Malignant otitis externa (MOE), also called necrotising otitis externa, is a rare condition in Spain, with the annual incidence recently being estimated at 1.30 cases per million population.1 It generally affects older patients with poorly controlled diabetes or immunosuppression.1 After originating in the squamous epithelium of the external auditory canal (EAC), MOE can invade adjacent bone structures and lead to life-threatening skull base osteomyelitis. Although over 90% of episodes are caused by Pseudomonas aeruginosa, MOE caused by Aspergillus spp. is well reported in patients with human immunodeficiency virus (HIV) infection or neutropenia, with other fungal aetiologies being rarer.2–4 We present a case of MOE due to Candida albicans complicated by skull base osteomyelitis in a patient without predisposing factors and we discuss the role of echinocandins in the treatment of this unusual scenario.
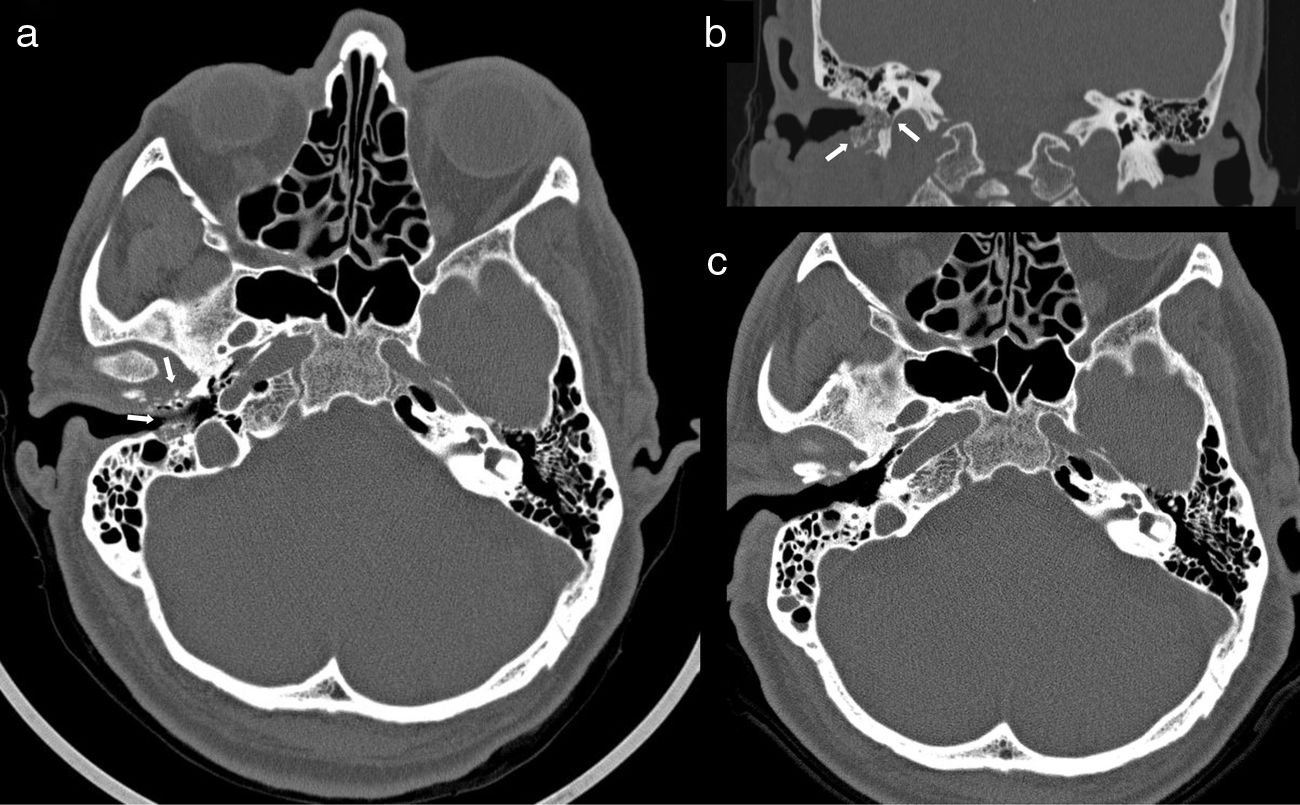
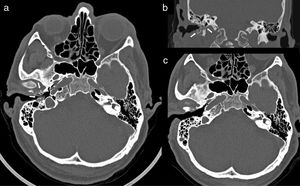
This was a 63-year-old male patient, originally from Ecuador but resident in Spain for over 20 years, whose previous medical history included hypertension, dyslipidaemia, subclinical hypothyroidism and gout. His usual treatment consisted of bisoprolol, simvastatin and acetylsalicylic acid. The symptoms had begun at least two months before the initial consultation and consisted of earache, hearing loss and otorrhoea from the right ear (RE). Otoscope examination at that time showed an EAC with oedematous and erythematous walls, with the eardrum intact. After a cycle of topical treatment with dexamethasone and gentamicin, repeat otoscope examination revealed a laceration in the floor of the EAC exposing bone tissue, with an inflammatory reaction and abundant otorrhoea. The patient reported progressive worsening of the earache and preauricular pain despite analgesic treatment. Once the clinical diagnosis of MOE was established, treatment was started with oral and topical ciprofloxacin and a computed tomography (CT) scan of the ears and mastoids was requested. The CT scan showed areas of osteolytic rarefaction affecting the walls of the right EAC, with bone sequestration and small gas bubbles, findings consistent with skull base osteomyelitis secondary to MOE (Fig. 1a and b). The patient was operated on using a retro-auricular approach, confirming the bone destruction of the posterior, inferior and anterior walls of the EAC, and was completed with meatoplasty and canaloplasty by milling (until a flat area of healthy bone tissue was obtained) and subsequent coverage with a temporal fascia graft. Culture of all three samples obtained during the procedure (including bone tissue and soft tissue) was positive for C. albicans, with a large number of colonies and no other microbiological isolates. The EAC skin biopsy showed fibrous connective tissue and necrobiotic material, with no evidence of malignancy or demonstration of fungal structures. Under direct questioning, the patient denied any history of ear trauma or gardening activities. Basic immunodeficiency screening was negative, and repeat basal blood glucose levels and glycated haemoglobin (5.7%) were both normal. The patient was given a three-week cycle of anidulafungin (200mg loading dose followed by 100mg every 24h), and treatment was subsequently completed with fluconazole (400mg every 24h) orally for three months and topical cleaning of the RE with boric acid alcohol solution. The patient's earache and otorrhoea gradually improved and finally disappeared, and he remains asymptomatic at the time of writing. A follow-up CT scan five months later showed the complete normalisation of the anatomy of the EAC and the mastoid cells (Fig. 1c).
Computed tomography (CT) of ears and mastoids with axial (a) and coronal images (b) performed at the time of diagnosis of skull base osteomyelitis associated with right malignant otitis externa (MOE), which shows areas of osteolysis affecting the anterior and posterior walls and the floor of the external auditory canal, with sequestrations and small gas bubbles inside the compact bone and increased soft tissue inflammation (white arrows); (c) follow-up CT performed five months after surgical debridement and after a 3-month cycle of antifungal treatment confirming the resolution of MOE and of the osteomyelitis component.
As a rare condition, the few examples of fungal MOE in the literature are mainly caused by Aspergillus fumigatus and are limited to patients with diabetes or with various forms of immunosuppression (advanced HIV infection, acute myeloid leukaemia with profound neutropenia, steroid treatment or primary phagocytic disorders).2–4 Although in our case there was no histological study of the bone tissue from the walls of the EAC to demonstrate tissue invasion by fungal structures, the isolation of C. albicans in all the intraoperative samples, the absence of alternative agents and the response to the antifungal treatment gave us a high degree of certainty about the diagnosis. In the largest series of fungal MOE published to date (nine cases diagnosed in a single centre over 18 years), 89% of patients had a history of diabetes.5 Compared to the cases produced by P. aeruginosa, patients with fungal MOE were more likely to have paralysis of the seventh cranial nerve and skull base involvement throughout the radiological follow-up.5 A recent systematic review of the literature from the year 2000 onwards identified 25 cases of fungal MOE, out of which Candida spp. was the causative microorganism in seven (all patients had diabetes).3 That makes the absence of apparent risk factors in our case quite striking. The Netea group investigated innate and adaptive immunity at a functional level in a series of six patients without underlying immunosuppression who had developed skull base osteomyelitis caused by Aspergillus. Compared to healthy controls, a lower production of interleukin (IL)-17 and IL-22 was observed in peripheral blood mononuclear cells subjected to a specific antigen stimulus for A. fumigatus and C. albicans, suggesting a deficient Th17 response against fungal pathogens, which could act as a predisposing factor.6
While there is no established optimal approach to fungal MOE, the reversal of the underlying factors (particularly adequate metabolic control of diabetes) combined with extensive surgical debridement and prolonged antifungal treatment seems to be key. In the aforementioned series, surgical debridement was necessary in 78% of fungal MOE cases, compared to only 18% of patients with bacterial aetiology infection (mostly P. aeruginosa) who required such a procedure.5 In terms of the antifungal regimen, most of the published cases were given a triazole (fluconazole, voriconazole or itraconazole), sometimes associated with amphotericin B for the first few weeks.2,3,5 The average duration of treatment in the systematic review of the literature was 178 days3, although it is not possible to establish a firm recommendation in this regard. An echinocandin (caspofungin) was only used in one case of MOE by A. flavus as part of the treatment (which also included hyperbaric therapy and a prolonged course of voriconazole), obtaining a favourable outcome.7 In our patient, fluconazole treatment was continued for a total of 110 days, in line with the average duration of 100 days reported in the Hamzany et al. series.5 It is possible that the absence of immunosuppression, the administration of an induction cycle with echinocandin and the lower virulence of Candida compared to filamentous fungi contributed to the marked clinical improvement, negating the need for longer courses of treatment. Although the treatment of MOE with skull base involvement is not specifically addressed, the most recent clinical practice guidelines recommend the use of fluconazole (6mg/kg every 24h) for 6–12 months, either alone or preceded by at least two weeks of an echinocandin, for Candida osteomyelitis.8 An experimental model of A. fumigatus otitis media showed that the use of caspofungin for seven days produced a clinical, microbiological and histological response comparable to that obtained with amphotericin B.9 As our experience shows, the fact that echinocandins are able to penetrate bone tissue10, combined with their good safety profile, makes this group of antifungals an attractive option for the treatment of Candida MOE extending to the skull base.
FundingMFR has a “Miguel Servet” contract (CP18/00073) and MRR a “Río Hortega” research training contract (CM17/00098), in both cases with Instituto de Salud Carlos III [Carlos III Health Institute], Ministerio de Ciencia, Innovación y Universidades [Spanish Ministry of Science, Innovation and Universities] (Spain).
Please cite this article as: Fernández-Ruiz M, Ruiz-Ruigómez M, Montojo J. Osteomielitis de la base del cráneo secundaria a otitis externa maligna por Candida albicans: papel del tratamiento con equinocandina asociado a desbridamiento quirúrgico. Enferm Infecc Microbiol Clin. 2020;38:89–91.