Over recent years we have witnessed an increase in the resistance of microorganisms to the available antimicrobials and a decrease in the number of new antimicrobials. Fosfomycin is a safe and cheap broad-spectrum antibiotic which has shown very promising results in combination therapy, mainly against gram-negative microorganisms. Little is known, however, about its clinical efficacy against gram-positive microorganisms.
MethodsWe performed a retrospective review of all patients with severe gram-positive infections who received fosfomycin as part of their treatment from 2011 to 2017. We also performed in vitro time-kill assays to study the behaviour of fosfomycin with different antimicrobials against two strains of methicillin-resistant Staphylococcus aureus (MRSA) and two strains of methicillin-susceptible S. aureus (MSSA).
ResultsSeventy-five patients were treated with different fosfomycin combinations. Among them, 61 (81%) were successfully treated. Daptomycin plus fosfomycin was the most effective combination. Overall, the treatment with fosfomycin was safe, and side effects were minor. There was only one major side effect that resolved after discontinuation of therapy. Time-kill studies demonstrated increased activity of fosfomycin combinations, with daptomycin•fosfomycin being the most active combination against both MRSA and MSSA strains.
ConclusionsOur results suggest that antimicrobial combinations including fosfomycin are an alternative and effective approach for gram-positive infections.
En los últimos años se ha ido produciendo un aumento de la resistencia de los microorganismos a los antimicrobianos disponibles, y una disminución en el número de nuevos antimicrobianos. La fosfomicina es un antibiótico seguro y barato con un amplio espectro de actividad, que ha mostrado resultados muy prometedores en terapia de combinación, principalmente contra microorganismos gramnegativos. Sin embargo, poco se conoce sobre su eficacia clínica frente a microorganismos grampositivos.
Mèc)todosRevisión retrospectiva de todos los pacientes con infecciones graves por microorganismos grampositivos que recibieron fosfomicina como parte de su tratamiento, entre los años 2011 y 2017. Tambièc)n se realizaron curvas de letalidad in vitro para estudiar el comportamiento de la fosfomicina con diferentes antimicrobianos, frente a 2 cepas de Staphylococcus aureus resistentes a meticilina (SARM) y 2 cepas de S. aureus sensible a la meticilina (SASM).
ResultadosSetenta y cinco pacientes recibieron tratamiento con diferentes combinaciones de fosfomicina. De ellos, 61 (81%) fueron tratados con èc)xito. Daptomicina más fosfomicina fue la combinación más efectiva. En general, el tratamiento con fosfomicina fue seguro, con efectos secundarios menores. Hubo solo un efecto secundario importante que se resolvió tras la suspensión del tratamiento. Las curvas de letalidad demostraron buena actividad de las combinaciones de fosfomicina, siendo la combinación daptomicina-fosfomicina la más activa, tanto frente a las cepas de SARM como de SASM.
ConclusionesNuestros resultados sugieren que combinaciones con fosfomicina, pueden ser un tratamiento alternativo y efectivo en infecciones por grampositivos.
Infections due to multidrug resistant microorganisms are an increasing problem and remain one of the biggest challenges in the treatment of infectious diseases. New multi-resistant profiles had been reported for gram-positive cocci. Methicillin-resistant Staphylococcus aureus (MRSA) and enterococci with vancomycin (VAN) and linezolid (LNZ) resistance or decreased susceptibility to daptomycin (DAP) had been notified. Moreover, new mechanisms of resistance have been recently found in Europe such as betalactamase production in Enterococcus faecium.1,2
The optimal treatment for serious infections caused by these organisms is still unknown, and therefore studies of new therapeutic alternatives are needed. Antibiotic combination has been used not only as a strategy to avoid the appearance of resistance, but as a way of enhancing the activity of each of the combined antibiotic.3
Fosfomycin is a natural antibiotic that acts through an inhibition of an early step in cell wall synthesis. Although the drug is mainly used for non-complicated urinary infections, there is large evidence regarding its efficacy against a broad spectrum of gram-positive cocci, including MRSA and VRE, and gram-negative rods, including those producing extended spectrum betalactamases.4•8 In S. aureus, methicillin resistance does not confer cross-resistance to fosfomycin, remaining susceptible >90% of the isolates.9 In addition, although not currently recommended, fosfomycin monotherapy has proven high efficacy against difficult-to-treat MRSA infections, like osteomyelitis.10
Fosfomycin has shown in different in vitro and in vivo models synergism with almost all families of antimicrobials, suggesting and enormous potential of this inexpensive drug.11•14 However, despite these promising in vitro and in vivo data, there is a paucity of data regarding the efficacy of FOS combinations in patients with gram-positive infections, and limited to case-reports of fosfomycin combinations as rescue therapy against MRSA.15,16 A multicentre open label study evaluating the efficacy of daptomycin plus fosfomycin has been completed in January 2018, but no results has been reported.17
MethodsIn vitro studiesMicroorganisms: Two MRSA and two MSSA clinical isolates recovered from patients with persistent bacteraemia were used for time-kill assays.
Susceptibility tests: Susceptibility testing was performed in duplicate by E-test. Quality control (QC) was performed using S. aureus ATCC 29213.
Antibiotics and regimens: Daptomycin (DAP), vancomycin (VAN), linezolid (LNZ), imipenem (IMP), gentamycin (GM), fosfomycin (FOS), oxacillin (OX) and levofloxacin (LEV) were commercially purchased from Sigma•Aldrich Co., Madrid, Spain (vancomycin, imipenem, gentamycin, oxacillin and levofloxacin), Novartis Pharmaceuticals, Basel. Switzerland (daptomycin), Pfizer Madrid, Spain (linezolid) and Laboratorios ERN S.A. Barcelona, Spain (fosfomycin).
Regimens evaluated against MRSA included DAP, VAN, LNZ and IMP at 1í and 4í MIC alone and in combination with GM and FOS at 0.5í and 2íMIC. Regimens evaluated against MSSA included DAP, VAN, OXA and LEV at 1í and 4í MIC alone and in combination with GM and FOS at 0.5í and 2íMIC.
Media: Mueller Hinton Broth supplemented with 25mg/L calcium and 12.5mg/L magnesium (SMHB; Difco Laboratories, Detroit, MI) was used for all susceptibility testing and time kill experiments. For experiments using daptomycin, SMHB was supplemented with 50mg/L calcium and 25mg/L magnesium.
Time-kill assay: Time-kill assays were performed using a starting inoculum of 108•9CFU/ml. Inoculum in stationary phase of growth was prepared using an appropriate dilution of a 0.5 McFarland. Quantification of viability was performed at 0, 1, 2, 4, 8 and 24h.
Samples were diluted in normal saline prior to drop plating onto Tryptic Soy Agar (TSA) plates. Antibiotic carryover was accounted using vacuum filtration. Plates were incubated at 35°C (5% CO2) for 18h prior to colony counting. All tests were performed in triplicate to account for biological variability. The limit of detection of these methods of colony count determination was 2log10CFU/ml extended to 1log10CFU/ml by vacuum filtration.
Synergy was defined as a reduction >3logCFU/ml over the most active antimicrobial agent alone, additive effect was defined as a reduction <3logCFU/ml over the most active antimicrobial agent alone.
Clinical dataFrom 2011 fosfomycin was used as an add-on therapy in those patients with persistent bacteraemia, and from 2013 as initial combination therapy in selected patients with gram-positive cocci bacteraemia. Data from all patients who received fosfomycin for gram-positive cocci bacteraemia was recovered from the bacteraemia database. Patients were considered successfully treated if blood cultures sterilization was achieved after fosfomycin therapy.
ResultsIn vitro studiesMRSA strainsDaptomycin demonstrated rapid bactericidal activity at 4í MIC. T99.9% was achieved at 8h. No other agent displayed similar activity in monotherapy.
In the synergy tests, the combination of DAP at 4íMIC with FOS at 2íMIC was the most active regimens achieving T99.9% at 4h. All daptomycin combinations achieved the limit of detection at 24h.
IMP-GM and IMP-FOS combination showed synergistic activity compared to that of IMP alone, however only IMP-FOS combinations achieved the limit of detection at 24h against MRSA.
Vancomycin at 4íMIC with either GM at 0.5 or 2íMIC achieved the limit of detection at 24h. However, VAN 1xMIC with either GM at 0.5 or 2íMIC did not achieved the limit of detection. On the other hand, VAN-FOS combinations did not reach the limit of detection at any concentration.
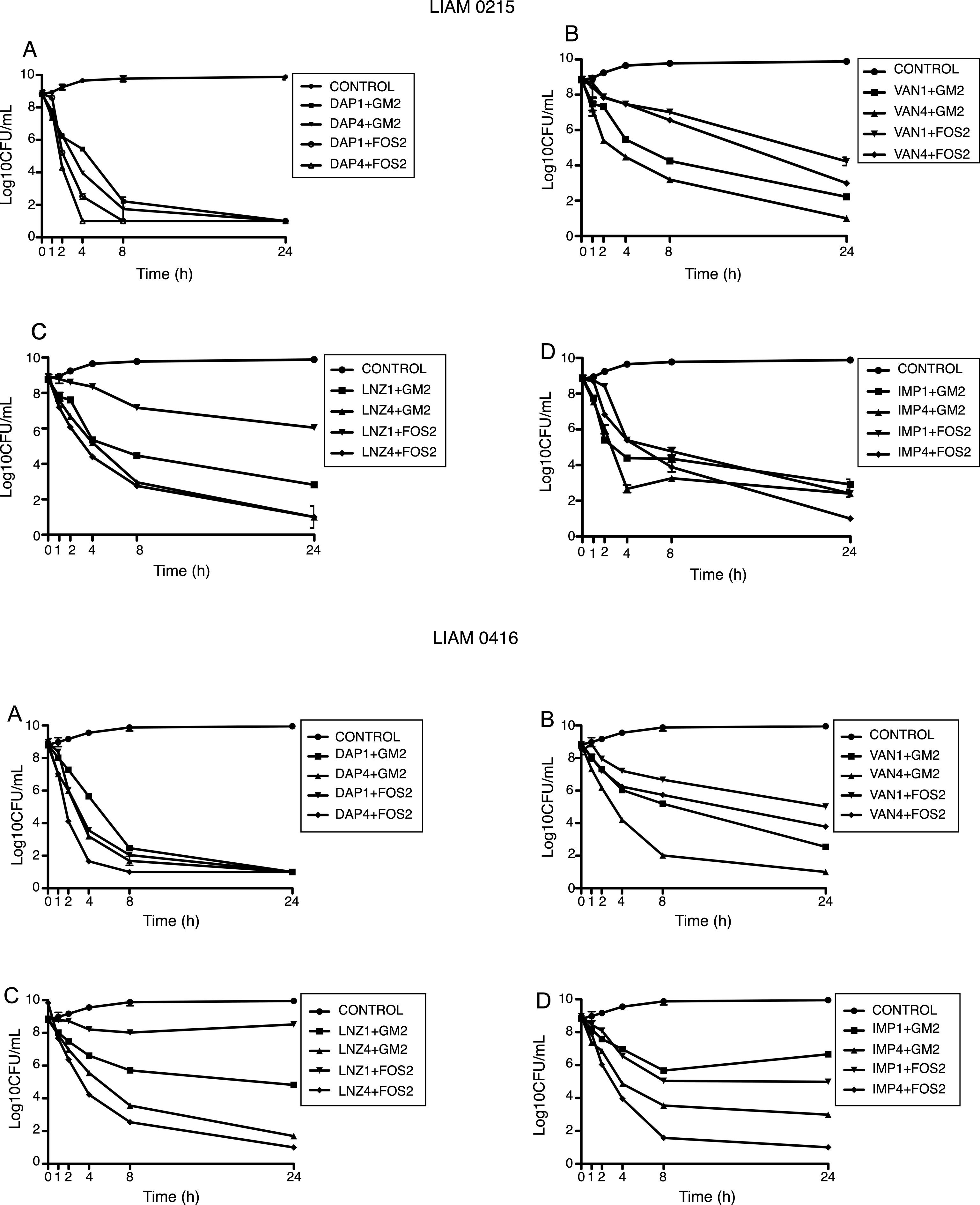
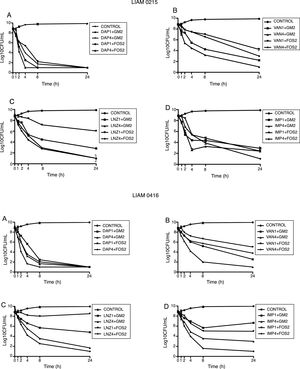
Finally, linezolid at 4í MIC combined with GM at 2íMIC achieved the limit of detection against one strain, but not against the other. Linezolid at 4íMIC combined with FOS at 4íMIC achieved the limit of detection against both strains at 24h (Fig. 1).
Time kill studies of two MRSA clinical strain versus daptomycin, vancomycin, imipenem and linezolid in combination with gentamycin and fosfomycin. (A) Daptomycin combinations. (B) Vancomycin combinations. (C) Linezolid combinations. (D) Imipenem combinations. DAP1: daptomycin 1íMIC. DAP4: daptomycin 4íMIC. GM2: gentamycin 2íMIC. FOS2: fosfomycin 2íMIC. VAN1: vancomycin 1íMIC. VAN4: vancomycin 4íMIC. LNZ1: linezolid 1íMIC. LNZ4: linezolid 4íMIC. IMP1: imipenem 1íMIC. IMP4: imipenem 4íMIC.
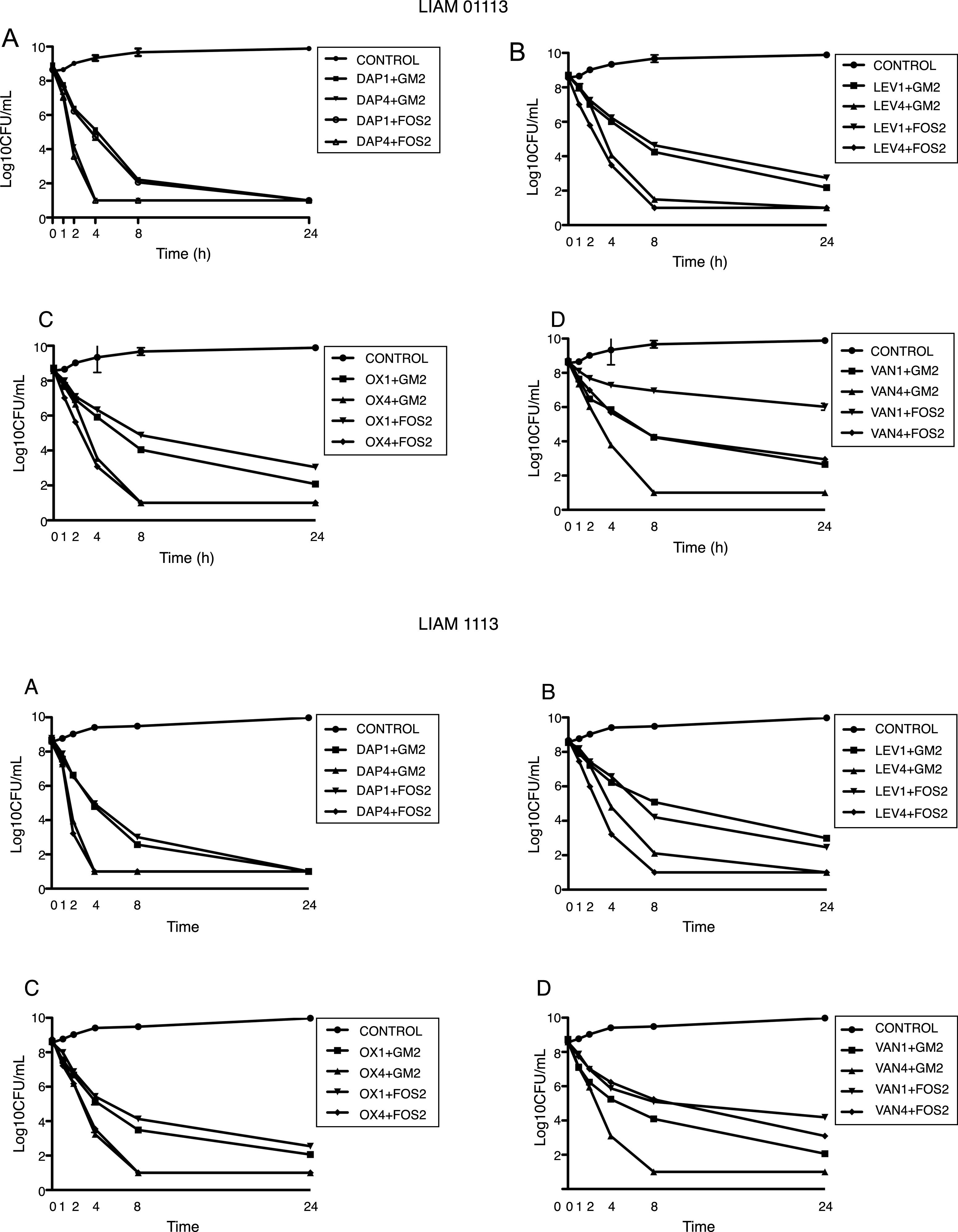
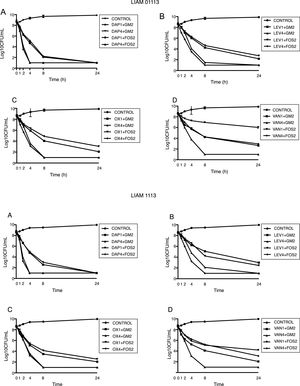
Daptomycin at 4íMIC was the most active regimens with T99.9% at 8h and bactericidal activity at 4h. At 1íMIC, daptomycin had bacteriostatic activity.
Daptomycin at 4íMIC plus either GM at 2íMIC or FOS at 2íMIC achieved the limit of detection at 4h. Daptomycin at 1íMIC plus either GM at 2íMIC or FOS at 2íMIC achieved limit of detection at 24h.
Levofloxacin and oxacillin displayed a very similar pattern, achieving the limit of detection at 4íMIC at 24h and bactericidal activity at 8h. At 1íMIC both agents did not achieve the limit of detection, being bacteriostatic at 24h. Both LEV and OX at 4íMIC, combined with either GM at 2íMIC and FOS at 2íMIC, showed synergistic activity achieving the limit of detection at 8h. The combination of LEV and OX at 1íMIC with GM and FOS at 2íMIC showed additive effect, being bactericidal at 8h.
Finally, VAN at 4íMIC achieved the limit of detection and bactericidal activity at 24h. At 1íMIC VAN displayed a bacteriostatic pattern. At 4íMIC, VAN combined with GM at 2íMIC achieved the limit of detection at 8h. On the contrary, VAN at 4íMIC combined with FOS at 2íMIC was not able to achieve the limit of detection. The combination of VAN at 1íMIC with GM at 2íMIC showed additive effect, being bactericidal at 24h. The combination of VAN at 1íMIC with FOS at 2íMIC, had indifferent effect (Fig. 2).
Time kill studies of two MSSA clinical strain versus daptomycin, levofloxacin, oxacillin, and vancomycin in combination with gentamycin and fosfomycin. (A) Daptomycin combinations. (B) Levofloxacin combinations. (C) Oxacillin combinations. (D) Vancomycin combinations. DAP1: daptomycin 1íMIC. DAP4: daptomycin 4íMIC. GM2: gentamycin 2íMIC. FOS2: fosfomycin 2íMIC. LEV1: levofloxacin 1íMIC. LEV4: levofloxacin 4íMIC. OX1: oxacillin 1íMIC. OX4: oxacillin 4íMIC. VAN1: vancomycin 1íMIC. VAN4: vancomycin 4íMIC.
From January 2011 to June 2017 seventy-five patients were treated with different FOS combinations. Among them, 61 (81%) were successfully treated. Eight out of the 14 failures, defined as persistence of bacteraemia despite combination therapy, were with vancomycin plus fosfomycin. Globally, the combination of daptomycin plus fosfomycin was the most effective one, with 93% success rate. On the contrary, vancomycin plus fosfomycin was the less effective combination, with a 47% success rate.
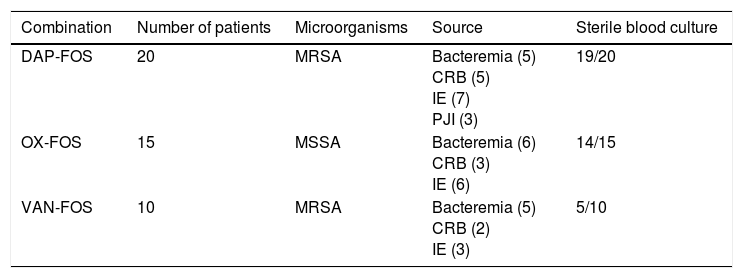
FOS was prescribed as add-on therapy in 45 patients (Table 1). Those patients were initially treated with oxacillin (15 patients with MSSA infection), daptomycin (20 patients with MRSA or MSSA infection) or vancomycin (10 patients with MRSA infection) and, after 72h of persistence of bacteraemia, FOS was added. Globally, after 48h, combination therapy resulted in sterilization of blood cultures in 38 patients (84%) and persistence of bacteraemia in 7 patients (16%). Patients treated with daptomycin plus fosfomycin had the highest rate of sterilization and those treated with vancomycin plus fosfomycin had the lowest rate.
Gram-positive bacteremia episodes treated with fosfomycin combinations as an add-on therapy.
| Combination | Number of patients | Microorganisms | Source | Sterile blood culture |
|---|---|---|---|---|
| DAP-FOS | 20 | MRSA | Bacteremia (5) CRB (5) IE (7) PJI (3) | 19/20 |
| OX-FOS | 15 | MSSA | Bacteremia (6) CRB (3) IE (6) | 14/15 |
| VAN-FOS | 10 | MRSA | Bacteremia (5) CRB (2) IE (3) | 5/10 |
DAP: daptomycin; FOS: fosfomycin; OX: cloxacillin; VAN: vancomycin; MRSA: methicillin-resistant S. aureus; MSSA: methicillin-susceptible S. aureus; CRB: catheter-related bacteremia; IE: infectious endocarditis; PJI: Prosthetic joint infection.
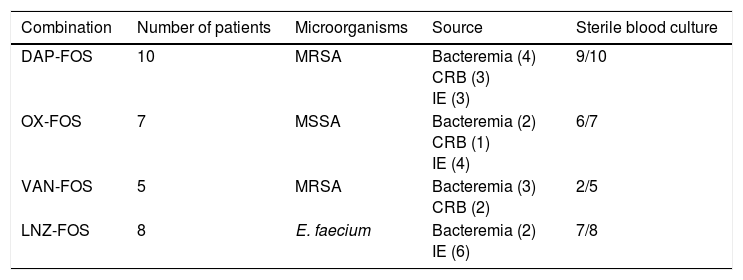
The other 30 patients received fosfomycin-based combination as initial therapy (Table 2). This group included patients treated with DAP-FOS combination (10 patients with MRSA infection), OX-FOS (7 patients with MSSA infection), VAN-FOS (5 patients with MRSA infection) and LNZ-FOS (8 patients with Enterococcus faecium infection). Globally, combination therapy resulted in sterilization of blood cultures in 24 patients (80%) after 48h. Similar to patients treated with the add-on strategy, those treated with daptomycin plus fosfomycin had the highest rate of sterilization and those treated with vancomycin plus fosfomycin had the lowest rate.
Gram-positive bacteremia episodes treated with fosfomycin combinations as initial therapy.
| Combination | Number of patients | Microorganisms | Source | Sterile blood culture |
|---|---|---|---|---|
| DAP-FOS | 10 | MRSA | Bacteremia (4) CRB (3) IE (3) | 9/10 |
| OX-FOS | 7 | MSSA | Bacteremia (2) CRB (1) IE (4) | 6/7 |
| VAN-FOS | 5 | MRSA | Bacteremia (3) CRB (2) | 2/5 |
| LNZ-FOS | 8 | E. faecium | Bacteremia (2) IE (6) | 7/8 |
DAP: daptomycin; FOS: fosfomycin; OX: cloxacillin; VAN: vancomycin; LNZ: linezolid; MRSA: methicillin-resistant S. aureus; MSSA: methicillin-susceptible S. aureus; CRB: catheter-related bacteremia; IE: infectious endocarditis.
Overall, FOS combinations were safe, with minor side effects like phlebitis or minor hypernatremia in nine patients. However, there was a severe side effect in one patient. An 81-year-old female suffering from aortic valve endocarditis, had acute cardiogenic pulmonary oedema secondary to sodium overload after 10 days of daptomycin•fosfomycin therapy that required ICU admission. Blood cultures were sterile at the moment of ICU admission, and the clinical situation resolved soon after combination therapy was discontinued. After ICU discharge, daptomycin monotherapy was maintained for another 4 weeks, and there was clinical cure without relapse.
DiscussionCombination regimens that include FOS, as an add-on therapy or as first-line therapy resulted in a high success rate when used to treat gram-positive bacteraemia.
We performed time-kill curves comparing the efficacy of fosfomycin combination to that of gentamycin combinations. In our study, daptomycin•fosfomycin combinations showed potent in vitro activity against MRSA and MSSA isolates. In addition, additive or synergistic effect was demonstrated with all FOS combinations at 0.5 and 2íMIC FOS concentrations.
Our results correlates with previous data published in the literature describing in vitro synergistic effect with fosfomycin combinations.18,19 Interestingly, when used at sub-MIC concentration and combined with other antibiotics, FOS showed an additive effect. Descourouez et al. also reported this effect. They tested the effect of sub MIC concentration of FOS and daptomycin, amoxicillin and linezolid against 32 strains of vancomycin-resistant Enterococcus faecium and found increased activity of all combinations.12
We also reviewed our clinical experience with fosfomycin in combination with other antibiotics like daptomycin, linezolid, oxacillin, and vancomycin. We used these combinations to treat 75 patients with gram-positive bacteraemia, showing good clinical outcomes even in those in which treatment with a prior first line regimen had failed. To our knowledge, this is the largest series of patients with infections caused by gram-positive microorganisms treated with antimicrobial combinations that included fosfomycin.
We decided to use the combination of linezolid plus fosfomycin based on in vitro data previously published demonstrating the efficacy of linezolid plus fosfomycin in an in vitro PK/PD model of simulated endocardial vegetations.20 The results obtained in our patients treated with linezolid-fosfomycin combination supports our in vitro results.
There is a paucity of data regarding fosfomycin combinations in patients with severe gram-positive infections, and most of them limited to clinical cases and case series. Portier et al. reported the efficacy of cefotaxime-fosfomycin combination against S. aureus meningitis. This combination sterilized all CSF samples evaluated and clinical recovery was achieved in 21 out of 22 patients treated (95.2%).21 The same group reported the efficacy of cefotaxime 25mg/kg plus fosfomycin 50mg/.g three to four times per day against a variety of MRSA infections (three patients with meningitis, six with bone and joint infections and seven with persistent bacteraemia). All patients were cured without relapses.22 More recently, Miro et al. described the efficacy of high dose daptomycin plus fosfomycin in three patients with left-sided endocarditis. All three patients (one with MSSA prosthetic aortic valve endocarditis and two with MRSA native valve endocarditis) were cured without relapses. Of interest, two patients had perivalvular abscesses and all three had failed prior antibiotic regimens (daptomycin and vancomycin alone).15 Similar to us, they also tested S. aureus strains to investigate the presence of synergism of the combination. Daptomycin plus fosfomycin showed synergism against 100% of MSSA strains tested (7/7), 60% of MRSA (3/5) and 50% of GISA (1/2).15
Finally, in a recent trial, patients with complicated MRSA bacteraemia or endocarditis who had failed first line therapy were offered rescue therapy with imipenem (1g q8h) plus fosfomycin (2g q6h). The authors included 16 patients (12 with endocarditis, 2 with vascular graft infection and 2 with complicated bacteraemia). All patients had negative blood cultures after 72h of initiation of imipenem plus fosfomycin with a 69% success rate.23 Our results are quite close to that described in this trial. We also had a very early negativization of cultures in most patients, suggesting a high bactericidal activity of fosfomycin combinations.
Regarding safety, del Rio et al. reported the safety of FOS combinations, being well tolerated. In their cohort, there was only one major side effect due to excessive sodium overload in a cirrhotic patient.23 Similarly, we did not have severe side effects with fosfomycin combinations, being safe in all patients but one, who suffered acute cardiogenic pulmonary oedema secondary to sodium overload. The patient had complete recovery after fosfomycin discontinuation.
Finally, we would like to highlight that clinical outcomes parallel what we observed in vitro, being daptomycin plus fosfomycin the most effective combination, with vancomycin plus fosfomycin showing poor efficacy. In addition, linezolid plus fosfomycin and oxacillin plus fosfomycin showed good results in our in vitro models. Similarly, in our series, good clinical outcomes were observed with daptomycin, oxacillin and linezolid combinations, whereas patients treated with vancomycin plus fosfomycin had poor clinical results, suggesting a good correlation of in vitro and in vivo data.
Despite the limitations related to the low number of episodes included, and the heterogeneity of cases included (different microorganisms, different infections and different comorbidities), we believe that our study provides valuable data regarding fosfomycin-based antimicrobial combinations as an alternative and effective approach for gram-positive infections.
Ethical approvalThe local Research Ethic Board of our Institution approved the study protocol and waived the requirement of informed consent because of its design.
Informed consentBased on the retrospective nature of the study, no informed consent was required.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors tm) contributionNMCA, and DP carried out the in vitro studies, reviewed clinical charts and helped to draft the manuscript. NMCA and JPR performed the statistical analysis. JPR conceived the study, participated in its design and coordination, and helped to draft the manuscript.
All authors read and approved the final manuscript.
Conflict of interestJPR has received honoraria, consultancy fees and funding research from Novartis, Pfizer, and ERN laboratories. DP and NMCA, nothing to declare.