There is a growing increase in prosthetic joint infection (PJI) incidence due to cephalosporin-resistant bacteria, used in surgical prophylaxis. The replacement of these with glycopeptides has not been shown to improve the results, but they have been shown to improve with their combination.
MethodsComparative study of combination of teicoplanin and cefazolin before arthroplasty surgery against cefazolin alone from a previous control group.
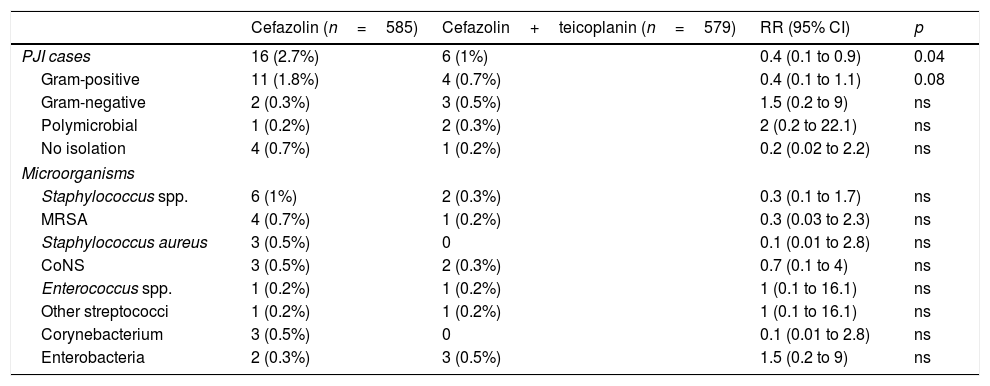
ResultsDuring the control period, there were 16 PJIs from 585 surgeries, while in the intervention group there were 6 from 579 (incidence 2.7% vs. 1.03%, RR 0.4, p=0.04). In control group, 11 of the infections were caused by Gram-positive bacteria vs. 4 in the intervention group (1.8% vs. 0.7%, p=0.08).
ConclusionsThe addition of teicoplanin to cefazolin in the prophylaxis of arthroplasty surgery was associated with a reduction in the incidence of PJI, thanks to a decrease in infections caused by Gram-positive bacteria.
Existe un creciente aumento de las infecciones de prótesis articular (IPA) por bacterias resistentes a las cefalosporinas utilizadas en la profilaxis quirúrgica. La sustitución de estas por glucopéptidos no ha demostrado mejorar los resultados pero sí su asociación.
MétodosEstudio comparativo de la asociación de teicoplanina y cefazolina antes de la cirugía de artroplastia frente a cefazolina sola de un grupo control previo.
ResultadosEn el periodo control hubo 16 IPA de 585 cirugías, mientras que en el grupo de intervención fueron 6 de 579 (incidencia 2,7% vs. 1,03%; RR 0,4, p=0,04). En el grupo control, 11 de las infecciones fueron causadas por bacterias grampositivas frente a 4 en el de intervención (1,8% vs. 0,7%, p=0,08).
ConclusionesLa adición de teicoplanina a cefazolina en la profilaxis de la cirugía de artroplastia se asoció a una reducción de la incidencia de IPA, a expensas de un descenso de las causadas por grampositivos.
Preoperative antibiotic prophylaxis with cephalosporins has demonstrated its efficacy in the prevention of prosthetic joint infection (PJI), and its use is recommended in all the surgical prophylaxis guidelines.1 Despite this, the number of cases of PJI has increased, due to the increasingly greater number of procedures which are performed, in an increasingly ageing population with a higher number of comorbidities, and increasingly more due to antibiotic-resistant bacteria, fundamentally Staphylococcus spp., both Staphylococcus aureus (S. aureus) and coagulase-negative staphylococci.2,3 For this reason, the possibility of using glycopeptides as prophylaxis instead of cephalosporins has been evaluated. This has not proven to be effective, since the benefit of achieving a reduction in gram-positive infections is compensated by a greater rate of gram-negative infections.4
At our centre, preoperative prophylaxis is performed with a single dose of cefazolin. However, 47% of PJIs were caused by bacteria resistant to this antibiotic. Several studies which have evaluated the association of cephalosporin with glycopeptide as prophylaxis in arthroplasty have shown a reduction in rates of PJI, at the expense fundamentally of those cases caused by gram-positive bacteria.5,6 Therefore, from 1 June 2015, the surgical prophylaxis protocol in elective arthroplasty was modified by adding a preoperative dose of teicoplanin to the cefazolin.
The objective of this study was to evaluate the efficacy and safety of this change to antibiotic prophylaxis in primary elective hip and knee arthroplasty.
MethodComparative before–after intervention study. Patients who were administered a dose of 800mg of teicoplanin and 2g of cefazolin before primary elective hip and knee arthroplasty from 1 June 2015 to 31 May 2017 were analysed. Patients operated on in the previous two years, in whom only one single dose of cefazolin was used, were used as the control group. The intervention was approved by the hospital's Infectious Diseases and Antibiotic Policy Committee.
In both periods, patients followed a selective decontamination of S. aureus protocol before surgery, implemented in our centre since 2011,7 in addition to the routine surgical wound prevention measures.
Patients allergic to beta-lactams and glycopeptides, and also prosthesis replacement surgeries and non-elective surgical procedures due to hip fracture were excluded.
The patients’ demographic variables, the main PJI risk factors, the onset of adverse events after the infusion of antibiotics and the development of postoperative complications such as acute kidney damage or Clostridium difficile (C. difficile) infection were recorded. We define acute kidney damage as an increase of 0.3mg/dl in creatinine levels or 1.5 times the baseline value, in accordance with the Acute Kidney Injury Network criteria.8 PJI was defined according to the latest published criteria9 and cases of it were defined as those that manifested in the first 12 months after the implantation of the prosthesis.
Statistical analysisThe description of the quantitative variables was performed with the mean and its 95% confidence interval (CI). We described the categorical variables with their number, percentage and 95% CI. We analysed the differences of means using the Student's t-test and the categorical variables using the relative risk (RR) calculation. Statistical significance was established at p<0.05. All calculations were performed with the SPSS 15.0 statistical package (Chicago, USA).
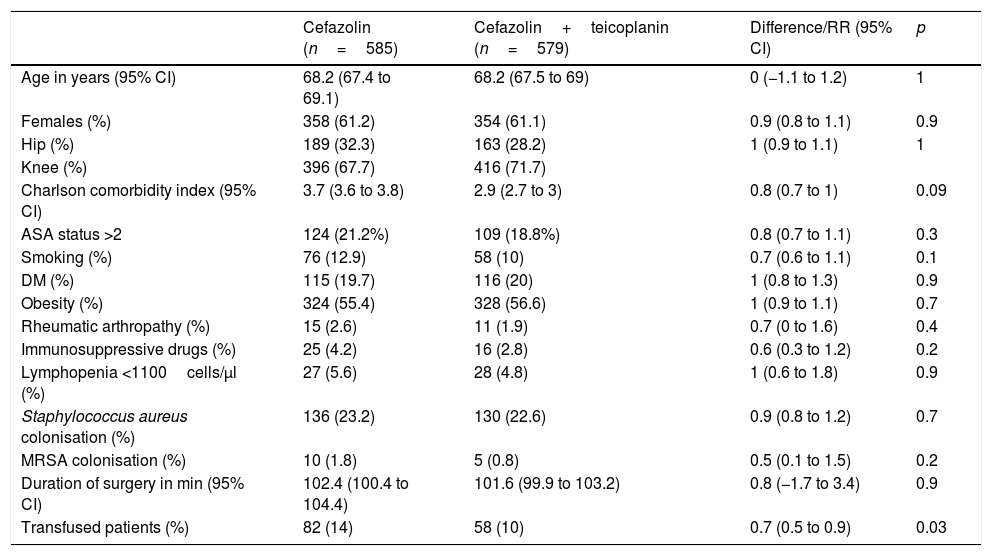
ResultsIn the control period, 585 surgical procedures were performed, of whom 16 (2.7%) developed PJI, while in the intervention group 579 patients with the teicoplanin–cefazolin regimen were included, of whom six (1%) developed complications with PJI (RR 0.4, 95% CI, 0.1–0.9, p=0.04). Table 1 shows the demographic data and the main risk factors for PJI of both groups. Table 2 shows the PJI aetiological agents of each group.
Comparison of patients in control group (cefazolin) and intervention group (cefazolin+teicoplanin).
| Cefazolin (n=585) | Cefazolin+teicoplanin (n=579) | Difference/RR (95% CI) | p | |
|---|---|---|---|---|
| Age in years (95% CI) | 68.2 (67.4 to 69.1) | 68.2 (67.5 to 69) | 0 (−1.1 to 1.2) | 1 |
| Females (%) | 358 (61.2) | 354 (61.1) | 0.9 (0.8 to 1.1) | 0.9 |
| Hip (%) | 189 (32.3) | 163 (28.2) | 1 (0.9 to 1.1) | 1 |
| Knee (%) | 396 (67.7) | 416 (71.7) | ||
| Charlson comorbidity index (95% CI) | 3.7 (3.6 to 3.8) | 2.9 (2.7 to 3) | 0.8 (0.7 to 1) | 0.09 |
| ASA status >2 | 124 (21.2%) | 109 (18.8%) | 0.8 (0.7 to 1.1) | 0.3 |
| Smoking (%) | 76 (12.9) | 58 (10) | 0.7 (0.6 to 1.1) | 0.1 |
| DM (%) | 115 (19.7) | 116 (20) | 1 (0.8 to 1.3) | 0.9 |
| Obesity (%) | 324 (55.4) | 328 (56.6) | 1 (0.9 to 1.1) | 0.7 |
| Rheumatic arthropathy (%) | 15 (2.6) | 11 (1.9) | 0.7 (0 to 1.6) | 0.4 |
| Immunosuppressive drugs (%) | 25 (4.2) | 16 (2.8) | 0.6 (0.3 to 1.2) | 0.2 |
| Lymphopenia <1100cells/μl (%) | 27 (5.6) | 28 (4.8) | 1 (0.6 to 1.8) | 0.9 |
| Staphylococcus aureus colonisation (%) | 136 (23.2) | 130 (22.6) | 0.9 (0.8 to 1.2) | 0.7 |
| MRSA colonisation (%) | 10 (1.8) | 5 (0.8) | 0.5 (0.1 to 1.5) | 0.2 |
| Duration of surgery in min (95% CI) | 102.4 (100.4 to 104.4) | 101.6 (99.9 to 103.2) | 0.8 (−1.7 to 3.4) | 0.9 |
| Transfused patients (%) | 82 (14) | 58 (10) | 0.7 (0.5 to 0.9) | 0.03 |
ASA: American Society of Anaesthesiologists; CI: confidence interval; DM: diabetes mellitus; MRSA: methicillin-resistant S. aureus; RR: relative risk.
Comparison of PJI aetiology in control group (cefazolin) and intervention group (cefazolin+teicoplanin).
| Cefazolin (n=585) | Cefazolin+teicoplanin (n=579) | RR (95% CI) | p | |
|---|---|---|---|---|
| PJI cases | 16 (2.7%) | 6 (1%) | 0.4 (0.1 to 0.9) | 0.04 |
| Gram-positive | 11 (1.8%) | 4 (0.7%) | 0.4 (0.1 to 1.1) | 0.08 |
| Gram-negative | 2 (0.3%) | 3 (0.5%) | 1.5 (0.2 to 9) | ns |
| Polymicrobial | 1 (0.2%) | 2 (0.3%) | 2 (0.2 to 22.1) | ns |
| No isolation | 4 (0.7%) | 1 (0.2%) | 0.2 (0.02 to 2.2) | ns |
| Microorganisms | ||||
| Staphylococcus spp. | 6 (1%) | 2 (0.3%) | 0.3 (0.1 to 1.7) | ns |
| MRSA | 4 (0.7%) | 1 (0.2%) | 0.3 (0.03 to 2.3) | ns |
| Staphylococcus aureus | 3 (0.5%) | 0 | 0.1 (0.01 to 2.8) | ns |
| CoNS | 3 (0.5%) | 2 (0.3%) | 0.7 (0.1 to 4) | ns |
| Enterococcus spp. | 1 (0.2%) | 1 (0.2%) | 1 (0.1 to 16.1) | ns |
| Other streptococci | 1 (0.2%) | 1 (0.2%) | 1 (0.1 to 16.1) | ns |
| Corynebacterium | 3 (0.5%) | 0 | 0.1 (0.01 to 2.8) | ns |
| Enterobacteria | 2 (0.3%) | 3 (0.5%) | 1.5 (0.2 to 9) | ns |
CI: confidence interval; CoNS: coagulase-negative staphylococci; MRSA: methicillin-resistant Staphylococcus aureus; ns: not significant; PJI: prosthetic joint infection; RR: relative risk.
One patient in each group developed a skin rash immediately after the infusion of antibiotics, with no angioedema or anaphylaxis. The rash was abated with antihistamines and they were not forced to discontinue the intervention. There were no cases of C. difficile infection in any of the groups during the hospital admission. In the intervention group there were 15 cases (2.6%) of acute kidney injury. All the cases were mild and reversible, while in the control group there were 12 (2.1%, RR 1.2, 95% CI, 0.6–2.7, p not significant). No cases of osteoarticular infection due to Enterococcus faecium resistant to glycopeptides occurred in the study period.
DiscussionIn our study, the addition of a preoperative dose of teicoplanin to the routine dose of cefazolin was associated with a statistically significant reduction of 60% of the PJI rate, at the expense of the decrease in cases caused by gram-positive bacteria, particularly Staphylococcus spp., without modifying the infections caused by gram-negative bacteria or increasing the adverse effects.
The growing number of infections caused by multidrug-resistant bacteria implies, among other consequences, a severe threat for the successes achieved with different surgical procedures or the implantation of devices (pacemakers, valves, etc.).10 Most antibiotic prophylaxis studies are from the 1970s and 1980s, where the main causative agents of postoperative or postimplant infection were sensitive.11,12
In the particular case of PJIs, as in other infections associated with biodevices, in recent decades a notable increase in cases caused by methicillin-resistant S. aureus and other beta-lactams has been reported, which, combined with enterococcal infections and others implies that currently a good part of PJIs are caused by antibiotic-resistant bacteria more routinely used in their prevention, cephalosporins.2,3 Accordingly, a change in the prevention strategies of these infections seems necessary.13 Decolonisation of patients carrying S. aureus, implemented in our centre several years ago with good results, is an effective option to reduce infections caused by this bacterium,7 but it does not act on coagulase-negative staphylococci, whose rate of resistance to methicillin, and by extension, to cephalosporins, is also greater. In this respect, the option of using glycopeptides to replace cephalosporins in preoperative antibiotic prophylaxis has been evaluated. However, the results have not offered benefits, since the reduction achieved in gram-positive infections is compensated by a greater rate of gram-negative infections. In addition, there are even more methicillin-susceptible S. aureus infections.4
Therefore, the following approach would be if the combination of cephalosporins with glycopeptides could be useful. Studies performed with vancomycin, alone or in association, have demonstrated moderate success at reducing the incidence of PJI, but with excess nephrotoxicity.6,14,15 Teicoplanin has a more favourable side effect profile than vancomycin. It is less nephrotoxic and can be administered over a shorter period of time, which is important in the operational logistics of operating theatre activity.16 In this sense, experience published with the teicoplanin–cefuroxime combination has been proven to improve results in the prevention of PJI.5
At our centre, as in others, almost half of PJIs were caused by cephalosporin resistant-bacteria. The surgical prophylaxis protocol was therefore changed, combining cefazolin and teicoplanin, after which an overall decrease was produced in the rate of PJIs, at the expense of infections caused by gram-positive bacteria, with a reduction in the incidence of Staphylococcus cases in general, and of methicillin-resistant S. aureus infections, in particular. The benefit of this new regimen could be attributed to the greater half-life of teicoplanin compared to cefazolin, which would extend its effect several hours after the performance of surgery, and not to the broadening of the antibiotic spectrum. However, it has been demonstrated that the use of successive doses of cephalosporins after surgery does not add benefit to that of a single preoperative dose.17 This fact can therefore be ruled out.
This procedure was also safe, without producing excess acute kidney injury (and all cases were also reversible), anaphylactic reactions or C. difficile superinfections, compared to the control group. Furthermore, to date, the greater use of teicoplanin has not impacted on the onset of cases of glycopeptide-resistant Enterococcus infections, prosthesis or other locations.
One of the limitations of this study is that it is single-centre and the results may not be applicable in hospitals with a high incidence of cases of teicoplanin-resistant S. aureus, of which there are currently none at our centre. Another limitation is that patients are not randomised, with the control group being a historical cohort, i.e. it is not a clinical trial, rather a quasi-experimental study. The control group patients have similar characteristics to those of the intervention group, although it is true that more patients in the control group received transfusions, which is a well-known risk factor of PJI, and this may be a bias. However, when analysing only the patients who received transfusions, the reduction of cases was maintained (6.1% vs 1.7%). In addition, our study shows similar results to those published, and it is consistent in that only gram-positive infections were reduced, with the gram-negative PJI rate being similar, since teicoplanin does not have activity on these, which strongly suggests causality of the intervention and seems to rule out that it was a causal effect.
Finally, the chosen follow-up period, 12 months, could also be a bias, as there could be cases of coagulase-negative staphylococcal infections which will be manifested later in this period. In fact, there have been cases of PJI after these 12 months in the intervention group, but also (and in a greater number) in the control group, meaning that the results are not affected.
In conclusion, the combination of teicoplanin and cephalosporin seems to be effective and well-tolerated in the prevention of elective PJI.
FundingThis study did not receive any funding.
Conflicts of interestJosé María Barbero declares that he has received fees for conferences from the pharmaceutical company Angelini. Jose Sanz Moreno declares that he has received fees due to teaching and consultancy collaborations from the pharmaceutical companies Gilead, Viid and Janssen. All other authors declare that they have no conflicts of interest.
Please cite this article as: Barbero-Allende JM, García-Sánchez M, Montero-Ruiz E, Vallés-Purroy A, Plasencia-Arriba MÁ, Sanz-Moreno J. Profilaxis dual con teicoplanina añadida a cefazolina en la prevención de la infección de prótesis articular. Enferm Infecc Microbiol Clin. 2019;37:588–591.