To describe the characteristics of patients diagnosed with Mycoplasma pneumoniae infection.
MethodsA retrospective study of clinical and epidemiological characteristics of acute infections by M. pneumoniae confirmed by PCR was carried out in the Navarra Health Service (Spain) in 2014–2018.
ResultsM. pneumoniae infection was confirmed in 9.5% of analyzed patients. Among 123 confirmed cases, 65% were 5–14 years old, 21.1% <5 years old, and 13.8% were ≥14 years old. Pneumonia was radiologically confirmed in 83.7% of cases, and 22.0% presented extra-respiratory manifestations. A total of 44.7% of cases required hospitalization. Bilateral pneumonia, asthmatic crisis and extra-respiratory manifestations were associated to higher risk of hospitalization (81.3, 72.2 and 66.7%, respectively). Microbiological targeted treatment was monotherapy with macrolides in 60.2% of cases and combined with other antibiotics in 13.0%.
ConclusionM. pneumoniae was the cause of acute respiratory infection affecting mainly to children younger than 14 years old and frequently required hospitalization.
Describir las características de pacientes diagnosticados de infección por Mycoplasma pneumoniae.
MétodosSe realizó un estudio retrospectivo de las características clínicas y epidemiológicas de las infecciones agudas por M. pneumoniae confirmadas por PCR en el Servicio Navarro de Salud entre 2014 y 2018.
ResultadosSe confirmó M. pneumoniae en el 9,5% de los pacientes analizados. Entre 123 casos confirmados, el 65% tenían 5-14 años; el 21,1%, <5 años y el 13,8%, ≥14 años. En el 83,7% se confirmó neumonía y el 22,0% presentó manifestaciones extrarrespiratorias. El 44,7% de los pacientes requirieron ingreso hospitalario. La neumonía bilateral, las crisis asmáticas y los síntomas extrarrespiratorios se asociaron a mayor riesgo de hospitalización (81,3; 72,2 y 66,7%, respectivamente). El tratamiento dirigido fue solo con macrólidos en el 60,2% y combinado con otro antibiótico en el 13,0%.
ConclusiónM. pneumoniae es causa de enfermedad respiratoria aguda principalmente en menores de 14 años y requiere, con frecuencia, hospitalización.
Mycoplasma pneumoniae is an intracellular bacterium to which 20%–40% of community-acquired pneumonias and 10%–20% of pneumonias in hospitalised children are attributed1. Pneumonia affects children and adults worldwide, with epidemic peaks every 3–7 years2.
The relationship of this bacterium with asthma has been demonstrated in different studies addressing both exacerbations and their severity3,4. The respiratory symptoms caused by M. pneumoniae are similar to those of other atypical pneumonias5. Extrapulmonary manifestations appear in up to 25% of cases6 and can be neurological, cutaneous, gastrointestinal, cardiovascular, musculoskeletal, haematological and renal, and may occur in isolation, before, during or after the respiratory symptoms7.
Hitherto, serology has been the diagnostic method par excellence, although molecular techniques (polymerase chain reaction [PCR]) offer a rapid and reliable diagnostic alternative.
Macrolides are the treatment of choice, especially in children, due to their limited side effects8.
The objective of this study was to describe the epidemiological and clinical characteristics of patients diagnosed with M. pneumoniae infection and the treatment they received.
Materials and methodsA retrospective descriptive study was conducted on all cases of acute M. pneumoniae infection diagnosed by PCR at the Navarre Health Service between 1 January 2014 and 31 December 2018.
The Microbiology databases were used to analyse the cases of M. pneumoniae infection diagnosed by PCR. In addition, an informative document outlining the objectives of the study was sent by email, requesting the patient’s or their guardian’s informed consent. Patients whose consent was obtained were included in the clinical history data study.
The samples analysed were: pharyngeal swabs, nasopharyngeal aspirates/exudates and sputum. DNA extraction was performed by the MagCore HF16 (RBC Bioscience)® or EZ1 Advanced (Quiagen®, Germany) automatic systems and amplification and detection by FTD Atypical CAP® (Fast-Track Diagnostics, Luxembourg). The sequence of the probe and the characteristics of the primers are unknown (not disclosed by the manufacturer).
In some patients, a serological study with determination of IgG or IgM by means of particle agglutination or chemiluminescence was also available.
Information on sex, age, date of diagnosis and manifestations was obtained from the computerised clinical history. The clinical manifestations taken into account were: maximum temperature measured; cough or bronchitis; nasal congestion; chest pain, vomiting; diarrhoea; hypertransaminasaemia; exanthema, urticaria; and neurological, cardiovascular and haematological manifestations.
Categorical variables are presented as number and percentage and continuous variables as median and interquartile range (IQR). The analyses were stratified by age groups. The variables associated with a higher probability of hospitalisation among the cases were analysed. The χ2 test and Fisher’s exact test were used to compare proportions.
ResultsDuring the study period, 1426 PCR determinations were performed on 1345 patients and a positive PCR for M. pneumoniae was obtained in 136 patients (9.5%).
The annual positivity between 2014 and 2018 was: 21.4% (25/117); 7.7% (14/182); 5.7% (18/318); 5.9% (19/317) and 12.2% (60/492), respectively.
Informed consent was obtained in 123 cases (90.4%). The incidence was higher in 2018, with 49 cases, compared to an annual median of 19 cases, and in the average across the years, peaks were apparent in June and August, when 36.6% more cases than the monthly average were recorded, and in December and January, with 26.8% more; troughs were observed in April and September, with 41.5% fewer cases than the monthly average.
The type of sample was nasopharyngeal exudate in 74 (60.1%), pharyngeal swab in 47 (38.2%) and sputum in 2 (1.6%). Serology was performed in only 29 patients, 19 (65.5%) of whom were positive for IgM, 7 (24.1%) were negative for IgM and IgG and 3 were positive for IgG and negative for IgM. The serology was repeated in 2 patients after 4 weeks, when seroconversion was confirmed.
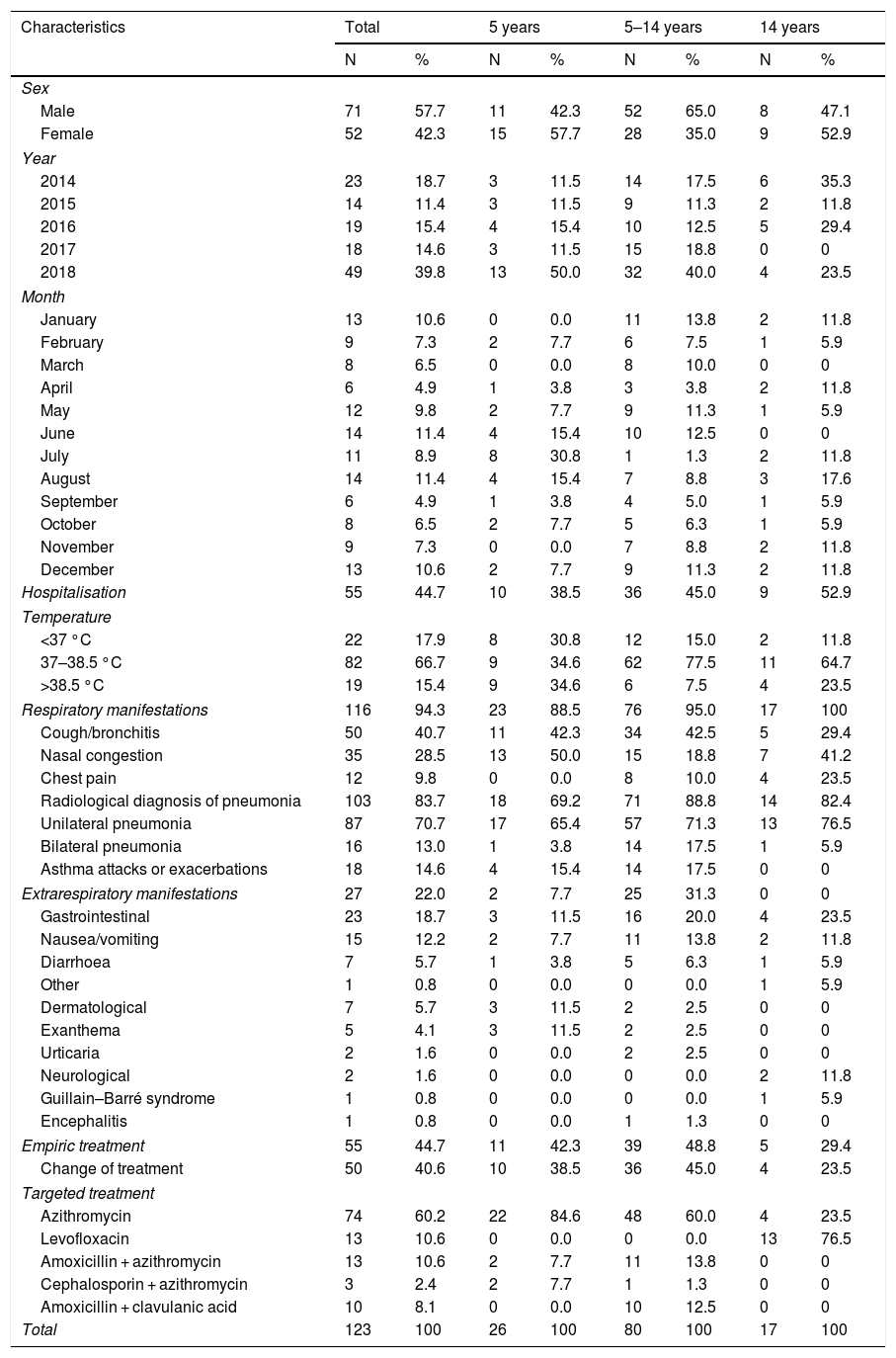
Of the 123 patients included in the study, 57.7% were male, 65.0% were between 5 and 14 years old, 19.5% were younger than 5 years old and 15.4% were older than 14 years (Table 1).
Epidemiological characteristics of the cases included in the study.
| Characteristics | Total | 5 years | 5–14 years | 14 years | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 71 | 57.7 | 11 | 42.3 | 52 | 65.0 | 8 | 47.1 |
| Female | 52 | 42.3 | 15 | 57.7 | 28 | 35.0 | 9 | 52.9 |
| Year | ||||||||
| 2014 | 23 | 18.7 | 3 | 11.5 | 14 | 17.5 | 6 | 35.3 |
| 2015 | 14 | 11.4 | 3 | 11.5 | 9 | 11.3 | 2 | 11.8 |
| 2016 | 19 | 15.4 | 4 | 15.4 | 10 | 12.5 | 5 | 29.4 |
| 2017 | 18 | 14.6 | 3 | 11.5 | 15 | 18.8 | 0 | 0 |
| 2018 | 49 | 39.8 | 13 | 50.0 | 32 | 40.0 | 4 | 23.5 |
| Month | ||||||||
| January | 13 | 10.6 | 0 | 0.0 | 11 | 13.8 | 2 | 11.8 |
| February | 9 | 7.3 | 2 | 7.7 | 6 | 7.5 | 1 | 5.9 |
| March | 8 | 6.5 | 0 | 0.0 | 8 | 10.0 | 0 | 0 |
| April | 6 | 4.9 | 1 | 3.8 | 3 | 3.8 | 2 | 11.8 |
| May | 12 | 9.8 | 2 | 7.7 | 9 | 11.3 | 1 | 5.9 |
| June | 14 | 11.4 | 4 | 15.4 | 10 | 12.5 | 0 | 0 |
| July | 11 | 8.9 | 8 | 30.8 | 1 | 1.3 | 2 | 11.8 |
| August | 14 | 11.4 | 4 | 15.4 | 7 | 8.8 | 3 | 17.6 |
| September | 6 | 4.9 | 1 | 3.8 | 4 | 5.0 | 1 | 5.9 |
| October | 8 | 6.5 | 2 | 7.7 | 5 | 6.3 | 1 | 5.9 |
| November | 9 | 7.3 | 0 | 0.0 | 7 | 8.8 | 2 | 11.8 |
| December | 13 | 10.6 | 2 | 7.7 | 9 | 11.3 | 2 | 11.8 |
| Hospitalisation | 55 | 44.7 | 10 | 38.5 | 36 | 45.0 | 9 | 52.9 |
| Temperature | ||||||||
| <37 °C | 22 | 17.9 | 8 | 30.8 | 12 | 15.0 | 2 | 11.8 |
| 37–38.5 °C | 82 | 66.7 | 9 | 34.6 | 62 | 77.5 | 11 | 64.7 |
| >38.5 °C | 19 | 15.4 | 9 | 34.6 | 6 | 7.5 | 4 | 23.5 |
| Respiratory manifestations | 116 | 94.3 | 23 | 88.5 | 76 | 95.0 | 17 | 100 |
| Cough/bronchitis | 50 | 40.7 | 11 | 42.3 | 34 | 42.5 | 5 | 29.4 |
| Nasal congestion | 35 | 28.5 | 13 | 50.0 | 15 | 18.8 | 7 | 41.2 |
| Chest pain | 12 | 9.8 | 0 | 0.0 | 8 | 10.0 | 4 | 23.5 |
| Radiological diagnosis of pneumonia | 103 | 83.7 | 18 | 69.2 | 71 | 88.8 | 14 | 82.4 |
| Unilateral pneumonia | 87 | 70.7 | 17 | 65.4 | 57 | 71.3 | 13 | 76.5 |
| Bilateral pneumonia | 16 | 13.0 | 1 | 3.8 | 14 | 17.5 | 1 | 5.9 |
| Asthma attacks or exacerbations | 18 | 14.6 | 4 | 15.4 | 14 | 17.5 | 0 | 0 |
| Extrarespiratory manifestations | 27 | 22.0 | 2 | 7.7 | 25 | 31.3 | 0 | 0 |
| Gastrointestinal | 23 | 18.7 | 3 | 11.5 | 16 | 20.0 | 4 | 23.5 |
| Nausea/vomiting | 15 | 12.2 | 2 | 7.7 | 11 | 13.8 | 2 | 11.8 |
| Diarrhoea | 7 | 5.7 | 1 | 3.8 | 5 | 6.3 | 1 | 5.9 |
| Other | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 5.9 |
| Dermatological | 7 | 5.7 | 3 | 11.5 | 2 | 2.5 | 0 | 0 |
| Exanthema | 5 | 4.1 | 3 | 11.5 | 2 | 2.5 | 0 | 0 |
| Urticaria | 2 | 1.6 | 0 | 0.0 | 2 | 2.5 | 0 | 0 |
| Neurological | 2 | 1.6 | 0 | 0.0 | 0 | 0.0 | 2 | 11.8 |
| Guillain–Barré syndrome | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 5.9 |
| Encephalitis | 1 | 0.8 | 0 | 0.0 | 1 | 1.3 | 0 | 0 |
| Empiric treatment | 55 | 44.7 | 11 | 42.3 | 39 | 48.8 | 5 | 29.4 |
| Change of treatment | 50 | 40.6 | 10 | 38.5 | 36 | 45.0 | 4 | 23.5 |
| Targeted treatment | ||||||||
| Azithromycin | 74 | 60.2 | 22 | 84.6 | 48 | 60.0 | 4 | 23.5 |
| Levofloxacin | 13 | 10.6 | 0 | 0.0 | 0 | 0.0 | 13 | 76.5 |
| Amoxicillin + azithromycin | 13 | 10.6 | 2 | 7.7 | 11 | 13.8 | 0 | 0 |
| Cephalosporin + azithromycin | 3 | 2.4 | 2 | 7.7 | 1 | 1.3 | 0 | 0 |
| Amoxicillin + clavulanic acid | 10 | 8.1 | 0 | 0.0 | 10 | 12.5 | 0 | 0 |
| Total | 123 | 100 | 26 | 100 | 80 | 100 | 17 | 100 |
At the time of diagnosis, 94.3% of the patients presented symptoms of acute respiratory infection. Pneumonia was radiologically confirmed in 103 patients (83.7%), which was unilateral in 87 (84.5%) and bilateral in 16 (15.5%) cases. 14.6% of the patients had asthma attacks or exacerbations.
Of the 123 patients, 27 (22.0%) had extrarespiratory symptoms, which were gastrointestinal in 23 (18.7%) patients, dermatological in 7 (5.6%) patients and serious neurological in 2 (1.6%) patients. One patient presented Guillain–Barré syndrome and the other encephalitis accompanied by pneumonia (Table 1). There were no cases with cardiovascular or haematological manifestations.
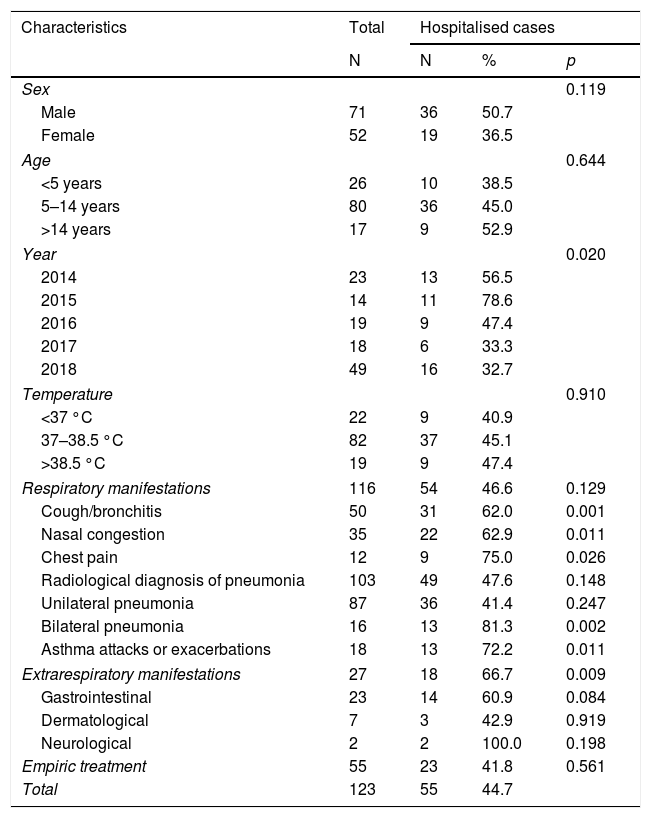
44.7% of the patients required hospitalisation, with a median stay of 4 days (IQR: 3–5). Bilateral pneumonia, asthmatic complications and extrarespiratory manifestations were associated with a higher risk of hospitalisation (81.3%, 72.2%, and 66.7%, respectively) (Table 2).
Hospitalisation of cases of Mycoplasma pneumoniae infection based on different characteristics.
| Characteristics | Total | Hospitalised cases | ||
|---|---|---|---|---|
| N | N | % | p | |
| Sex | 0.119 | |||
| Male | 71 | 36 | 50.7 | |
| Female | 52 | 19 | 36.5 | |
| Age | 0.644 | |||
| <5 years | 26 | 10 | 38.5 | |
| 5–14 years | 80 | 36 | 45.0 | |
| >14 years | 17 | 9 | 52.9 | |
| Year | 0.020 | |||
| 2014 | 23 | 13 | 56.5 | |
| 2015 | 14 | 11 | 78.6 | |
| 2016 | 19 | 9 | 47.4 | |
| 2017 | 18 | 6 | 33.3 | |
| 2018 | 49 | 16 | 32.7 | |
| Temperature | 0.910 | |||
| <37 °C | 22 | 9 | 40.9 | |
| 37–38.5 °C | 82 | 37 | 45.1 | |
| >38.5 °C | 19 | 9 | 47.4 | |
| Respiratory manifestations | 116 | 54 | 46.6 | 0.129 |
| Cough/bronchitis | 50 | 31 | 62.0 | 0.001 |
| Nasal congestion | 35 | 22 | 62.9 | 0.011 |
| Chest pain | 12 | 9 | 75.0 | 0.026 |
| Radiological diagnosis of pneumonia | 103 | 49 | 47.6 | 0.148 |
| Unilateral pneumonia | 87 | 36 | 41.4 | 0.247 |
| Bilateral pneumonia | 16 | 13 | 81.3 | 0.002 |
| Asthma attacks or exacerbations | 18 | 13 | 72.2 | 0.011 |
| Extrarespiratory manifestations | 27 | 18 | 66.7 | 0.009 |
| Gastrointestinal | 23 | 14 | 60.9 | 0.084 |
| Dermatological | 7 | 3 | 42.9 | 0.919 |
| Neurological | 2 | 2 | 100.0 | 0.198 |
| Empiric treatment | 55 | 23 | 41.8 | 0.561 |
| Total | 123 | 55 | 44.7 | |
Empiric treatment was started prior to microbiological diagnosis in 55 (44.7%) patients, in 50.9% with ß-lactam antibiotics. After diagnosis, treatment was changed in 50 patients. Targeted treatment was initiated in a median of 3 days (IQR: 2–4 days), and azithromycin was used in 74 (60.2%) patients, 95% of whom were under 14 years of age; levofloxacin was used in 13 (10.6%) patients, all older than 15 years; and amoxicillin combined with azithromycin in 13 (10.6%) patients and ceftriaxone with azithromycin in 3 (2.4%) patients. Following the initiation of active antibiotic treatment, the evolution was favourable in all cases.
DiscussionIn the series of patients analysed, M. pneumoniae presented mainly as a cause of atypical pneumonia in children and adolescents. Without microbiological confirmation, the clinical symptoms may be indistinguishable from those caused by other bacteria or viruses1,5.
Significant annual and seasonal oscillations in the number of cases were detected, the highest incidence being in 2018 and in July–August and December–January. Epidemic outbreaks are described in the literature every 3–7 years5.
In line with previous studies, M. pneumoniae infections were more common in children and adolescents9,10.
In our study, 44.7% of the patients required hospitalisation, a situation that was associated with the diagnosis of bilateral pneumonia, asthma complications or significant extrarespiratory manifestations. This concurs with other studies, which have also related it to ineffective initial treatment, immunosuppression and comorbidities1.
There is no consensus on a reference technique for the diagnosis of M. pneumoniae. Therefore, to improve sensitivity, it is recommended that molecular techniques and serology be combined11. In this work, we studied cases confirmed by PCR, and the proportion of cases with confirmatory serology was low.
14.6% of the cases studied presented asthmatic manifestations. Other studies had associated it with exacerbations, worsening and decreased function and with the risk of developing asthma without a history of atopy12.
Extrarespiratory manifestations occur in about 25% of patients6; dermatological manifestations are noteworthy for being the most common12 and neurological manifestations for being the most serious6. In our study, dermatological manifestations were infrequent, and evolution was favourable after the administration of the antibiotic. Guillain–Barré syndrome was recorded in one adult patient, who healed without sequelae, and encephalitis in another, who also developed pneumonia. Gastrointestinal symptoms (vomiting and diarrhoea) were the most common extrarespiratory manifestations in our study, in line with studies by other authors1.
Although M. pneumoniae infections may resolve without treatment, treatment is mostly necessary for the symptoms to improve, particularly in cases with extrapulmonary manifestations13. The antibiotics of choice are macrolides, which can be used in children14,15. Azithromycin is currently preferred because it is better tolerated and easy to administer. All our patients were treated with antibiotics, of which azithromycin was the most frequently used, while levofloxacin was used in patients over 14 years.
The main limitations of the study are its retrospective nature in a single centre, the limited sensitivity of the samples, the small number of cases and the fact that mild cases may have been under-represented.
In conclusion, in our study, 9.5% of the samples analysed by PCR were positive for M. pneumoniae, which was the cause of acute respiratory disease mainly in children under 14 years of age and frequently required hospitalisation. Most of the cases presented pneumonia, and 22.0% extrarespiratory manifestations. Bilateral pneumonia, asthma attacks and extrarespiratory symptoms were associated with a higher risk of hospitalisation. The importance of targeted treatment based on microbiological diagnosis has been demonstrated.
Conflicts of interestNone.
Please cite this article as: Álvaro Varela AI, Aguinaga Pérez A, Navascués Ortega A, Castilla J, Ezpeleta Baquedano C. Características clínicas de pacientes con infección por Mycoplasma pneumoniae. Enferm Infecc Microbiol Clin. 2022;40:449–452.