Infectious gastroenteritis remains a public health problem. The most severe cases are of bacterial origin. In Spain, Campylobacter and Salmonella are the most prevalent bacterial genus, while Yersinia and Shigella are much less frequent. Most cases are usually self-limiting and antibiotic therapy is not generally indicated, unless patients have risk factors for severe infection and shigellosis. Ciprofloxacin, third generation cephalosporins, azithromycin, ampicillin, cotrimoxazole and doxycycline are the most recommended drugs. The susceptibility pattern of the different bacteria determines the choice of the most appropriate treatment. The aim of this review is to analyse the current situation, developments, and evolution of resistance and multidrug resistance in these 4 enteric pathogens.
La gastroenteritis infecciosa continúa siendo un problema de salud pública. La etiología bacteriana es la responsable de la mayoría de los casos graves. En nuestro país, Campylobacter y Salmonella son los géneros bacterianos más prevalentes, mientras que Yersinia y Shigella son mucho menos frecuentes. La mayoría de los casos suelen ser autolimitados y, en general, el tratamiento antibiótico no está indicado, salvo en pacientes con factores de riesgo de infección grave y en shigelosis. Ciprofloxacino, cefalosporinas de tercera generación, azitromicina, ampicilina, cotrimoxazol y doxiciclina son los fármacos más recomendados. El patrón de sensibilidad de las diferentes bacterias determina la elección del tratamiento antibiótico más adecuado. El objetivo de esta revisión es analizar la situación, las novedades y la evolución de la resistencia y la multirresistencia en estos 4 enteropatógenos.
Infectious gastroenteritis remains a public health problem. Bacterial causes, which are less prevalent than viral causes, are responsible for most severe episodes.1
Antibiotic treatment of infectious diarrhoea of known causes, and empirical antibiotic therapy, must be considered in specific cases in order to shorten the duration of the illness, decrease transmission and prevent the onset of complications.1 It is therefore important to be aware of the most common pathogens and their antibiotic susceptibility profile.
In Spain, Campylobacter and Salmonella are the most common aetiologic agents, followed by Yersinia and Shigella,2 which is why this study will focus on the latest antibiotic developments against these pathogens.
Non-typhoidal Salmonella entericaSigns and symptoms of gastroenteritis due to non-typhoidal S. enterica (NTS) are usually mild and self-limiting. The use of antibiotics is not recommended and may even prolong carriage of the pathogen.1,3 However, in patients with risk factors for bacteraemia, severe diarrhoea or signs of systemic infection, the treatment of choice is an oral fluoroquinolone or azithromycin for cases of enteritis and third-generation cephalosporins, aztreonam or ciprofloxacin for cases of bacteraemia or localised infection.4
Salmonella serotypes Enteritidis and Typhimurium account for more than 80% of isolates obtained in clinical practice.2 Both in Spain and worldwide, Enteritidis has been the predominant serotype for a very long time. However, over recent years, a change has been observed, with figures increasing for serotype Typhimurium.5–8 The importance of this change is based on the susceptibility profile since serotype Typhimurium is associated with higher antibiotic resistance and multi-drug resistance rates.6,9
Ampicillin and amoxicillin/clavulanic acid resistance in NTS is attributed to classic plasmid-mediated β-lactamases, with TEM-1, PSE-1 and OXA-1 being the most common, although the latter two seem to be more limited to serotype Typhimurium.6,10,11
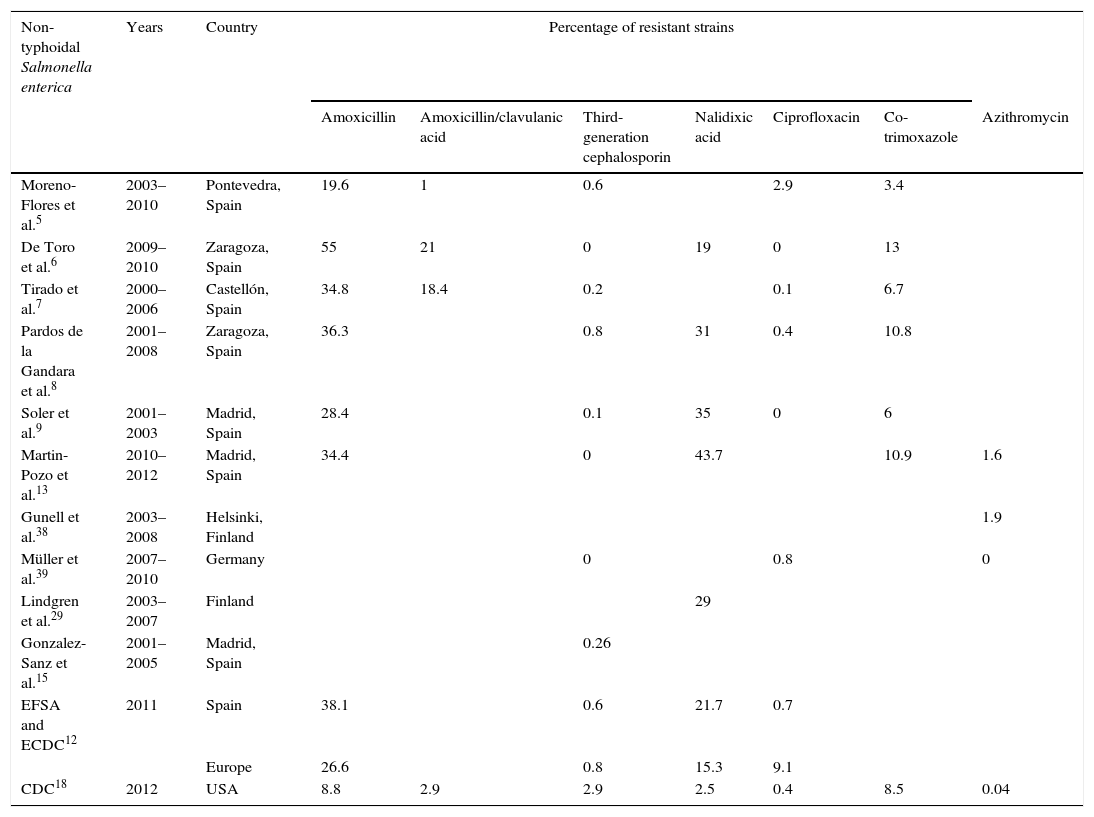
According to European data, approximately 26% of NTS strains are resistant to ampicillin, with variations from 0% to 59% based on geographical region.12 Resistance rates in Spain are greater than 30%, which is higher than the European average.5,6,8,13 Resistance to amoxicillin/clavulanic acid is on the increase in Spain, although it is still relatively low, with rates below 1% in 2001–2003 and around 7–16% in recent years.5–7
Numerous extended spectrum β-lactamase (ESBL)- and cephamycinase-producing NTS strains have been described, with CMY-25,8,10,14–17 being the most common. In Spain, resistance rates to ceftriaxone are lower than 0.5%.8,9,15 In the USA, 3% of strains were resistant in 2012.18 Some studies show that serotype Virchow accounts for a high percentage of ESBL-producing strains, primarily CTX-M-9.8,15
In rare cases, KPC, OXA-48, NDM and VIM-type carbapenemase producers have been reported.16,19–21 Given their capacity to spread and how difficult phenotypic detection can sometimes be, these may pose a threat in the future. Resistance to carbapenems among Salmonella species has also been associated with other mechanisms, such as cephamycinase production and loss of porins and also loss of OmpC expression during treatment with ertapenem.22,23
Resistance to co-trimoxazole in Spain has remained constant over the past decade, varying between 5 and 13%.6,7,9,13
High-level fluoroquinolone resistance, which was defined until recently as MIC>1mg/l or MIC≥4mg/l by EUCAST and CLSI, respectively, is still rare, which may be due to fitness costs, which could limit its survival in the absence of antibiotic selection pressure.24
The spread of S. enterica serotype Kentucky displaying high-level ciprofloxacin resistance (MIC>1mg/l) over the past decade deserves special attention. Le Hello et al.16 described an increase in the number of isolates in Europe and Africa between 2002 and 2010. Despite the fact that absolute figures are not high, its geographic spread and temporal evolution is concerning, as is the higher rate of hospitalisation compared with ciprofloxacin-susceptible strains of this same serotype. All isolates belonged to the ST198 clonal complex, isolated from chickens and turkeys from different African countries, suggesting that these animals are the possible source of infection. Resistance was due to a double mutation in gyrA and a single parC mutation. In addition, strains have appeared since 2009 that have also acquired a variety of β-lactamases, with resistance to broad-spectrum cephalosporins and carbapenems. Among those enzymes described are ESBLs CTX-M-1 and CTX-M-15, cephamycinase CMY-2 and carbapenemases VIM-2 and OXA-48.16,25,26
The phenotype that currently predominates is characterised by its high-level resistance to nalidixic acid and decreased susceptibility to ciprofloxacin (MIC=0.12–1mg/l). Most have a single mutation in gyrA27; this phenotype is related to low clinical response and increased mortality in patients with systemic infections treated with fluoroquinolones.28 However, we also found strains that are susceptible to nalidixic acid with decreased susceptibility to ciprofloxacin due to plasmid-mediated resistance mechanisms associated with qnr and aac-6′-Ib-cr genes.29–31 Although there is still no information on the clinical impact that this phenotype may have, its decreased susceptibility to ciprofloxacin seems to be the most important factor for predicting treatment response.32 Prevalence of plasmid-mediated resistance is low in the USA33,34 and somewhat higher in Europe and Asia.29–31,35
The method recommended by both CLSI and EUCAST for evaluating and reporting quinolone susceptibility in Salmonella was based on the use of nalidixic acid as a screening test. The discovery of other mechanisms of quinolone resistance in this genus has meant that this method is no longer suitable for inferring resistance in all cases. Therefore, EUCAST (since 2014) and CLSI (since 2015) have recommended the use of 5-μg pefloxacin disks. However, CLSI considers all strains with MIC≥1mg/l to be ciprofloxacin-resistant, unlike EUCAST whose breakpoint has fallen to 0.06mg/l.36,37
With regards to azithromycin, Martin-Pozo et al.13 found that 98.4% (63/64) of NTS strains had a MIC below the EUCAST epidemiological cut-off value (MIC≤16mg/l). Likewise, only 1.9% (24/1237) of the strains studied by Gunell et al.38 had a MIC≥32mg/l and most were between 4 and 8mg/l. In the study conducted by Müller et al.39 (No.=125), none of the strains showed resistance to azithromycin.
The phenomenon of multi-drug resistance, which is defined as resistance to 3 or more groups of antimicrobial classes, is currently of great concern as it is on the increase and limits treatment options. This is the case of Salmonella typhimurium definitive phage type (DT) 104, which has common resistance to ampicillin, chloramphenicol, streptomycin, sulphonamides and tetracycline (ACSSuT resistance type) and began to spread during the mid-1980s all over the world.40 De Toro et al.6 observed a high percentage of resistance to ACSSuT, especially in this serotype. They found multi-resistant phenotypes in most ampicillin-resistant isolates, with the most common patterns being ACSSuT and AACSTCSu (AC: amoxicillin/clavulanic acid). Soler et al.9 found that 74% (896/1211) of Salmonella typhimurium isolates at the beginning of the last decade were resistant to 4 or more antibiotics and only 13.5% (163/1211) were susceptible to all antibiotics evaluated. Only 18.5% (387/2092) of the strains studied by Pardos de la Gandara et al.8 were susceptible to all of the antibiotics analysed and a multi-drug resistant phenotype was found in all ESBL-producing strains. The association of different families of β-lactamases found on conjugative plasmids increases resistance and the capacity to spread. Examples include associations of CTX-M-14 with OXA-1 and CTX-M-9 with SHV-12.15 Even more concerning is the simultaneous presence of CMY-2 and OXA-48 in serotype Kentucky ST198-X1, which is resistant to ciprofloxacin.16
The data analysed (Table 1) show low resistance rates to antibiotics of choice to date, especially in the case of third-generation cephalosporins and azithromycin. With regard to quinolones, high-level resistance to ciprofloxacin is still rare, unlike resistance to nalidixic acid. Furthermore, the new screening system allows a more exhaustive analysis of resistance to this group of antimicrobials to be carried out and adequate guidance for antibiotic treatment.
Percentage of resistance in non-typhoidal Salmonella enterica.
| Non-typhoidal Salmonella enterica | Years | Country | Percentage of resistant strains | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amoxicillin | Amoxicillin/clavulanic acid | Third-generation cephalosporin | Nalidixic acid | Ciprofloxacin | Co-trimoxazole | Azithromycin | |||
| Moreno-Flores et al.5 | 2003–2010 | Pontevedra, Spain | 19.6 | 1 | 0.6 | 2.9 | 3.4 | ||
| De Toro et al.6 | 2009–2010 | Zaragoza, Spain | 55 | 21 | 0 | 19 | 0 | 13 | |
| Tirado et al.7 | 2000–2006 | Castellón, Spain | 34.8 | 18.4 | 0.2 | 0.1 | 6.7 | ||
| Pardos de la Gandara et al.8 | 2001–2008 | Zaragoza, Spain | 36.3 | 0.8 | 31 | 0.4 | 10.8 | ||
| Soler et al.9 | 2001–2003 | Madrid, Spain | 28.4 | 0.1 | 35 | 0 | 6 | ||
| Martin-Pozo et al.13 | 2010–2012 | Madrid, Spain | 34.4 | 0 | 43.7 | 10.9 | 1.6 | ||
| Gunell et al.38 | 2003–2008 | Helsinki, Finland | 1.9 | ||||||
| Müller et al.39 | 2007–2010 | Germany | 0 | 0.8 | 0 | ||||
| Lindgren et al.29 | 2003–2007 | Finland | 29 | ||||||
| Gonzalez-Sanz et al.15 | 2001–2005 | Madrid, Spain | 0.26 | ||||||
| EFSA and ECDC12 | 2011 | Spain | 38.1 | 0.6 | 21.7 | 0.7 | |||
| Europe | 26.6 | 0.8 | 15.3 | 9.1 | |||||
| CDC18 | 2012 | USA | 8.8 | 2.9 | 2.9 | 2.5 | 0.4 | 8.5 | 0.04 |
Y. enterocolitica primarily causes enterocolitis in children in winter months and more rarely mesenteric lymphadenitis, terminal ileitis, septicaemia (in individuals with depressed immune systems) and reactive arthritis. It is associated with the consumption of contaminated water and foods, especially pork, as pigs are the most common reservoir. In Europe, serotypes O:3 and O:9, and rarely O:5.27 and O:8, cause infections in humans, with O:3 being the most common. Serotype O:8 is primarily detected in the USA.41
Most symptoms of enterocolitis are self-limiting and do not require antibiotic therapy. If necessary, the drug of choice for gastroenteritis is ciprofloxacin, and for systemic infections, a third-generation cephalosporin with gentamicin. Co-trimoxazole and doxycycline are accepted alternatives.4
The main mechanism of resistance to β-lactams in Y. enterocolitica is the production of chromosomal β-lactamases, a constitutive class A penicillinase (Bla-A) and an inducible class C cephalosporinase (Bla-B) that is not inhibited with β-lactamase inhibitors. The genes Bla-A and Bla-B are found in most of the strains, but expression of one or the other depends on the serotype.42,43 Expression of both genes would result in resistance to aminopenicillins, carboxypenicillins, first-generation cephalosporins, cefoxitin and amoxicillin/clavulanic acid. Despite being found in the chromosome of practically all strains of Y. enterocolitica, expression of both enzymes, of only one enzyme or of neither enzyme is due, among other possible factors, to point mutations.44,45
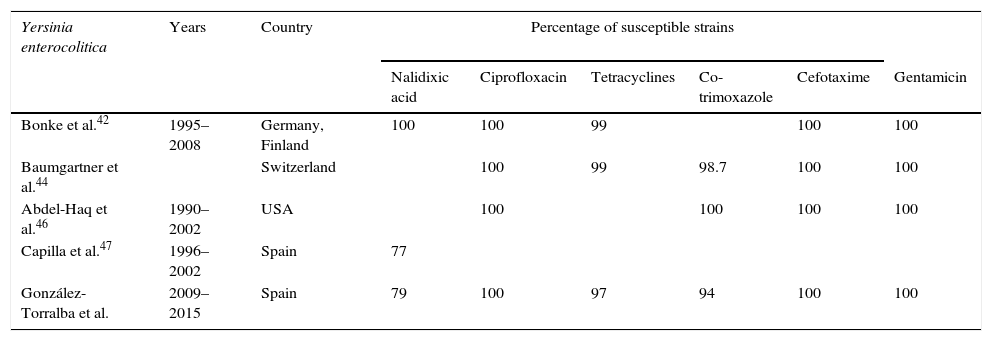
Most strains are currently susceptible to a large number of antibiotics, including third- and fourth-generation cephalosporins, carbapenems, aminoglycosides and fluoroquinolones. The resistance rate for co-trimoxazole is less than 2%, while that for tetracyclines is less than 1%.13,42–44,46
Capilla et al.47 detected that 23% of serotype O:3 strains were nalidixic acid-resistant, of which practically all showed a single mutation in gyrA with high heterogeneity in the mutations observed. They found that high-level resistance to nalidixic acid is associated with a mutation in gyrA plus the overexpression of efflux pumps. Decreased susceptibility to ciprofloxacin, however, was only associated with the presence of a mutation in gyrA. All strains were susceptible to ciprofloxacin according to current breakpoints.
In our experience, we have observed an increase in resistance to nalidixic acid over recent years, increasing from 13.5% in the 2009–2011 period to 32% in 2012–2015, and an increase in co-trimoxazole-resistant strains (González-Torralba, manuscript being drafted).
According to the data analysed (Table 2), we can conclude that the aforementioned treatments of choice are still displaying excellent in vitro activity against Y. enterocolitica, although the increase in resistance to nalidixic acid deserves special attention due to its possible involvement in fluoroquinolone resistance in the future.
Percentage of susceptibility in Yersinia enterocolitica.
| Yersinia enterocolitica | Years | Country | Percentage of susceptible strains | |||||
|---|---|---|---|---|---|---|---|---|
| Nalidixic acid | Ciprofloxacin | Tetracyclines | Co-trimoxazole | Cefotaxime | Gentamicin | |||
| Bonke et al.42 | 1995–2008 | Germany, Finland | 100 | 100 | 99 | 100 | 100 | |
| Baumgartner et al.44 | Switzerland | 100 | 99 | 98.7 | 100 | 100 | ||
| Abdel-Haq et al.46 | 1990–2002 | USA | 100 | 100 | 100 | 100 | ||
| Capilla et al.47 | 1996–2002 | Spain | 77 | |||||
| González-Torralba et al. | 2009–2015 | Spain | 79 | 100 | 97 | 94 | 100 | 100 |
There are about 165 million cases of gastroenteritis due to Shigella worldwide each year. According to the ECDC Annual Epidemiological Report 2013,48 7322 cases were reported in Europe in 2011, showing a gradual decrease from 2007, in which 8380 cases were reported.
It is transmitted through contaminated food or water and person-to-person contact. It has recently become evident that shigellosis in men who have sex with men is basically a sexually-transmitted infection. One of the main risk factors associated with Shigella infection is recent travel to an endemic area. Therefore, in Europe, approximately 60% of patients acquired the infection on other continents, primarily Africa and Asia.48
Of the 4 species included in the Shigella genus, S. sonnei and S. flexneri currently account for the most cases.49 However, while S. flexneri predominates in developing countries, developed countries have more cases caused by S. sonnei. Therefore, in Europe, in 2011 S. sonnei was detected in 61% of cases and S. flexneri was detected in 32%.48 Likewise, countries experiencing recent socio-economic growth, such as Vietnam, showed an increase from 29% to 78% in S. sonnei cases between 1995 and 2008.50
Clinical manifestations of Shigella infection are primarily determined by the Shigella serotype involved. While S. sonnei tends to be associated with milder symptoms of gastroenteritis, S. flexneri and especially S. dysenteriae may cause severe cases. The main complications are bacteraemia, neurological symptoms and haemolytic uraemic syndrome.
Antibiotic treatment in shigellosis reduces the risk of potential complications, shortens the duration of symptoms and reduces excretion of the microorganism in faeces. The use of antibiotics is therefore indicated.51 It is a fundamental measure for preventing transmission, especially in the event of an outbreak, due to the low infectious dose required to cause Shigella infection.
Empiric treatment currently includes fluoroquinolones (ciprofloxacin), azithromycin and third-generation cephalosporins (ceftriaxone) as first-line drugs, all of which have low global resistance rates. Alternatives include ampicillin and co-trimoxazole.4
S. flexneri tends to be more resistant to ampicillin and is more multi-drug resistant to antibiotics than S. sonnei. However, S. sonnei is more likely to be resistant to co-trimoxazole than S. flexneri.52–57
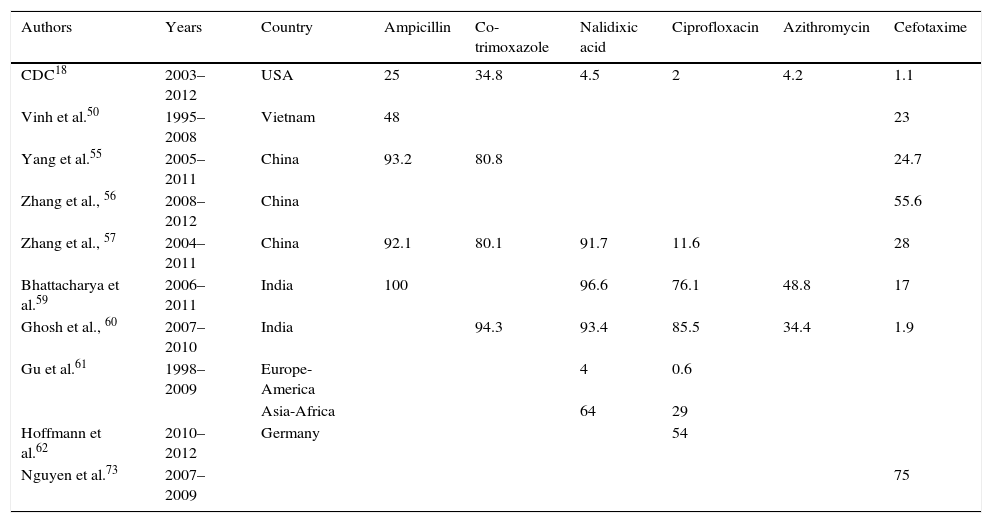
Ampicillin resistance was first detected in the 1970s, a few years after being recognised as the treatment of choice for shigellosis.58 The highest resistance rates (>90%) have been detected in China55,57 and India.59 Different studies in other countries have observed a gradual decrease over recent years. Consequently, in the USA, the resistance rate in 2003 was 79%, but by 2012 had fallen to 25%.18 Likewise, the rate fell from 75% to 48% in Vietnam between 1995 and 2008.50
Co-trimoxazole emerged as an alternative treatment to ampicillin, but high resistance rates were observed within just a few years.58 Today, in countries such as the USA, resistance rates vary between 30% and 50%, while in China they reach 70–80% and exceed 90% in India.18,55,57,60
After the appearance of these high resistance rates to both drugs, nalidixic acid began to be used followed by fluoroquinolones.58 Different studies show a high degree of heterogeneity in resistance rates to quinolones worldwide. Gu et al.61 conducted a meta-analysis comparing resistance rates in 2 different geographical areas (Europe-America and Africa-Asia) from 1998 to 2009. In Africa-Asia, resistance to nalidixic acid reached 64% in 2009, which was 5 times higher than in 1998, and resistance to ciprofloxacin increased almost 50 times to a final rate of 29%. In Europe-America, resistance rates to nalidixic acid and ciprofloxacin did not exceed 4% and 0.6%, respectively, throughout the study period, with few changes observed. In the NARMS study, in the USA, 4.5% of isolates in 2012 were resistant to nalidixic acid and 2% were resistant to ciprofloxacin, with a statistically significant increase in resistance to nalidixic acid from 2003.18 In Germany, higher resistance rates to ciprofloxacin (54%) have been detected in men who have sex with men (MSM); resistance was even higher in HIV-infected MSM (66 vs 24%).62 In India, resistance rates to nalidixic acid exceed 90%, while resistance rates to fluoroquinolones are 70–85%.59,60 In China, studies also detected that more than 90% of isolates were resistant to nalidixic acid. Although global resistance to ciprofloxacin was 11.6%, the species analysis showed higher rates and an upward trend in S. flexneri, with figures reaching 54% in 2011.57
Another first-line drug that can be administered orally is azithromycin. Although there are currently no clinical breakpoints for Shigella, those strains with MIC≥32mg/l are considered to have decreased susceptibility.18 The mechanism responsible for this is the presence of the mphA gene, which encodes the phosphotransferase that inactivates the antibiotic.63 Resistance to this antibiotic in the USA varied between 3.1% and 4.2% in 2011–2012.18 These figures are similar to those reported by Pons et al.53 between 1995 and 2010 at a tropical medicine unit in Spain. A higher proportion of strains with reduced susceptibility to azithromycin has been detected in men who have sex with men, especially those infected with HIV.64–66 Over recent years, more alarming levels of resistance have been detected with rates in India reaching 34–49%.59,60
The first case of resistance to third-generation cephalosporins in Shigella was reported in 2001.67 Later, new cases were detected in Iran, India, Bangladesh, Vietnam and even Spain.54,68–71 Folster et al.72 studied 3880 strains in the USA between 1999 and 2007, 6 of which were ESBL producers. The blaCTX-M-15 gene was detected in 4 of the strains and the blaCTX-M-14 gene was detected in the other 2. At present, resistance to third-generation cephalosporins in the USA is stable at very low levels, accounting for 1.1% in 2012.18 In India, Ghosh et al.60 discovered that 1.9% of S. flexneri isolates were resistant to third-generation cephalosporins, while no S. sonnei resistant strains were detected. However, another study conducted in India, in the Bay of Bengal, detected that 17% of strains were resistant between 2006 and 2011.59 In China, resistance rates to cefotaxime of 28–30% have been reported.55,57 Zhang et al.56 analysed isolates between 2008 and 2012 and found that 55.6% were resistant to cefotaxime, mainly due to ESBL production. The presence of AmpC was identified in 2 isolates. In Vietnam, a major increase in third-generation cephalosporin-resistant strains has been detected, increasing from 1% in 2000 to 23% in 2008 in one study,50 and from 0% to 75% in another study conducted between 2007 and 2009. 94.5% of the strains were S. sonnei and CTX-M-15 and CTX-M-24 production was detected in 92% and 8%, respectively.73
At present, the presence of carbapenemases has not been detected in Shigella.
Resistance of US strains to 3 classes of antibiotics was 37%, to 4 classes was 19%, and to 5 classes was 8%.18 In Chile, multi-drug resistance in S. sonnei has increased since 1997; in 2008–2009, 100% of strains were resistant to at least ampicillin, co-trimoxazole, tetracycline and chloramphenicol.74 Fifty-three per cent of strains with decreased susceptibility to azithromycin among men who have sex with men were resistant to 5 or more classes of antibiotics and 4% were also resistant to ciprofloxacin.65 Strains that are resistant to more than 10 different antibiotics have been described in 45% of cases in India,59 and multi-drug resistance has been detected in 90% of the strains studied in China, with the following patterns being the most common: ampicillin-tetracycline-co-trimoxazole in 70.8% of strains and ampicillin-co-trimoxazole-ciprofloxacin in 24%.55 Ninety-seven per cent of third-generation cephalosporin-resistant isolates in Vietnam also displayed resistance to co-trimoxazole and tetracyclines.73 Furthermore, strains that are resistant to fluoroquinolones and third-generation cephalosporins have also been detected, reaching levels of up to 74% in S. flexneri isolates.56
In Europe, Shigella infection is primarily associated with recent travel to endemic areas. Since there is considerable geographical variability between the different resistance patterns (Table 3), it is vital to know the patient's epidemiological information in order to prescribe a suitable empiric therapy. Currently, no resistance to carbapenems has been detected, and third-generation cephalosporins usually have low resistance rates. Fluoroquinolones and azithromycin are alternative oral therapies.
Percentage of resistance in Shigella.
| Authors | Years | Country | Ampicillin | Co-trimoxazole | Nalidixic acid | Ciprofloxacin | Azithromycin | Cefotaxime |
|---|---|---|---|---|---|---|---|---|
| CDC18 | 2003–2012 | USA | 25 | 34.8 | 4.5 | 2 | 4.2 | 1.1 |
| Vinh et al.50 | 1995–2008 | Vietnam | 48 | 23 | ||||
| Yang et al.55 | 2005–2011 | China | 93.2 | 80.8 | 24.7 | |||
| Zhang et al., 56 | 2008–2012 | China | 55.6 | |||||
| Zhang et al., 57 | 2004–2011 | China | 92.1 | 80.1 | 91.7 | 11.6 | 28 | |
| Bhattacharya et al.59 | 2006–2011 | India | 100 | 96.6 | 76.1 | 48.8 | 17 | |
| Ghosh et al., 60 | 2007–2010 | India | 94.3 | 93.4 | 85.5 | 34.4 | 1.9 | |
| Gu et al.61 | 1998–2009 | Europe-America | 4 | 0.6 | ||||
| Asia-Africa | 64 | 29 | ||||||
| Hoffmann et al.62 | 2010–2012 | Germany | 54 | |||||
| Nguyen et al.73 | 2007–2009 | 75 |
Since 2005, Campylobacter has been the enteropathogen with the highest number of reported cases in the European Union. In recent years, an increase in cases of up to 14% has been detected in the USA.75–77
Infections in humans are mainly caused by thermotolerant species Campylobacter jejuni and Campylobacter coli. In Europe, almost 90% of the 214,268 confirmed cases reported in 2012 were associated with these species.76
The main clinical symptom of most infections is self-limiting acute diarrhoea. However, severe, prolonged cases or relapse may occur, especially in very young or elderly patients, immunodeficient patients and pregnant women. Extra-intestinal manifestations, such as bacteraemia or meningitis, and post-infection complications (reactive arthritis, Guillain-Barré syndrome) are rare.
Whenever antibiotic therapy is indicated, erythromycin and azithromycin are considered first-line therapeutic agents.1 Fluoroquinolones and tetracyclines are among the available alternatives. In the event of systemic infection, patients should be treated with imipenem alone or in combination with gentamicin.4,78
Resistance to macrolides is mainly due to point mutations in the 23S rRNA gene, primarily at positions 2074 and 2075. When all 3 copies of the gene, which are carried by both C. jejuni and C. coli, are affected, MICs are high79; strains have been described that only have 2 mutated gene copies, with lower MICs to macrolides.80 Other mechanisms include mutations in ribosomal proteins L4 and L22, which are associated with low-level resistance, and efflux pumps, mainly CmeABC, which act synergistically with 23S rRNA mutations to generate high resistance.78,79,81 The presence of gene ermB, which encodes a ribosomal methylase, has recently been detected.82 In one study involving 1554 strains, including 10 human strains, this gene was found in 3.7% of the strains, mainly in C. coli. All strains with ermB were resistant to erythromycin, most with MIC>512mg/l, and to clindamycin, ciprofloxacin and tetracyclines. The distribution over time shows a recent appearance and upward trend; horizontal transmission of this gene has also been shown between C. coli and C. jejuni.83
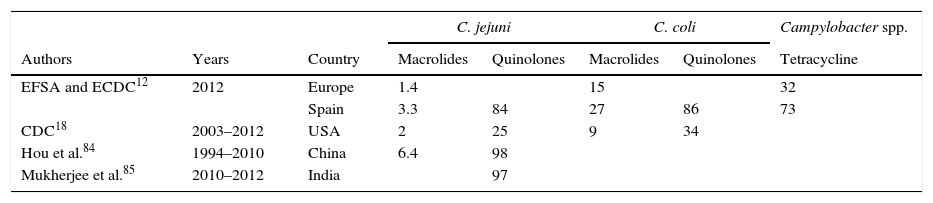
Globally, the resistance rate to macrolides has remained at a low and stable level. In Europe, erythromycin-resistant strains account for 1.4% in C. jejuni and 15% in C. coli. In Spain, these figures are higher, currently standing at 3.3 and 27%, respectively.12 In the USA, figures reach 2% for C. jejuni and 9% for C. coli.18 The highest erythromycin resistance rates in C. jejuni are 6.4%, which have been reported in China.84
One of the main problems when treating Campylobacter infections is the increased resistance to fluoroquinolones and tetracyclines detected worldwide. Resistance to fluoroquinolones is mainly due to mutations in the quinolone-resistance-determining region of the gene that encodes the gyrA subunit of DNA gyrase. The most common mutation involves a Thr86Ile amino acid change, which generates high resistance to nalidixic acid and ciprofloxacin (MIC>16mg/l); other mutations cause a Thr86Ala amino acid change, which results in high resistance to nalidixic acid but low resistance to ciprofloxacin. Alterations have also been described in the CmeABC efflux pump system, which acts synergistically with other mechanisms, producing a high-level resistance to fluoroquinolones.79–81
Fluoroquinolone resistance rates cover a wide range among the different countries. In the USA, between 1997 and 2011, 25% of C. jejuni and 34% of C. coli were resistant to ciprofloxacin, showing an increase over time of 12%.18 In Spain, resistance rates are especially high, with rates of 84% in C. jejuni and 86% in C. coli.12 However, the highest rates have been reported in India, 97%,85 and China, 98%.84 Between 1994 and 2006, Vlieghe et al.86 detected norfloxacin resistance in 70% of the strains from Asia, 60.6% of the strains from Latin America and 30% of the strains from Africa in patients with a history of international travel. The most notable increase over time was for the Indian subcontinent, increasing from 29% to 78%.
Resistance to tetracyclines is measured by modification of the ribosome binding site as a result of production of an encoded protein in the tetO gene, which confers high MICs. Although this gene is normally of plasmid origin, it has also been detected in the chromosome. Mechanisms of efflux pumps, such as CmeABC and CmeG, also play a role.79–81 In Europe, resistance rates of 32% were detected in 2012, with particularly high rates in Spain (73%).12
Other antibiotics that can be used as treatment are aminoglycosides and β-lactams. With regard to aminoglycosides, enzymatic modifications, which decrease affinity for the 30S subunit binding site, are responsible for conferring resistance. Multiple enzymes (which are normally plasmid-borne) have been described, but the most common in both C. jejuni and C. coli is a phosphotransferase.79
Mechanisms of resistance to β-lactams are less well known. The presence of β-lactamases, including a class D β-lactamase that is not inhibited by clavulanic acid, OXA-61, has been detected. Alterations in porins, and the existence of efflux pumps such as CmeABC, have also been described.80
Resistance to gentamicin and carbapenems is rare. Fernandez-Cruz et al.87 analysed Campylobacter bacteraemia over 23 years. All isolates were susceptible to imipenem and 94% were susceptible to aminoglycosides.
The development of macrolide resistance is important as it is associated with resistance to fluoroquinolones and other groups of antibiotics. Eighteen per cent of C. jejuni and 6.4% of C. coli isolates from Europe were susceptible to all the antibiotics tested, while in Spain only 1.6% and 0%, respectively, were susceptible. Multidrug-resistant strains (resistant to more than 3 groups of antibiotics) were detected in 24% of C. jejuni and 35% of C. coli isolates. 1.4% and 16%, respectively, also showed co-resistance to both erythromycin and ciprofloxacin.12 Wang et al.83 found that all ermB-carrying isolates were also resistant to fluoroquinolones and tetracyclines. Lehtopolku et al.88 detected 1.1% of macrolide-resistant strains, of which 95%, 74% and 32% showed co-resistance to ciprofloxacin, tetracyclines and amoxicillin/clavulanic acid, respectively. Due to this limitation of the therapeutic arsenal, different studies have analysed the usefulness of tigecycline as an alternative antibiotic. The above-mentioned study found that all multidrug-resistant strains studied were susceptible. Similar results have recently been reported in Kuwait, Spain and Poland.89–92
Furthermore, increased Campylobacter resistance reinforces the importance of in vitro susceptibility testing. Despite being recognised as a human pathogen in 1972, the susceptibility testing method was not standardised until 2004. While CLSI37 recommends agar dilution or broth microdilution as the method of choice, EUCAST has recently standardised a disk diffusion method.36 There are also variations in the criteria used to interpret results, including different breakpoints for C. jejuni and C. coli, and it would therefore be beneficial to harmonise isolate interpretation criteria.78
Despite observing increased resistance over recent years, macrolides are still the main therapeutic option for Campylobacter. However, oral alternatives are limited due to current resistance rates to quinolones and tetracyclines (Table 4).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: González-Torralba A, García-Esteban C, Alós J-I. Enteropatógenos y antibióticos. Enferm Infecc Microbiol Clin. 2018;36:47–54.