Invasive pneumococcal disease (IPD) typically presents as bacterial pneumonia, meningitis or primary bacteraemia. However, Streptococcus pneumoniae can produce infection at any level of the body (endocarditis, arthritis, spontaneous bacterial peritonitis, etc.), which is also known as unusual IPD (uIPD). There are very limited data available about the clinical and microbiological profile of these uncommon manifestations of pneumococcal disease. Our aim was to analyse clinical forms, microbiological profile, epidemiology and prognosis of a cohort of patients with unusual invasive pneumococcal disease (uIPD).
MethodsWe present a retrospective study of 389 patients (all adult and paediatric patients diagnosed during the period) diagnosed with IPD at our hospital (Complejo Hospitalario Universitario de Vigo) between 1992 and 2014. We performed an analysis of clinical, microbiological and demographical characteristics of patients comparing the pre-pneumococcal conjugate vaccine (PCV) period with the post-vaccination phase. IPD and uIPD were defined as follows; IPD: infection confirmed by the isolation of S. pneumoniae from a normally sterile site, which classically presented as bacterial pneumonia, meningitis or primary bacteraemia; uIPD: any case of IPD excluding pneumonia, meningitis, otitis media, rhinosinusitis or primary bacteraemia.
ResultsA total of 22 patients (6%) met the criteria of uIPD. A Charlson index >2 was more prevalent in uIPD patients than IPD patients (45% vs 24%; p=0.08). The most common clinical presentation of uIPD was osteoarticular infection (8 patients, 36%), followed by gastrointestinal disease (4 patients, 18%). Infection with serotypes included in PCV-13 was significantly higher in IPD patients (65%) than in patients with uIPD, 35% (p=0.018). Conversely, infection with multidrug-resistant strains was higher among patient with uIPD (27% vs 9%; p=0.014). The all-cause mortality rate was 15%, 13% in the IPD group and 32% among patients with uIPD (p=0.07). According to the multivariate analysis, a Charlson Index >2 (OR 5.1, 95% CI, 1.8•14.0) and a Pitt Score >2 (OR 1.4, 95% CI, 1.2•1.9) were independent predictors of mortality.
ConclusionuIPD is a rare entity that affects patients with more comorbidities than typical IPD and it is usually caused by non-vaccine serotypes with greater antimicrobial resistance.
La enfermedad neumocócica invasiva (ENI) se presenta típicamente como neumonía bacteriana, meningitis o bacteriemia primaria. Sin embargo, Streptococcus pneumoniae puede producir infección a cualquier nivel del organismo (endocarditis, artritis, peritonitis bacteriana espontánea…), tambièc)n conocida como ENI inusual (ENIi). Hay pocos datos sobre el perfil clínico y microbiológico de estas manifestaciones poco frecuentes de enfermedad neumocócica. Nuestro objetivo fue analizar las formas clínicas, el perfil microbiológico, la epidemiología así como el pronóstico de una cohorte de pacientes con ENIi.
Mèc)todosPresentamos un estudio retrospectivo de 389 pacientes (todos los adultos y pacientes pediátricos diagnosticados durante el período) con ENI diagnosticados en nuestro hospital (Complejo Hospitalario Universitario de Vigo) entre 1992 y 2014. Realizamos un análisis de las características clínicas, microbiológicas y demográficas de los pacientes que comparan el período de preintroducción de la vacuna neumocócica conjugada con la fase postimplantación. Las definiciones de ENI y ENIi fueron las siguientes: ENIes una infección confirmada con aislamiento de Streptococcus pneumoniae de un sitio normalmente estèc)ril y con presentación típica como neumonía bacteriana, meningitis o bacteriemia primaria; ENIies cualquier caso de ENI, excluyendo neumonía, meningitis, otitis media, sinusitis de rinoceronte o bacteriemia primaria.
ResultadosUn total de 22 pacientes (6%) cumplieron los criterios de ENIi. Los pacientes con uIPD presentaron mayor proporción de índice de Charlson > 2 (45 vs. 24%; p=0,08). La presentación clínica más frecuente de ENIi fue la infección osteoarticular (8 pacientes; 36%), seguida de enfermedad gastrointestinal (4 pacientes; 18%). La infección con serotipos incluidos en VNC-13 fue significativamente mayor en pacientes con ENI (65%) que en pacientes con ENIi (35%; p=0,018). Por el contrario, la infección con cepas multirresistentes fue más frecuente entre los pacientes con ENIi (27 vs. 9%; p=0,014). La tasa de mortalidad por todas las causas fue del 15% (13% en el grupo IPD y 32% entre los pacientes con uIPD; p=0,07). Por análisis multivariante, el índice de Charlson > 2 (OR: 5,1; IC 95%: 1,8-14,0) y el Pitt score > 2 (OR: 1,4; IC 95%: 1,2-1,9) fueron predictores independientes de mortalidad.
ConclusiónLa ENIi es una entidad rara que afecta a pacientes con más comorbilidades que la ENI típica y es generalmente causada por serotipos no vacunales con mayor nivel de resistencia a los antimicrobianos.
Invasive pneumococcal disease (IPD) is defined as isolation of Streptococcus pneumoniae in blood or another organic sterile fluid. Despite the reduction in incidence of IPD following the introduction of pneumococcal conjugate 7-valent and 13-valent vaccines (PCV7/PCV13) IPD remains an important health problem with an incidence of 6.6•14.2 cases per 100,000 patients per year1,2 and with a mortality ranging between 10% and 30%.3,4
Even though S. pneumoniae can produce infection in any site of the organism,5 more frequent clinical presentations of IPD are bacteremic pneumonia, meningitis and primary bacteraemia. Other clinical presentations, such as arthritis, spontaneous bacterial peritonitis or endocarditis are occasionally seen in clinical practice.6,7
Host-related factors, such as age or predisposing comorbidities (human immunodeficiency virus infection, chronic liver disease, diabetes mellitus, splenectomy or connective tissue disease) influence clinical presentation and outcome of patients diagnosed with IPD.8,9
Also, different pneumococcal serotypes are known to cause different pneumococcal clinical syndromes (i.e. serotypes 1 or 7 and empyema)4,10 and different outcomes.11 On the other hand, following the introduction of 7-valent pneumococcal conjugate vaccine in Europe and US, different authors have observed a change in IPD clinical presentations in the last few years with increase in empyema and severe pneumonia cases and a decrease in incidence of meningitis.12•14 These changes have been linked to a replacement of serotypes included in conjugate vaccine to others, like 35B or 11A.
The objectives of our study were to analyse clinical forms, microbiological profile, epidemiology and outcome of patients with unusual invasive pneumococcal disease (uIPD) as well as to evaluate the possible correlation between the profile of serotypes and the clinical forms of this type of IPD.
Materials and methodsThe study was carried out in Complejo Hospitalario Universitario de Vigo, a 1300 bed tertiary teaching hospital, serving a population of almost 600,000 inhabitants. All S. pneumoniae isolated from blood or another steril fluid between January 1992 and December 2014, in adult and paediatric patients, were studied. Epidemiological data and comorbidities of IPD patients were collected, as well as characteristics of the infection (clinical form, severity of infection, empirical treatment, hospital stay and outcome). In our area the implantation of PCV7 and PCV13 vaccine occurred in 2002 and 2011 respectively.
DefinitionsIPD cases were defined as disease in persons with S. pneumoniae detected in cultures of specimens from normally sterile sites. uIPD was defined as any case of IPD excluding pneumonia, meningitis, otitis media, rhinosinusitis or primary bacteraemia.
Alcohol abuse was set as an ethanol intake of more than 40g/day in men and more than 32g/day in women, associated or not to organic damage and dependence. Chronic liver disease was defined as presence of liver failure with a illness duration of more than 26 weeks, including at least two of these items: hypoalbuminemia, coagulopathy, ascitis, hepatic encephalopathy and portal hypertension. Patients were considered to have a pulmonary disease with a previous diagnosis of asthma, chronic obstructive pulmonary disease (COPD) and/or bronchiectasis.
This study has been performed with the approval of Local Ethics Committee of Complejo Hospitalario Universitario de Vigo. In the development of data base and use of different variables anonymity of every patient was respected. Anonimity of patients was preserved during extraction of data and analysis, and through manuscript elaboration.
Microbiological studiesS. pneumoniae isolates were stored at ∧80°C. They were recovered by sample inoculation in chocolate agar and culture for 24•48h at 35°C in a 5% CO2 atmosphere. Identification of S. pneumoniae strains was performed through blood agar colonies morphology, solubility tests in bile and optoquine susceptibility >15mm. Penicillin, erythromycin, clindamycin, levofloxacin and cefotaxime susceptibility tests were determined in Mñ/4eller-Hinton blood at 5%, in 5% CO2 atmosphere by E-test according to CLSI 2014 breakpoints and recommendations.15 Intermediate susceptibility or resistant isolates were classified as non-susceptible strains. We considered multiresistant strains those with non susceptibility to 2 or more different antibiotic clases.16 Serotyping was performed with agglutination and Quellun reaction (Pneumotest-Latex, Statens Serum Institut, Copenhague, Denmark) or multiple, sequential, polymerase chain reaction (QIAGEN Multiplex PCR Kit, Qiagen, Hilden, Germany).17 Fourteen more prevalent serotypes in Spain were selected11 and they were pooled in 5 multiple reactions depending on the size of PCR products to differentiate serotypes.
Statistical analysisTo show differences in serotype incidences between prevaccine and postvaccine years, we compared data from prevaccine years (1992•2001) to postvaccine years (2002•2014). Statistical analysis was carried out using SPSS 22.0 (SPSS, Chicago, IL). Normally distribution of continuous variables were checked using Kolmogorv•Smirnov test. To asses differences in disease characteristics between the two periods we compared overall and age group patients. Continuous variables were compared using t-student test or U Mann•Whitney test and they were described as medium±standard deviation or as median and interquartilic range (IQR) according to normally or non-normally variables distribution. Chi-square test (χ2) and Fisher's exact test were used to compared categorical variables.
Multivariate analysis was performed using a binary logistic regression model to assess factors that it might have influence in study variables. We included in multivariate analysis all variables with a p-value <0.05 in univariate analysis. Variables with a two-sided p-value <0.05 were considered statistically significant.
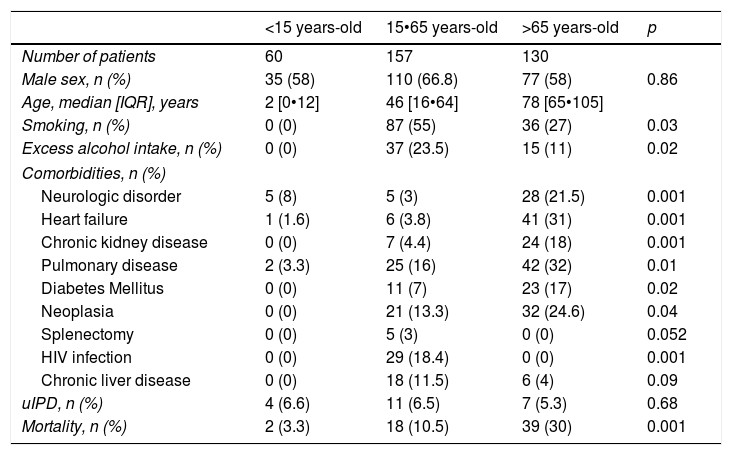
ResultsA total 389 patients with IPD were identified, 22 (6%) of them met uIPD criteria. Epidemiological characteristics of IPD and uIPD patients adjusted by age-groups are shown in Table 1. Data from 47 patients were not complete so they were excluded from the analysis.
Clinical and epidemiological baseline characteristics patients age-adjusted.
| <15 years-old | 15•65 years-old | >65 years-old | p | |
|---|---|---|---|---|
| Number of patients | 60 | 157 | 130 | |
| Male sex, n (%) | 35 (58) | 110 (66.8) | 77 (58) | 0.86 |
| Age, median [IQR], years | 2 [0•12] | 46 [16•64] | 78 [65•105] | |
| Smoking, n (%) | 0 (0) | 87 (55) | 36 (27) | 0.03 |
| Excess alcohol intake, n (%) | 0 (0) | 37 (23.5) | 15 (11) | 0.02 |
| Comorbidities, n (%) | ||||
| Neurologic disorder | 5 (8) | 5 (3) | 28 (21.5) | 0.001 |
| Heart failure | 1 (1.6) | 6 (3.8) | 41 (31) | 0.001 |
| Chronic kidney disease | 0 (0) | 7 (4.4) | 24 (18) | 0.001 |
| Pulmonary disease | 2 (3.3) | 25 (16) | 42 (32) | 0.01 |
| Diabetes Mellitus | 0 (0) | 11 (7) | 23 (17) | 0.02 |
| Neoplasia | 0 (0) | 21 (13.3) | 32 (24.6) | 0.04 |
| Splenectomy | 0 (0) | 5 (3) | 0 (0) | 0.052 |
| HIV infection | 0 (0) | 29 (18.4) | 0 (0) | 0.001 |
| Chronic liver disease | 0 (0) | 18 (11.5) | 6 (4) | 0.09 |
| uIPD, n (%) | 4 (6.6) | 11 (6.5) | 7 (5.3) | 0.68 |
| Mortality, n (%) | 2 (3.3) | 18 (10.5) | 39 (30) | 0.001 |
Patients in 15•65 years-old group and as compared IPD patients with patients with uIPD, these last had a higher proportion of Charlson Index >2 (54.5% vs 24%; p=0.03); more chronic liver disease (36.3% vs 8.5%, p=0.002) and lower prevalence of pulmonary disease (0% vs 9.2%; p=0.02). However, these differences were not observed in <15 or >65 years-old groups.
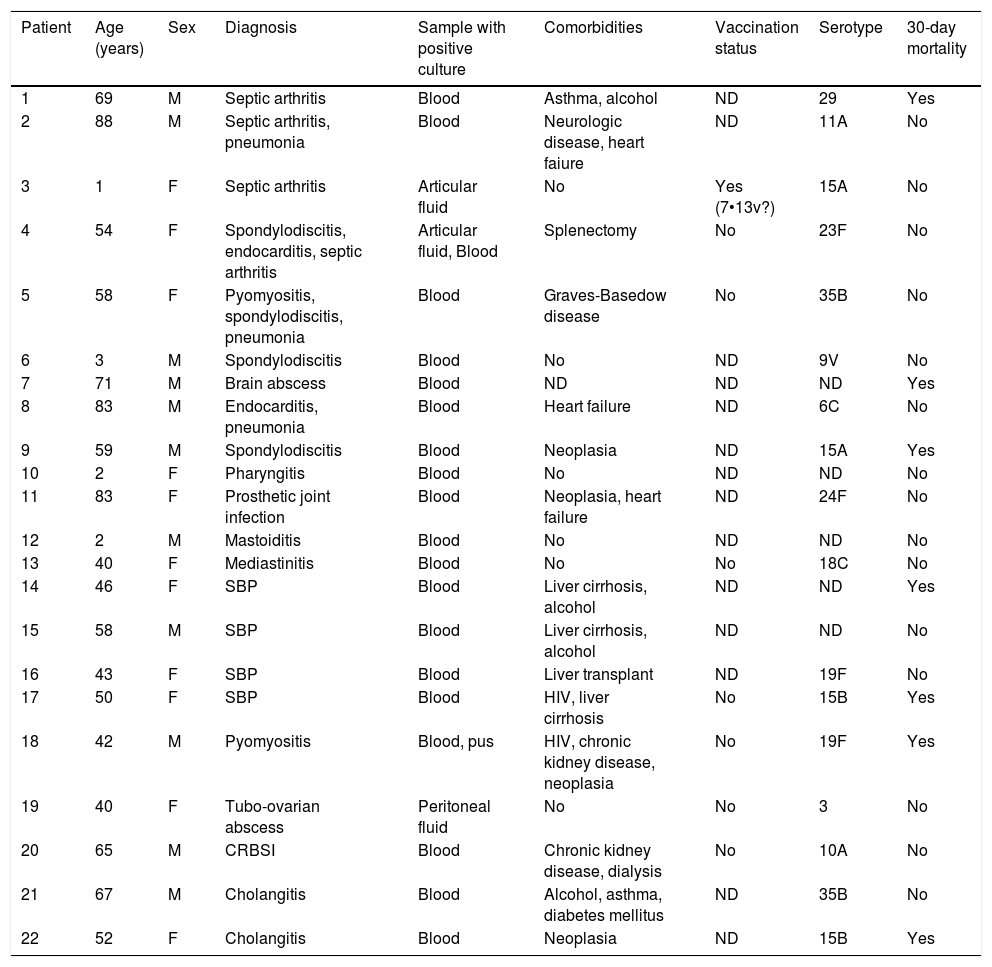
Clinical characteristics of atypical forms of IPD are shown in Table 2. The most frequent clinical presentation was osteoarticular infection in 8 patients (36%), either in form of septic arthritis, spondylodiscitis or prosthetic joint infection. Gastrointestinal infection was the second most common form or uIPD, in 4 patients (18%) with cirrhosis and spontaneous bacterial peritonitis, (all of them with advanced liver chronic disease), one of them coinfected with HIV. Also, two cases of endocarditis and two of pyomyositis were identified. In 4 patients, two or more foci of infection were present.
Clinical and microbiological characteristics of uIPD patients.
| Patient | Age (years) | Sex | Diagnosis | Sample with positive culture | Comorbidities | Vaccination status | Serotype | 30-day mortality |
|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | Septic arthritis | Blood | Asthma, alcohol | ND | 29 | Yes |
| 2 | 88 | M | Septic arthritis, pneumonia | Blood | Neurologic disease, heart faiure | ND | 11A | No |
| 3 | 1 | F | Septic arthritis | Articular fluid | No | Yes (7•13v?) | 15A | No |
| 4 | 54 | F | Spondylodiscitis, endocarditis, septic arthritis | Articular fluid, Blood | Splenectomy | No | 23F | No |
| 5 | 58 | F | Pyomyositis, spondylodiscitis, pneumonia | Blood | Graves-Basedow disease | No | 35B | No |
| 6 | 3 | M | Spondylodiscitis | Blood | No | ND | 9V | No |
| 7 | 71 | M | Brain abscess | Blood | ND | ND | ND | Yes |
| 8 | 83 | M | Endocarditis, pneumonia | Blood | Heart failure | ND | 6C | No |
| 9 | 59 | M | Spondylodiscitis | Blood | Neoplasia | ND | 15A | Yes |
| 10 | 2 | F | Pharyngitis | Blood | No | ND | ND | No |
| 11 | 83 | F | Prosthetic joint infection | Blood | Neoplasia, heart failure | ND | 24F | No |
| 12 | 2 | M | Mastoiditis | Blood | No | ND | ND | No |
| 13 | 40 | F | Mediastinitis | Blood | No | No | 18C | No |
| 14 | 46 | F | SBP | Blood | Liver cirrhosis, alcohol | ND | ND | Yes |
| 15 | 58 | M | SBP | Blood | Liver cirrhosis, alcohol | ND | ND | No |
| 16 | 43 | F | SBP | Blood | Liver transplant | ND | 19F | No |
| 17 | 50 | F | SBP | Blood | HIV, liver cirrhosis | No | 15B | Yes |
| 18 | 42 | M | Pyomyositis | Blood, pus | HIV, chronic kidney disease, neoplasia | No | 19F | Yes |
| 19 | 40 | F | Tubo-ovarian abscess | Peritoneal fluid | No | No | 3 | No |
| 20 | 65 | M | CRBSI | Blood | Chronic kidney disease, dialysis | No | 10A | No |
| 21 | 67 | M | Cholangitis | Blood | Alcohol, asthma, diabetes mellitus | ND | 35B | No |
| 22 | 52 | F | Cholangitis | Blood | Neoplasia | ND | 15B | Yes |
ND: no data. HIV: human immunodeficiency virus; CRBSI: catheter-related bloodstream infection; SBP: spontaneous bacterial peritonitis.
No statistically significant differences were observed in uIPD diagnosis rate between before and after conjugate vaccine implementation. 4.8% (3 patients) were diagnosed in pre-conjugate vaccine implementation period, 5.2% (7 patients) in PCV7 period and 8.5% (12 patients) in PCV13 period (p=0.402). No temporal changes were observed in the IPD incidence rate during the three analysed periods (T-trend test, p=0.244).
Serotype information was available in 237/367 (65%) of patients with IPD and in 17/22 (77%) of those with uIPD and this proportion is uniform over time with the exception of 2012•2014 period in which serotyping of all strains is available. The proportion of PCV7 serotypes was similarly low in both IPD (25%) and uIPD (29%) patients (p=0.773). However, PCV13 serotypes were significantly more prevalent in IPD patients, 65%, as compared to uIPD patients, 35% (p=0.018). Serotypes 3 (15%), 19A (8%), 7F (9%) and 14 (6%) were the most prevalent in IPD patients. In contrast, only serotype 3 was isolated among uIPD patients, in whom serotypes 15A, 15B, 19F and 35B (twice each of them) were the most frequent isolated.
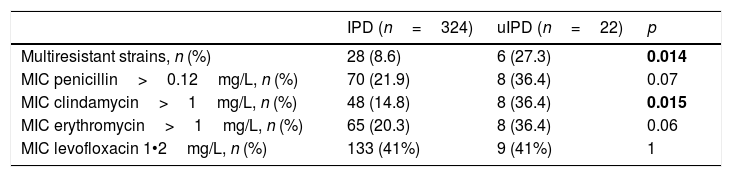
We observed a higher number of strains with a Minimum Inhibitory Concentration (MIC) of penicillin >0.12mg/L (36% vs 22%; p=0.122), erythromycin >1mg/L (36% vs 20%; p=0.103) and clindamycin >1mg/L (36% vs 15%; p=0.015) in patients with uIPD (Table 3). Isolates with a MIC of levofloxacin between 1 and 2mg/l were 41% in both groups. We also identified a higher rate of multidrug-resistant strains in uIPD patients compared to those with IPD (27% vs 9%; p=0.014). Analysing exclusively group of patients between 15 and 65 years-old these differences remain (27.2% in uIPD vs 7.1% in IPD group; p=0.018).
Antimicrobial profile of uIPD and IPD strains.
| IPD (n=324) | uIPD (n=22) | p | |
|---|---|---|---|
| Multiresistant strains, n (%) | 28 (8.6) | 6 (27.3) | 0.014 |
| MIC penicillin>0.12mg/L, n (%) | 70 (21.9) | 8 (36.4) | 0.07 |
| MIC clindamycin>1mg/L, n (%) | 48 (14.8) | 8 (36.4) | 0.015 |
| MIC erythromycin>1mg/L, n (%) | 65 (20.3) | 8 (36.4) | 0.06 |
| MIC levofloxacin 1•2mg/L, n (%) | 133 (41%) | 9 (41%) | 1 |
Values in bold are statistically significant.
In the whole IPD cohort, multiresistant strains were associated with age below 5 years-old (17% vs 8%, p=0,044), PCV 13 serotypes (18% vs 7%, p=0.021), 19F serotype (46% vs 8%; p<0.001), serotype 19A (25% vs 9%, p=0.040) and uIPD (27% vs 9%, p=0.014). Multivariate analysis showed an independent association of multiresistant strains with serotype 19A (OR 4.0, 95% CI, 1.3•12.5) and 19F serotype (OR 9.7, 95% CI, 2.3•39.6).
Overall, 30-day all-cause mortality was 15%, numerically higher in uIPD patients (32%) than in those with IPD, 13% (p=0.07). Among patients between 15 and 65 years old, 30-day all-cause mortality rate was significantly higher in the uIPD group (46% vs 9%; p=0.003) but no differences were observed between uIPD and IPD in patients older than 65 years-old (28.5% vs 29.4%; p=0.98) or in children under 15 years-old (0% vs 2%; p=0.77).
In the univariate analysis, risk factors associated with mortality were sepsis (18% vs 6.5%; p=0.013), renal failure (23.1% vs 7.3%; p=0.001), neoplasia (37% vs 12.5%; p<0.001), Charlson Index >2 (54% vs 25%; p<0.001) and a Pitt Score >2 (43% vs 8%; p<0.001). In multivariate analysis, factors associated with higher mortality rate were Charlson Index >2 (OR 5.1, 95% CI, 1.8•14.0) and Pitt Score >2 (OR 1.4, 95% CI, 1.2•1.9).
DiscussionFollowing the implementation of conjugate vaccines, the incidence of IPD dramatically decrease globally around the world.18,19 Nevertheless some authors13,17,28 like Burgos et al.14 reported an increase in the overall incidence of invasive pneumococcal pneumonia in adults, associated with serotype replacement, following the introduction of the PCV7 vaccine in Spain. In addition, there was a trend towards greater proportion of case-fatalities in patients aged 51•65 years, while in younger people this has tended to decrease. Vaccine coverage, serotypes fluctuations or infection outbreaks in a specific geographical area might contribute to explain these differences.14
Limited data, mainly from case reports or short case series, is available in regard with clinical and microbiological characteristics of less frequent manifestations of invasive pneumococcal disease.5•7,20•26 To our knowledge, our study is the first that analyse clinical and microbiological characteristics of a cohort of patients diagnosed with uIPD, after the implementation of pneumococcal conjugate vaccines.
In our cohort, in keeping with data from a systematic literature review,5 osteoarticular infection, especially septic arthritis, was the most common clinical form of uIPD. Concomitant pneumonia and/or meningitis are frequent in patients with pneumococcal septic arthritis.22 In our cohort, we identified 3 patients with arthritis and infection at other level: simultaneous pneumonia in 2 patients and coexisting endocarditis in other one.
Also, in agreement with previously reported data,24 gastrointestinal disease was the second most frequent manifestation of uIPD: spontaneous bacterial peritonitis in four patients and two cases with acute cholangitis.
Although advanced liver disease and/or alcoholism are known risk factors for IPD,25 it remains an intriguing fact the gastrointestinal infection by S. pneumoniae. Haematogenous seeding of the gastrointestinal wall and/or mucosal translocation,23 or ascension from the female genitourinary tract25 have been advocated as potential routes for gastrointestinal pneumoccocal infection (although this fact would hardly explain the cases of cholangitis).
Some recent studies have described an increase in the IPD incidence caused by non-PCV13 serotypes, that have replaced the vaccine serotypes.27,28 This has also been observed in uIPD series, although microbiological data and serotype information in these studies are scarce.5,20•26,29
In our cohort, we observed a non-statistically significant increase in uIPD incidence, from 4.3% in the prevaccination period to 6.9% following the implementation of pneumococcal conjugate vaccines, and most isolates in the uIPD group, mainly among patients older than 65 year-old, were not PCV13 serotypes.
It is tempting to especulate that less virulent strains could infect patients with higher medium age and more comorbidities. This fact, added to an expected replace of vaccine serotypes, could lead to a change in clinical presentations forms of IPD. However, until now it has not been observed as marked increase in unusual invasive pneumococcal disease.
Even though the incidence of 19A serotype has decreased after PCV13 implementation,12 it is still one of most prevalent serotypes in patients with IPD.30 In our series, two uIPD cases were caused by 19F serotype, rarely identified in recent reports,12 but we observed not any 19A serotype infection in patients with uIPD. Conversely, a high proportion of infrequent non PCV-included serotypes like 15A, 15B and 35B were seen in our cohort.
Thirty-day mortality rate was similar to that described in other studies,30 but it was higher in uIPD patients even after exclusion of paediatric population. However no association was found between uIPD presentation and mortality. Age older than 65 and primary bacteraemia presentation have been associated with a worse prognosis in this group of patients.12 In our cohort a Pitt Score >2 and a Charlson Index >2 were the only independent predictors of mortality in patients with uIPD. Conversely, isolation of multiresistant strains and empiric or targeted antimicrobial treatment did not impact in patients outcome.
The study has some limitations, one of them is the retrospective design. However, this fact has allowed us to analyse a large number of patients over an extended period of time (including PCV7 and PCV13 post-implementation period). Another limitation is that we could not serotype all S. pneumoniae isolates, but missing data was randomly distributed between two groups (77% uIPD vs 65% IPD; p=0.655).
In conclusion, unusual invasive pneumococcal disease is a rare entity that predominantly affects patients with more comorbidities than typical IPD. Osteoarticular infection and gastrointestinal diseases were the most frequent clinical forms. It is usually caused by non-vaccine serotypes with higher antimicrobial resistance level.
FundingNone to declare.
Conflict of interestNone of the authors have any disclosure.