To analyse and compare 30-day mortality prognostic power of several biomarkers (C-reactive protein, procalcitonin, lactate, suPAR and pro-adremomedullin) in elderly patients seen in Emergency Departments (ED) due to infections. Secondly, if these could improve the prognostic accuracy of sepsis criteria (systemic inflammatory response syndrome and quick Sepsis-related Organ Failure Assessment [qSOFA]).
MethodsA prospective, observational, multicentre and analytical study. Patients aged 75 years and older who were treated for infection in the ED of 8 participating hospitals were enrolled consecutively. An assessment was made of 25 independent variables (epidemiological, comorbidity, functional, clinical and analytical variables) that could influence short-term mortality (at 30 days).
ResultsThe study included 136 patients, 13 (9.5%) of whom died within 30 days of visiting the ED. MR-proADM is the biomarker with the best area under the curve ROC to predict 30-day mortality (0.864; 95% CI 0.775–0.997; p<0.001) with a prognostic cut-off>2.07nmol/l, sensitivity of 77% and specificity of 96%. The qSOFA score≥2 had an area under the curve ROC of 0.763 (95% CI 0.623–0.903; p=0.002), sensitivity of 76% and specificity of 75%. The mixed model (MR-proADM plus qSOFA≥2) improved the area under the curve ROC to 0.878 (95% CI 0.749–1; p<0.001) with the best prognostic performance with sensitivity of 69% and specificity of 97%.
ConclusionsMR-proADM showed the best performance for 30-day mortality prognostic power compared to other biomarkers in elderly patients seen in EDs due to infections. qSOFA score achieves better results than systemic inflammatory response syndrome, and the mixed model (qSOFA≥2 plus MR-proADM>2.07nmol/l) increased the predictive power of qSOFA.
Analizar y comparar el poder predictivo de mortalidad a 30 días de varios biomarcadores (proteína C reactiva, procalcitonina, lactato, suPAR y proadrenomedulina) en los pacientes ancianos que acuden al servicio de Urgencias (SU) por un episodio de infección. Y, secundariamente, comprobar si estos mejoran la capacidad pronóstica de los criterios de sepsis (síndrome de respuesta inflamatoria sistémica y quick Sepsis-related Organ Failure Assessment [qSOFA]).
MétodosEstudio observacional, prospectivo, multicéntrico y analítico. Se incluyó consecutivamente a pacientes de 75 o más años atendidos en 8 SU por un proceso infeccioso. Se analizaron 25 variables independientes (epidemiológicas, de comorbilidad, funcionales, clínicas y analíticas) que pudieran influir en la mortalidad a corto plazo (30 días).
ResultadosSe incluyó a 136 pacientes, de los que 13 (9,5%) habían fallecido a los 30 días tras su consulta en el SU. La MRproADM es el biomarcador que consigue la mayor área bajo la curva ROC para predecir mortalidad a los 30 días (0,864; IC 95% 0,775–0,997; p<0,001), con un punto de corte de mayor capacidad predictiva de 2,07nmol/l, que ofrece una sensibilidad del 77% y una especificidad del 96%. La escala qSOFA≥2 consigue un área bajo la curva ROC de 0,763 (IC 95% 0,623–0,903; p=0,002), con una sensibilidad del 76% y una especificidad del 75%. El modelo combinado (MRproADM con qSOFA≥2) mejora el área bajo la curva ROC a 0,878 (IC 95% 0,749–1; p<0,001) y ofrece el mejor rendimiento pronóstico, con una sensibilidad del 69% y una especificidad del 97%.
ConclusionesEn los pacientes ancianos que acuden al SU por un episodio de infección, la MRproADM presenta una capacidad pronóstica de mortalidad a los 30 días superior al resto de los biomarcadores, la qSOFA obtiene mayor rendimiento que los criterios de síndrome de respuesta inflamatoria sistémica, y el modelo combinado qSOFA≥2 con MRproADM>2,07nmol/l mejora el poder predictivo de qSOFA.
Care in patients over 75 years of age and the incidence of infectious processes in this age group at hospital emergency departments (HEDs) has significantly increased over the past decade.1 At present, they represent 31.7% of all patients seen with infections at these units.1,2 Moreover, there have also been increases in the severity of their clinical presentations and the short-term mortality recorded (30 days).1–3 Immunosenescence leads to immune cell function deterioration and diminished humoral immune function, as well as a chronic proinflammatory state that alters the production of cytokines, chemokines and some biomarkers (BMs).4
Given that clinical manifestations are less specific4 and that early diagnosis and the prognostic assessment of severe infectious processes are more complicated5,6 in elderly patients, the availability of additional objective tests that help the clinician has become one of the main lines of research among various groups and scientific societies.7–9 Thus, in recent years new prognostic mortality scales have been published,10,11 along with novel uses of different BMs,12–16 which, alone or combined,13,16 successfully increase the limited prognostic capacity of both classical sepsis definitions—specifically the systemic inflammatory response syndrome (SIRS) criteria17—and the new definition and prognostic assessment recommended for HEDs (Sepsis-3) through the quick Sepsis-related Organ Failure Assessment (qSOFA).18
The detection and prognostic assessment of severe bacterial infections have also significantly improved,19 as was the early and adequate administration of antibiotic treatments,20 with the introduction of multidisciplinary sepsis units and different information systems that are activated from the patient's first assessment in the HED and which now, at many sites, include new scales and BMs for detecting and predicting the patient's clinical severity.19–22
Among the additional tests available at HEDs are inflammatory and infection BMs. However, much like the sepsis criteria, these do not obtain the same results in the elderly, versus adult patients. Sepsis criteria have been proven sensitive but are somewhat unspecific, so the validity thereof in elderly patients has been discussed and they have been deemed insufficient.7,18 Likewise, it has been published that using the SOFA prognostic scale in low-risk patients is insufficient for predicting their prognosis, so it would become necessary to supplement it with other clinical and analytical variables, in particular BMs.23 Thus, previous studies have already shown the lack of reliability of the classical sepsis criteria for identifying high-risk elderly patients11,24 and, moreover, new predictive models and factors have been proposed in elderly patients with infections seen in HEDs.10
C-reactive protein (CRP) is the most widely used BM in HEDs, but offers the lowest diagnostic and prognostic performance.13 Procalcitonin (PCT) is synthesised in situations of bacterial infection and sepsis, and concentrations thereof are linked to bacterial load and/or endotoxin concentration, the existence of bacteraemia and the mortality prognosis.13,25,26 Lactate is considered the best biomarker for tissue hypoperfusion and hypoxia and values >2mmol/l constitute a powerful independent mortality factor.12,13 On the other hand, given that pro-adrenomedullin (proADM) is hard to measure (short half-life and extensive receptor binding), mid-regional pro-adrenomedullin (MR-proADM) is used, which increases in situations of cellular stress.13 Capable even of distinguishing between bacterial and viral infections, and of diagnosing sepsis and its progression to septic shock, MR-proADM is noted for its predictive power in relation to mortality.16,25,26 Similarly, different studies have evaluated the role of the soluble urokinase-type plasminogen activator receptor (suPAR) in diagnosing sepsis and predicting mortality, readmission and the length of hospital stay.14,25
The endpoint of this study was to analyse the utility and capacity of various BMs (CRP, PCT, Mr-proADM, suPAR and lactate) to predict short-term mortality (30 days) in elderly patients seen at the HED due to an episode of infection. Secondly, it sought to verify whether BMs could improve the prognostic capacity of classical sepsis criteria (SRIS)17 and the qSOFA.18
Patients and methodsA prospective, multicentre, analytical and observational study was designed and conducted at the eight HEDs of the participating investigators (Appendix A), belonging to the INFURG-SEMES (Emergency Department Infections Study Group of the Spanish Society of Emergency Medicine) network of sites. It was put together as a sub-study of another multicentre study by the INFURG-SEMES group24 which was carried out on 1 and 22 October 2015, 12 and 19 January 2016 and 13 and 27 April 2016. Thus, in this study we included patients who had agreed to donate an extra sample to their hospital's biobank for the purpose of carrying out this sub-study.
Patients ≥75 years of age who completed 30 days of follow-up with a diagnosis of infection, and who gave their consent to participate in this study and to donate an extra plasma sample to the corresponding hospital biobank, were consecutively enrolled by chance (when the investigators were on duty) upon clinical diagnosis of the infection in said HEDs.
The patients enrolled in this sub-study were asked to donate a 10-ml blood sample for research purposes, which was deposited at the biobanks of the participating hospitals. Eight of the sites participating in the main study24 received sample donations from these patients (Appendix A). All of the patients signed the informed consent form for donation at the local biobank.
The samples donated to the biobank were processed in a centrifuge and frozen at −80°C at each site. The patients’ frozen plasma samples were then stored and transported to the Hospital Clínico San Carlos, in Madrid, to perform a BM panel with the same system, reagents and technique using immunofluorescence methods (Kryptor®) for all of the samples, and in order to collate these results with the clinical database that was established beforehand for the conduct of the first study.24
The variables were recorded using an encrypted electronic case report form (online) which included the BM and haemodynamic results, as well as the existing clinical characteristics and analytical results. The various criteria, definitions and parameters were defined in advance by the INFURG-SEMES scientific committee and were agreed by the investigators.
The study was approved by the Independent Ethics Committee of the Hospital Universitario Clínico San Carlos of Madrid and met the ethical standards of all the participating sites. All the encrypted data were kept strictly confidential. Patients or family members were informed both orally and in writing, and informed consent was required prior to enrolment. The study did not involve any therapeutic procedure or have any clinical implications.
Unadjusted 30-day mortality was considered a dependent variable. The independent variables collected by consensus were those deemed interesting and which might influence the patient's prognosis and progression in the 30 days subsequent to their visit to the HED. Table 1 details: demographic variables (age, gender), comorbidity (Charlson index27 and dichotomised index ≥3), performance status variables (Barthel index28 and dichotomised index ≤60), clinical variables (altered level of consciousness defined as less than 15 points on the Glasgow Coma Scale, systolic blood pressure [SBP] and SBP <90mmHg, sepsis, severe sepsis or septic shock criteria and their defining variables according to the 2001 International Sepsis Definitions Conference,17 a qSOFA sepsis score ≥2 and its constituting variables according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)18 and analytical variables. In relation to the latter, the following were recorded: kidney failure in case of urea >50mg/dl or Cr >1.2mg/dl, leucocyte count, concentration of serum lactate (mmol/l), CRP in mg/l and PCT in ng/ml. For BMs that are widely available in HEDs, we adopted the normal reference values agreed between the participating sites for patients aged ≥75 years and these were dichotomised according to the recommendations made by various recent reviews13,25,26 and similar studies previously undertaken by the group10: for serum lactate, 0.55–2mmol/l was considered normal (and it was dichotomised for ≥2mmol/l and if ≥4mmol/l, as well as by the cut-off point found to have the best performance). PCT was considered normal if <0.5ng/ml (and dichotomised ≥1ng/ml) and, for CRP, 0–18mg/l (and dichotomised ≥68mg/l).
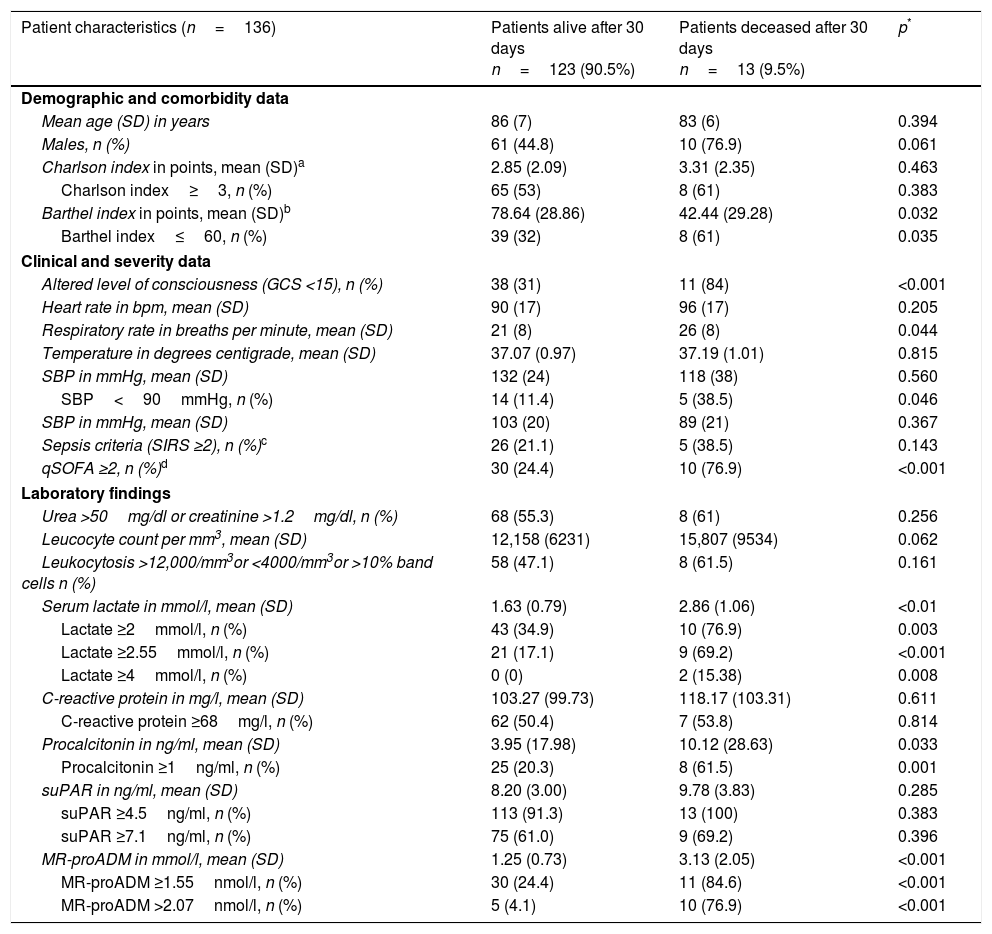
Baseline, functional, comorbidity, clinical–epidemiological and analytical characteristics studied during the patient's initial assessment in the HED (univariate analysis).
| Patient characteristics (n=136) | Patients alive after 30 days n=123 (90.5%) | Patients deceased after 30 days n=13 (9.5%) | p* |
|---|---|---|---|
| Demographic and comorbidity data | |||
| Mean age (SD) in years | 86 (7) | 83 (6) | 0.394 |
| Males, n (%) | 61 (44.8) | 10 (76.9) | 0.061 |
| Charlson index in points, mean (SD)a | 2.85 (2.09) | 3.31 (2.35) | 0.463 |
| Charlson index≥3, n (%) | 65 (53) | 8 (61) | 0.383 |
| Barthel index in points, mean (SD)b | 78.64 (28.86) | 42.44 (29.28) | 0.032 |
| Barthel index≤60, n (%) | 39 (32) | 8 (61) | 0.035 |
| Clinical and severity data | |||
| Altered level of consciousness (GCS <15), n (%) | 38 (31) | 11 (84) | <0.001 |
| Heart rate in bpm, mean (SD) | 90 (17) | 96 (17) | 0.205 |
| Respiratory rate in breaths per minute, mean (SD) | 21 (8) | 26 (8) | 0.044 |
| Temperature in degrees centigrade, mean (SD) | 37.07 (0.97) | 37.19 (1.01) | 0.815 |
| SBP in mmHg, mean (SD) | 132 (24) | 118 (38) | 0.560 |
| SBP<90mmHg, n (%) | 14 (11.4) | 5 (38.5) | 0.046 |
| SBP in mmHg, mean (SD) | 103 (20) | 89 (21) | 0.367 |
| Sepsis criteria (SIRS ≥2), n (%)c | 26 (21.1) | 5 (38.5) | 0.143 |
| qSOFA ≥2, n (%)d | 30 (24.4) | 10 (76.9) | <0.001 |
| Laboratory findings | |||
| Urea >50mg/dl or creatinine >1.2mg/dl, n (%) | 68 (55.3) | 8 (61) | 0.256 |
| Leucocyte count per mm3, mean (SD) | 12,158 (6231) | 15,807 (9534) | 0.062 |
| Leukocytosis >12,000/mm3or <4000/mm3or >10% band cells n (%) | 58 (47.1) | 8 (61.5) | 0.161 |
| Serum lactate in mmol/l, mean (SD) | 1.63 (0.79) | 2.86 (1.06) | <0.01 |
| Lactate ≥2mmol/l, n (%) | 43 (34.9) | 10 (76.9) | 0.003 |
| Lactate ≥2.55mmol/l, n (%) | 21 (17.1) | 9 (69.2) | <0.001 |
| Lactate ≥4mmol/l, n (%) | 0 (0) | 2 (15.38) | 0.008 |
| C-reactive protein in mg/l, mean (SD) | 103.27 (99.73) | 118.17 (103.31) | 0.611 |
| C-reactive protein ≥68mg/l, n (%) | 62 (50.4) | 7 (53.8) | 0.814 |
| Procalcitonin in ng/ml, mean (SD) | 3.95 (17.98) | 10.12 (28.63) | 0.033 |
| Procalcitonin ≥1ng/ml, n (%) | 25 (20.3) | 8 (61.5) | 0.001 |
| suPAR in ng/ml, mean (SD) | 8.20 (3.00) | 9.78 (3.83) | 0.285 |
| suPAR ≥4.5ng/ml, n (%) | 113 (91.3) | 13 (100) | 0.383 |
| suPAR ≥7.1ng/ml, n (%) | 75 (61.0) | 9 (69.2) | 0.396 |
| MR-proADM in mmol/l, mean (SD) | 1.25 (0.73) | 3.13 (2.05) | <0.001 |
| MR-proADM ≥1.55nmol/l, n (%) | 30 (24.4) | 11 (84.6) | <0.001 |
| MR-proADM >2.07nmol/l, n (%) | 5 (4.1) | 10 (76.9) | <0.001 |
GCS: Glasgow Coma Scale; MAP: mean arterial pressure; MR-proADM: mid-regional pro-adrenomedullin; qSOFA: quick Sepsis-related Organ Failure Assessment; SBP: systolic blood pressure; SD: standard deviation; SIRS: systemic inflammatory response syndrome; suPAR: soluble urokinase-type plasminogen activator receptor.
MR-proADM, on the other hand, was measured with Time-Resolved Amplified Cryptate Emission (TRACE) technology, using immunofluorescence techniques (Kryptor Compact Plus Analyser, BRAHMS, Hennigsdorf, Germany) with a sensitivity (SEN) of 0.05nmol/l and reference values (median) <0.39nmol/l, 97.5% percentile <0.55nmol/l. Moreover, it was dichotomised for ≥1.55nmol/l and by the cut-off point found to have the best performance. suPAR was measured along with MR-proADM using the suPARnostic® AUTO Flex ELISA kit in accordance with the manufacturer's instructions (ViroGates A/S, Birkerød, Denmark). Concentrations under 3.5ng/ml in women and under 3ng/ml in men were defined as normal concentrations, and were dichtomised for ≥4.5ng/ml and ≥7.1ng/ml.
For the statistical analysis of the association between mortality and the independent variables, means and their standard deviations (SD) were used for quantitative variables, and percentages for qualitative variables. The chi-square test, Fisher's exact test, Student's t-test and Mann–Whitney U test were used, as applicable, to investigate the correlation between mortality and the independent variables (and the dichotomised variables). A p-value <0.05 was considered to be significant and all the tests were two-tailed.
The comparison results were expressed as p-values and their odds ratios (OR), with a 95% CI. A p-value <0.05 or when the 95% CI of the OR excluded the value 1 was accepted as significant.
The efficacy for predicting 30-day mortality of the various BMs and sepsis definition criteria was studied by analysing ROC (receiver operating characteristic) curves with a 95% CI of the area under the ROC curve (AUROC), which was compared against the neutral value (0.5). The standard errors of the AUC were calculated by non-parametric methods.
Youden's index was used to determine the BM value cut-off points with the highest diagnostic capacity that maximised the difference between the true positive rate and the false positive rate. The sensitivity (SEN), specificity (SPE), positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+) and negative likelihood ratio (LR−) were found for all the results studied. Their 95% CI were found by exact binomial methods and by the Taylor series method in the case of the likelihood ratios.
To complete the study on the capacity for predicting 30-day mortality, a new variable was devised (quantitative predictor designed with a fitting logistic regression model), resulting from the combination of the best result yielded with a BM (in this case, MR-proADM) and a qSOFA score ≥2. The likelihoods calculated as detailed above were subjected to a ROC curve analysis and the same procedures as the individual markers. To check whether the differences between the AUROCs of the combined model (MR-proADM+qSOFA) and the best one achieved by the BM were significant, the differences between the AUROCs and their 95% CIs were analysed using the DeLong test variance–covariance matrices.29,30
The statistical analysis was performed using IBM-SPSS® Statistics 22 for Windows and STATA 12.0, MS Excel and the Statistical Analysis of ROC Curves (StAR)31 calculator to assess the 95% CIs of the differences between the AUROCs (available at: http://protein.bio.puc.cl/star/roc_analysis.php).
ResultsDuring the study period, 136 cases meeting the inclusion criteria were eventually gathered, on whom follow-up could be carried out for up to 30 days maintaining the infection diagnosis, and in whom a sample for the biobank was obtained and a BM panel performed. Of these, 13 (9.5%) died within 30 days of their consultation in the HED.
The baseline, functional, comorbidity, clinical–epidemiological and analytical characteristics of the HED patient are shown in Table 1 with the univariate analysis. Significant differences were found among the following variables: Barthel index (and dichotomised index ≤60 points), altered level of consciousness, respiratory rate, SBP<90mmHg, qSOFA≥2, serum lactate (and dichotomised for ≥2, ≥2.55 and ≥4mmol/l), PCT (and dichotomised if ≥1ng/ml) and MR-proADM (and dichotomised for ≥1.55 and >2.07nmol/l).
In relation to the type of infection and mortality, 51.47% (70 cases) were lower respiratory tract infections, of which 10% (7) died. 33.82% (46) were urinary tract infections, of which 8.7% (4) died, and 8 (5.88%) were intra-abdominal infections, of which only one patient died (12.5%). 7 (5.14%) were skin and soft tissue infections, with no deaths, and 5 cases corresponded to other infections (3.67%), with one death after 30 days (20%).
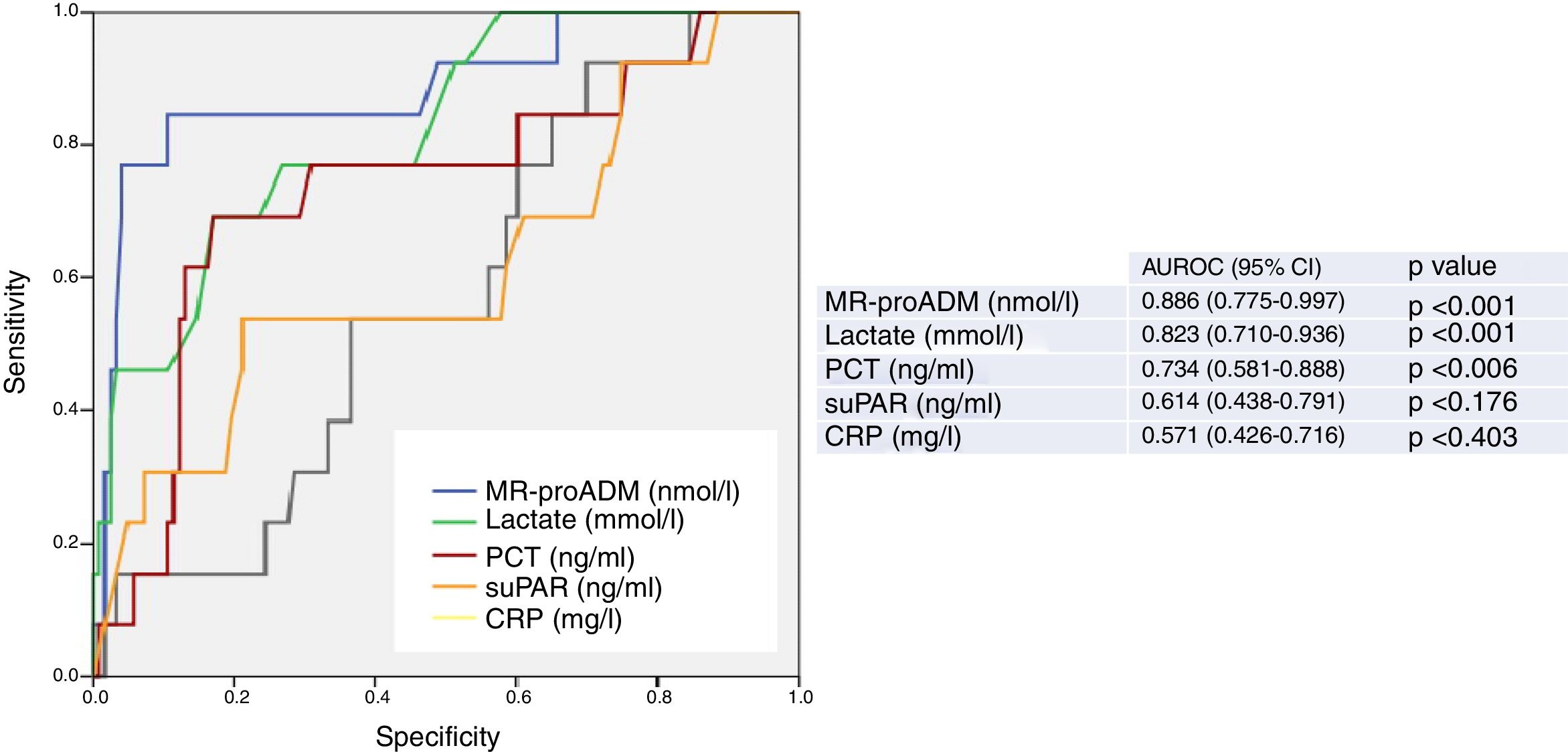
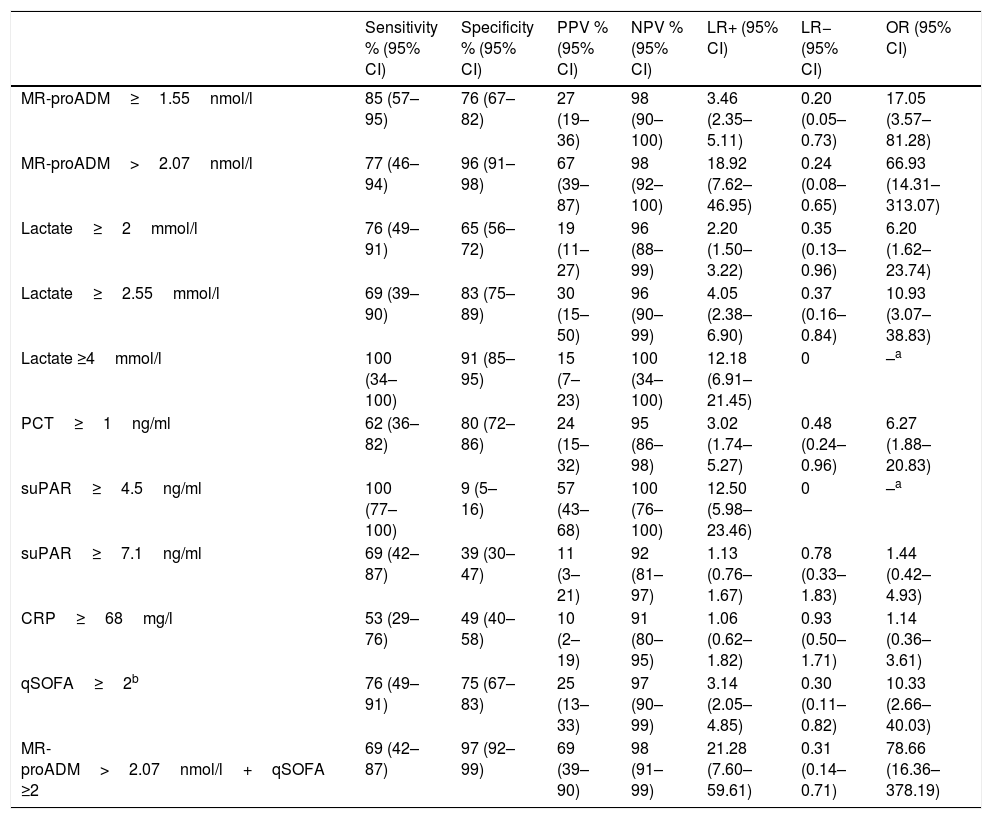
Fig. 1 presents the AUROC values of the BMs studied (CRP, PCT, MR-proADM, suPAR and lactate) for their predictive capacity regarding 30-day mortality in patients aged ≥75 years seen in the HED. The best results and greatest performance were achieved by the three BMs that obtained significant differences in the univariate analysis (Table 1): MR-proADM, lactate and PCT.
Predictive capacity of the biomarkers of 30-day mortality in patients aged ≥75 years seen in the emergency department due to infection. The p-value indicates the risk of a type 1 error in the null hypothesis test where the AUROC is 0.5. AUROC: area under the receiver operating characteristic curve; 95% CI: 95% confidence interval; CRP: C-reactive protein; MR-proADM: mid-regional pro-adrenomedullin; PCT: procalcitonin; suPAR: soluble urokinase-type plasminogen activator receptor.
The greatest AUROC—0.886—was achieved for MR-proADM (95% CI 0.775–0.997; p<0.001) and the cut-off point found to have the greatest predictive capacity was 2.07nmol/l, providing 77% SEN and 96% SPE (all of the diagnostic performance values are shown in Table 2). When a cut-off point ≥1.55ng/ml is assessed, SEN is 85% and SPE drops to 76%, with an AUROC of 0.801 (95% CI 0.678–0.924; p<0.001).
Cut-off points and performance for predicting 30-day mortality.
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | LR+ (95% CI) | LR− (95% CI) | OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| MR-proADM≥1.55nmol/l | 85 (57–95) | 76 (67–82) | 27 (19–36) | 98 (90–100) | 3.46 (2.35–5.11) | 0.20 (0.05–0.73) | 17.05 (3.57–81.28) |
| MR-proADM>2.07nmol/l | 77 (46–94) | 96 (91–98) | 67 (39–87) | 98 (92–100) | 18.92 (7.62–46.95) | 0.24 (0.08–0.65) | 66.93 (14.31–313.07) |
| Lactate≥2mmol/l | 76 (49–91) | 65 (56–72) | 19 (11–27) | 96 (88–99) | 2.20 (1.50–3.22) | 0.35 (0.13–0.96) | 6.20 (1.62–23.74) |
| Lactate≥2.55mmol/l | 69 (39–90) | 83 (75–89) | 30 (15–50) | 96 (90–99) | 4.05 (2.38–6.90) | 0.37 (0.16–0.84) | 10.93 (3.07–38.83) |
| Lactate ≥4mmol/l | 100 (34–100) | 91 (85–95) | 15 (7–23) | 100 (34–100) | 12.18 (6.91–21.45) | 0 | –a |
| PCT≥1ng/ml | 62 (36–82) | 80 (72–86) | 24 (15–32) | 95 (86–98) | 3.02 (1.74–5.27) | 0.48 (0.24–0.96) | 6.27 (1.88–20.83) |
| suPAR≥4.5ng/ml | 100 (77–100) | 9 (5–16) | 57 (43–68) | 100 (76–100) | 12.50 (5.98–23.46) | 0 | –a |
| suPAR≥7.1ng/ml | 69 (42–87) | 39 (30–47) | 11 (3–21) | 92 (81–97) | 1.13 (0.76–1.67) | 0.78 (0.33–1.83) | 1.44 (0.42–4.93) |
| CRP≥68mg/l | 53 (29–76) | 49 (40–58) | 10 (2–19) | 91 (80–95) | 1.06 (0.62–1.82) | 0.93 (0.50–1.71) | 1.14 (0.36–3.61) |
| qSOFA≥2b | 76 (49–91) | 75 (67–83) | 25 (13–33) | 97 (90–99) | 3.14 (2.05–4.85) | 0.30 (0.11–0.82) | 10.33 (2.66–40.03) |
| MR-proADM>2.07nmol/l+qSOFA ≥2 | 69 (42–87) | 97 (92–99) | 69 (39–90) | 98 (91–99) | 21.28 (7.60–59.61) | 0.31 (0.14–0.71) | 78.66 (16.36–378.19) |
95% CI: 95% confidence interval; CRP: C-reactive protein; LR+: positive likelihood ratio; LR−: negative likelihood ratio; MR-proADM: mid-regional pro-adrenomedullin; NPV: negative predictive value; OR: odds ratio; PCT: procalcitonin; PPV: positive predictive value; qSOFA: quick Sepsis-related Organ Failure Assessment; suPAR: soluble urokinase-type plasminogen activator receptor.
The cut-off points with the best diagnostic performance were used, as well as others chosen by the authors (values defined by the laboratory as normal or significant) in order to perform comparisons with other studies.
Not assessable: results conditioned by the limited number of positive and/or negative cases with this criterion and the PPVs and NPVs due to prevalence.
qSOFA ≥2 sepsis criteria according to the Third International Consensus Definitions for Sepsis and Septic Shock (Singer et al.18).
For lactate, the AUROC is 0.823 (95% CI 0.710–0.936; p<0.001) and the cut-off point with the best performance is 2.55mmol/l, with 69% SEN and 83% SPE. The results obtained for the standard cut-off points used, ≥2mmol/l and ≥4mmol/l, are detailed in Table 2. A cut-off point ≥2mmol/l generates 76% SEN and 65% SPE. And although we only recorded two patients with serum lactate ≥4mmol/l, interestingly we observed 100% SEN and 91% SPE.
PCT, on the other hand, with an AUROC of 0.734 (95% CI 0.581–0.888; p=0.006) and a chosen cut-off point ≥1ng/ml, obtained 62% SEN and 80% SPE.
CRP and suPAR did not achieve an AUROC with a significant performance (see Fig. 1 and Table 2) and the cut-offs chosen show a very poor balance between SEN and SPE.
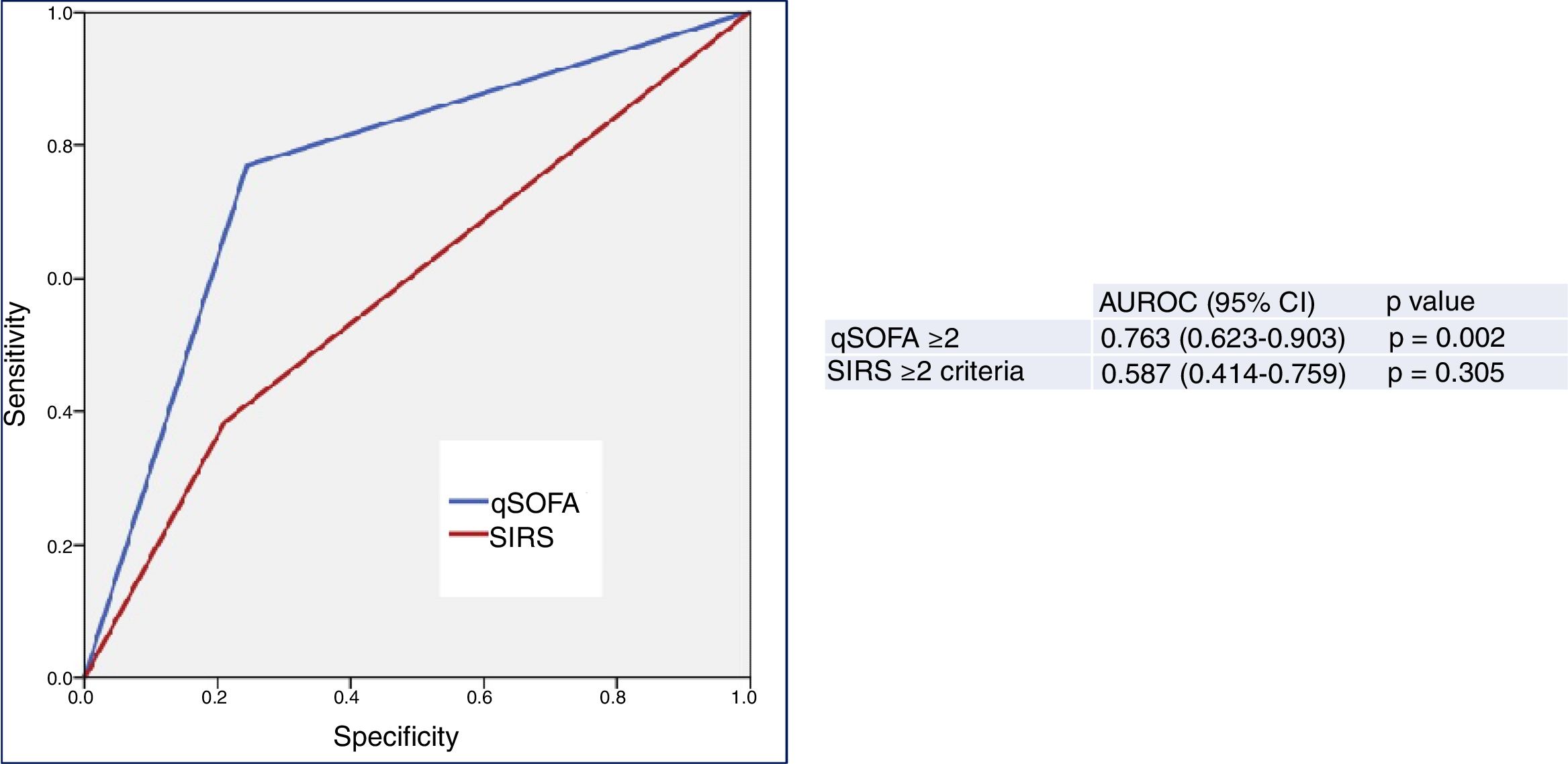
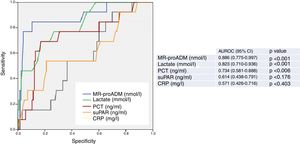
Fig. 2 describes the AUROC values of the classical sepsis criteria (SIRS≥2) and the Third Consensus (qSOFA≥2) for their capacity in predicting 30-day mortality among patients aged ≥75 years seen in the HED due to infection. The classical sepsis criteria (SIRS≥2) do not achieve a predictive performance. A qSOFA score ≥2, on the other hand, achieves a good performance, with an AUROC of 0.763 (95% CI 0.623–0.903; p=0.002). A qSOFA score ≥2 presents 76% SEN and 75% SPE and an OR of 10.33 (95% CI 2.66–40.03).
Predictive capacity of the classical sepsis definition criteria (SRIS≥2) and third consensus (qSOFA≥2) of 30-day mortality in patients aged ≥75 years seen in the emergency department due to infection. The p-value indicates the risk of a type 1 error in the null hypothesis test where the AUROC is 0.5. AUROC: area under the receiver operating characteristic curve; 95% CI: 95% confidence interval; qSOFA: quick Sepsis-related Organ Failure Assessment (qSOFA ≥2 sepsis criteria according to the Third International Consensus Definitions for Sepsis and Septic Shock; Singer et al.18); SIRS: systemic inflammatory response syndrome (≥2 sepsis criteria according to the 2001 International Sepsis Definitions Conference; Levy et al.17).
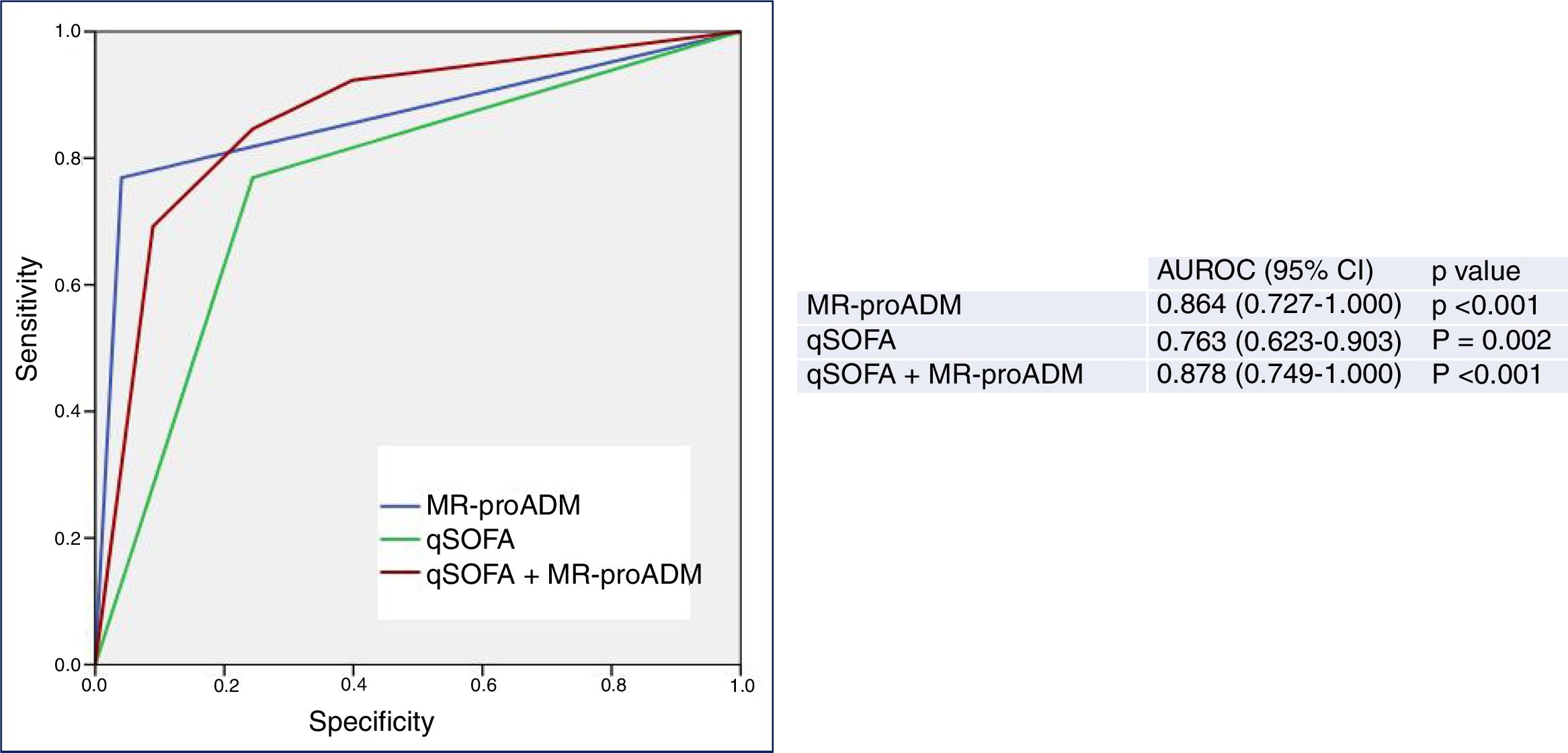
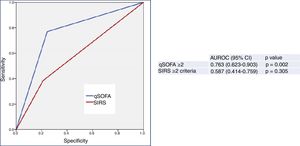
Finally, Fig. 3 shows the predictive performance of the new predictor—a model combining a qSOFA score ≥2 with MR-proADM >2.07nmol/l—regarding 30-day mortality in patients aged ≥75 years seen in the HED due to infection, which slightly improves the performance achieved individually by MR-proADM, and greatly improves that obtained with qSOFA alone. All of the diagnostic performance values are shown in Table 2. Thus, the combined model offers the best diagnostic performance, with 69% SEN, 97% SPE, a 98% NPV, a 69% PPV, a LR+ of 21.28 and an OR of 78.66 (95% CI 16.36–378.19), increasing the AUROC to 0.878 (95% CI 0.749–1.000; p<0.001).
Predictive capacity of the combined qSOFA≥2 and MR-proADM model of 30-day mortality in patients aged ≥75 years seen in the emergency department due to infection. The p-value indicates the risk of a type 1 error in the null hypothesis test where the AUROC is 0.5. AUROC: area under the receiver operating characteristic curve; 95% CI: 95% confidence interval; MR-proADM: mid-regional pro-adrenomedullin (cut-off point >2.07nmol/l); qSOFA: quick Sepsis-related Organ Failure Assessment (≥2 sepsis criteria according to the Third International Consensus Definitions for Sepsis and Septic Shock; Singer et al.18).
However, the differences between the AUROCs and their 95% CIs of the new predictor (MR-proADM+qSOFA) and MR-proADM, calculated using the variance-covariance matrices of the DeLong test, were not significant (difference of 0.00437774; 95% CI −0.0573669, 0.0661224). However, on comparing MR-proADM+qSOFA to a qSOFA score ≥2 alone, differences were in fact found between the AUROCs (0.09699997) and their 95% CIs (0.01890964, 0.11508997).
DiscussionThis study enables us to confirm the great predictive capacity offered by some BMs regarding mortality risk in elderly patients assessed at HEDs, and specifically MR-proADM,13,15,16,26 which is regarded as the BM that yields the best prognostic performance for short-term mortality (30 days) on its own. It also demonstrates the superiority of a qSOFA score≥2 for these patients versus the classical sepsis criteria (SRIS≥2), as shown previously on many occasions for adult patients7,23,32,33 but not for the elderly, now constituting a cause for dispute and a reason for study by various groups.10,11,24 Thus, according to the results of our study, we can highlight that after the emergency assessment conducted on elderly patients with infections in the HED, MR-proADM and a qSOFA score ≥2 are significantly correlated independent factors and the strongest prognostic indicators for short-term mortality (30 days). Moreover, these two factors combined (MR-proADM+qSOFA) achieve the best prognostic performance, so the urgent assessment thereof may become an effective tool to guide the clinician in making suitable decisions, such as deciding whether to discharge or admit patients, to obtain microbiological samples (and particularly blood cultures) or to immediately administer adequate antimicrobial treatment (especially in the most critically ill patients, where this will prove more decisive for the vital prognosis and in whom it is more important to get these early decisions right).7,20,22,34 Although the differences between the AUROCs of the combined model (MR-proADM >2.07nmol/l+qSOFA≥2) and MR-proADM are not significant (possibly due to our limited sample size and because the number of deaths at 30 days was only 13), they are if we compare it to qSOFA alone, which seems to indicate that the BM has a greater predictive power than the scale.
In this regard, the utility and capacity of MR-proADM in predicting mortality is known, as is its high performance with score systems that predict severity in patients with pneumonia seen in HEDs.13 This has given rise to mixed models that are recommended and already in use by various authors, as shown in a recent review,26 and which propose that a cut-off point of 1.5nmol/l generates the best performance, with this being somewhat lower than the figure we obtained (2.07nmol/l). Similarly, and in this case for elderly patients with pneumonia, it was also highlighted that the MR-proADM BM (compared to CRP, PCT and lactate) is the greatest prognostic indicator of short-term mortality, maintaining an AUROC of 0.858 (95% CI 0.722–0.993),35 which is very similar to the result obtained in young adults and our study. In relation to patients with sepsis, a recent study by Andaluz-Ojeda et al.16 confirms that MR-proADM is the BM with the greatest prognostic capacity for mortality, but also provides interesting data showing how this is maintained over time (on comparing measurements at admission, as well as on days 3 and 7, concentrations remain significantly raised, maintaining an AUROC of between 0.75 and 0.84) and in the different groups based on clinical severity and organ failure (according to a SOFA scale score of ≤6, 7–12 and ≥13). In other words, its utility is confirmed in the first determination, over time and for all patients, which was not the case for the SOFA scale with low-risk23 and elderly patients in HEDs.24 Another very significant finding from this study is that by combining MR-proADM with the SOFA scale in low-risk patients (SOFA≤6), the AUROC for said scale, which was 0.70 (95% CI 0.58–0.82), significantly increases to 0.77 (95% CI 0.66–0.88), as in our study. However, on this occasion, we added the BM to a qSOFA score ≥2 to obtain even better results for elderly patients, with an AUROC of 0.878, 97% SPE, a 97% NPV and an OR of 66.93. All this was with a cut-off point of 2.07nmol/l, which is slightly higher than what was deemed ideal by Andaluz-Ojeda et al.16 (1.79nmol/l) which achieved 83% SEN, 61% SPE and a 96% NPV.
Meanwhile, lactate is also included in the recommendations for assessing all septic patients at HEDs.22 This should also be the case in elderly patients, whose condition we would be obliged to monitor and observe on a closer basis in light of concentrations ≥2mmol/l, even in those without hypotension.13,15,36 Although few studies have been conducted specifically on elderly patients, Del Portal et al.12 found serum lactate >2mmol/l in the HED to be associated with a relative risk of 30-day mortality of 1.7–2.6. This was consistent with other studies and reviews where significant differences were also found in adult patients.13 Thus, as in our results, significant differences are obtained with a serum lactate cut-off point ≥2 or ≥4mmol/l. In a recently-published study on elderly patients with pneumonia with or without hypotension, Julián-Jiménez et al.15 found serum lactate concentrations > 2.5mmol/l to be correlated to 30-day mortality, with an AUROC of 0.85 (95% CI 0.78–0.92), which is very much in line with our results (where an OR of 10.93, 69% SEN and 83% SPE were obtained with a cut-off point ≥2.55, and an OR of 6.20, 76% SEN and 65% SPE were obtained with a serum lactate concentration ≥2mmol/l). Given that only two of our patients had serum lactate values ≥4mmol/l, albeit with 100% SEN and 91% SPE, we cannot draw conclusions in this regard, although similar findings have been published in adults.13
In relation to the other BMs compared in our study, it is important to note that PCT, despite having a greater capacity than CRP and suPAR, with significant differences found in the univariate analysis, is clearly inferior to MR-proADM in the prediction of mortality. However, since it is superior to the latter in the detection of severe bacterial infections and bacteraemia, the latest reviews recommend that they be used in conjunction, being termed “synergy BMs”.13,25,26 In any case, as we have found in elderly patients, PCT ≥1ng/ml would indicate a greater likelihood of 30-day mortality, bacterial infection, the existence of bacteraemia and the need for admission, at least under the observation of the HED, to adapt and improve treatment in elderly patients with sepsis.13,25,26,37
Finally, with respect to the results of the suPAR, a promising BM for predicting mortality, hospital stay and readmission in patients discharged from the HED,13,14,25 it should be noted that with the best-performing cut-off point (≥7.1ng/ml) 69% SEN and 39% SPE are achieved. We believe that our results are conditioned by the power of our study and our patients’ age, so it is thus not possible to extrapolate conclusions in our case.
Moreover, our results confirm that, for elderly patients seen due to infections at the HED, as was recently published for adults,7,38 classical sepsis criteria (SIRS≥2) are insufficient and less valid than a qSOFA score≥2 for assessing the patients’ prognoses. In our study, qSOFA's 76% SEN and 75% SPE are similar to recently published results.24 The latest mortality prediction scales published, such as GYM11 or LIPAS,10 obtain even better results for assessing prognosis among elderly patients in HEDs than a qSOFA score ≥2 and, of course, SIRS ≥2.24
Our study has various limitations. The main limitations are, on the one hand, the power of the study (since the small sample of 136 patients conditions the SEN, SPE, PPV and NPV results, which depend on prevalence). On the other hand, since it was a multicentre study with patients enrolled by chance, there is a possibility of selection bias resulting from enrolling patients only when each site's investigator was on duty, despite the fact that enrolment was consecutive during the recruitment period. Moreover, given that the various criteria, definitions and parameters were defined in advance by the INFURG-SEMES scientific committee and agreed upon by the investigators, some infection type classification errors may have occurred in the clinical diagnoses. Furthermore, patient characteristics and differences in mortality between different infection types were not analysed as they did not form part of the study endpoint. As such, some infection types, such as respiratory infections, were assumed to be heterogeneous and comprised both viral and bacterial infections, which could explain the differences found between them. Likewise, the selection of clinical variables could also have been more comprehensive (if it were not for the lack of data). Finally, since the mixed model was generated using the data of the 136 study patients, it is necessary to validate their results with an external series.
Despite these limitations, we believe that the study is a true reflection of actual clinical practice in our HEDs.
In light of the foregoing, we believe that since BMs have been shown to increase and improve the performance of various prognostic scales, it would be interesting to conduct multicentre studies with the necessary power to assess the utility of models that combine said scales and BMs. These future studies should compare and take into account variables that, up until now, have been proven independent factors for 30-day mortality in recently published studies7,10,11,13,25,26: altered state of consciousness, SBP, RR, Charlson index, serum lactate, Barthel index, kidney failure, PCT, MR-proADM and, possibly, other “promising” BMs such as presepsin and suPAR.
In terms of the conclusions drawn from this study, we can highlight that, in order to predict 30-day mortality in elderly patients seen at the HED due to an episode of infection, MR-proADM presents a superior prognostic capacity versus other BMs, the qSOFA scale performs better than the SIRS criteria, and the combined qSOFA ≥2 and MR-proADM >2.07nmol/l model improves the predictive power achieved individually by qSOFA, with 69% SEN, 97% SPE and a 97% NPV. Future external validation studies of the model are required.
FundingThe reagents for measuring pro-adrenomedullin and suPAR were provided by BRAHMS Thermo Scientific and ViroGates, respectively.
Neither of these two companies took part in designing the study or evaluating its results; they also had no influence on any of the planning stages. No funding was received from any public or private body to complete this manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest with regards to this article.
AJJ took part in scientific meetings organised by Roche, Thermo Scientific Biomarkers, BRAHMS AG and bioMérieux.
JGC participated in scientific meetings organised by Thermo Fisher, Merck, Tedec Meiji and AstraZeneca.
The Sponsor of this study was the Emergency Department Infections Study Group of the Spanish Society of Emergency Medicine (INFURG-SEMES). This group has received funding for organising scientific meetings on behalf of Merck, Tedec Meiji, Pfizer, Thermo Fisher, Laboratorios Rubio and Novartis in the past year. No authors received any payment for participating in this study.
To Mr Rafael Cuena Boy for his invaluable assistance with the statistical analysis of the data.
María Ángeles del Dedo Torres, Isabel Ortega Madueño and Ana Iglesias del Barrio (Clinical Analysis Department, Hospital Universitario Clínico San Carlos, Madrid), Francisco Javier Martín-Sánchez (Emergency Department, Hospital Universitario Clínico San Carlos, Madrid), Miguel Moreno Fernández (Hospital Regional Universitario de Málaga), Eva Fragero (Hospital Universitario Virgen de la Victoria, Málaga).
More information on the INFURG-SEMES group can be found in Appendix A.
Please cite this article as: Julián-Jiménez A, Yañez MC, González-del Castillo J, Salido-Mota M, Mora-Ordoñez B, Arranz-Nieto MJ, et al. Poder pronóstico de mortalidad a corto plazo de los biomarcadores en los ancianos atendidos en Urgencias por infección. Enferm Infecc Microbiol Clin. 2019;37:11–18.