No cases of human brucellosis caused by Brucella suis has been reported in Spain.
MethodsThis study involved interviews with the case and his co-workers, inspection of their workplace, checking infection control measures, and typing the Brucella strain isolated in the blood culture.
ResultsBrucella suis biovar 1 strain 1330 was isolated from a patient who worked in a waste treatment plant. Food borne transmission, contact with animals, and risk jobs were ruled out. An accidental inoculation with a contaminated needle from a research laboratory waste container was identified as the most probable mode of transmission.
ConclusionThere should be controls to ensure that waste containers are sealed.
En España no se habían comunicado casos humanos de brucelosis por Brucella suis anteriores a este.
MétodosLa investigación incluyó entrevistas con el caso y sus compañeros de trabajo, inspección del lugar de trabajo, comprobación de las medidas de control de la infección, y tipificación de la cepa de Brucella aislada en el hemocultivo.
ResultadosSe aisló Brucella suis biovariedad 1 cepa 1330 en un paciente que trabajaba en una empresa de tratamiento de residuos. Se descartó la fuente alimentaria, el contacto con animales y trabajos de riesgo. Un pinchazo accidental con una aguja contaminada de un contenedor procedente de un laboratorio de investigación fue la forma de transmisión más probable.
ConclusiónSe deben realizar controles para asegurar que los contenedores de residuos están sellados.
The main etiological agent of human brucellosis in Spain is Brucella melitensis, whose reservoirs are sheep and goats.1 The incidence rate has decreased sharply from 5.3 cases per 105 inhabitants in 1996 to 0.22 cases per 105 inhabitants in 2011.2 This is related to the successful implementation of compulsory control and elimination measures on the animal reservoir, carried out by the Spanish Health Administration and co-financed by the European Union.3
So far no human cases due to Brucella suis had been reported in Spain. However, B. suis biovar 2 is frequently isolated in wild boar, European hares (both considered the wild reservoirs of this biovar), and occasionally, also from pigs.4 Despite not considered a relevant cause of human brucellosis, B. suis biovar 2 infection has been reported in immunocompromised patients.5 On the other hand, B. suis biovar 1 has been never reported previously in any species in the European Union, with the exception of Croatia,6 despite being a major cause of human brucellosis in America, Oceania and Asia.7–10
On March 11, 2014 a brucellosis case was reported to the Epidemiological Surveillance Unit of Zaragoza (Spain). The patient developed typical brucellosis symptoms (fever, headache and joint pain) on February 3, 2014, and was admitted to the Miguel Servet Hospital (Zaragoza, Spain), where Brucella spp was isolated from hemoculture.
The aims of this study were to identify the infecting strain as well as the source of infection and the mode of transmission.
MethodsThe research involved interviews with the case and his co-workers, inspection of their workplace, checking infection control measures, and typing the Brucella strain isolated.
The interview focused on possible sources of exposure, up to six months before the onset of symptoms.
Two epidemiologists inspected the plant and met with the company's heads of management, occupational hazards and laboratory, as well as all the workers with the same task (autoclave operators). A complete list of suppliers of sanitary waste was requested.
Blood samples of the patient were submitted for hemoculture to the laboratory of Miguel Servet Hospital. The samples were processed using automated continuous monitoring system BD BACTEC™ FX. Bacterial growth was obtained after 48h incubation in aerobic culture vials. The strain was sent to the reference laboratory (National Center for Microbiology, Madrid, Spain). The Center for Food Research and Technology of Aragon (CITA) conducted further typing studies using both standard microbiological procedures, a PCR Multiplex11 and MLVA analysis.12
ResultsThe patient was a 53-year old male, living in the Zaragoza province (Northeast Spain), and suffering Wegener's granulomatosis, being treated with azathioprine (50mg/day) and prednisone (2.5mg/day).
We discarded the consumption of unpasteurized milk or dairy products, or undercooked meat. Direct contact with domestic (sheep, goats, cows, swine, horses and dogs) or wild animals was also ruled out. There were neither antecedents of traveling to B. suis biovar 1 endemic countries, nor history of transplantation. The patient had never worked previously in risky areas potentially associated with brucellosis, as veterinary clinics, slaughterhouses, farms or laboratories.
During the exposure period, the patient worked in a medical waste treatment plant, involved in the collection and sterilization of hazardous medical waste, using a dynamic rotary autoclave. The resulting product is recycled, compacted and disposed as municipal solid waste.
On December 23, 2013, 42 days before the onset of symptoms, the patient had an accident at the workplace. He poked his foot with a needle that was lying on the floor. The accident was recorded after receiving health care in the company's medical service.
Autoclave operators work in three stages:
- 1.
Closed waste containers are unloaded from trucks.
- 2.
Closed containers are introduced in the autoclave and sterilized.
- 3.
Waste from the containers is processed manually to recycle plastics.
The accident occurred in the second stage. The needle was not submitted to bacteriological analysis.
Workers wear always protective equipment, consisting of filter mask, apron, boots and gloves. Moreover, goggles and face shields are used where splashing fluids may occur (introduction of containers in the autoclave and waste manipulation after sterilization).
Interviews revealed that despite using boots and gloves, punctures were frequent during the third stage and infrequent during the first and second. Only in these two stages injured workers are headed to the company's medical services. Serological examination (using the standard Rose Bengal agglutination test) was performed in 12 co-workers, all resulting negative. Moreover, none developed symptoms compatible with brucellosis in the following six months after the accident.
Laboratory controls for the sterilization include:
- -
Physicochemical control in every autoclave cycle (temperature: 130°C; time: 10min; pressure: 2.5–3bar; and test strip color change).
- -
Biological control: daily using Bacillus stearotermophilus.
- -
Microbiological examination of autoclave leachates: once a week (Escherichia coli, total fecal enterobacteria, total aerobes and sulphite-reducing bacteria).
Additionally, an external laboratory performs microbiological control of autoclave leachates once a year.
All controls during the exposure period were considered appropriate.
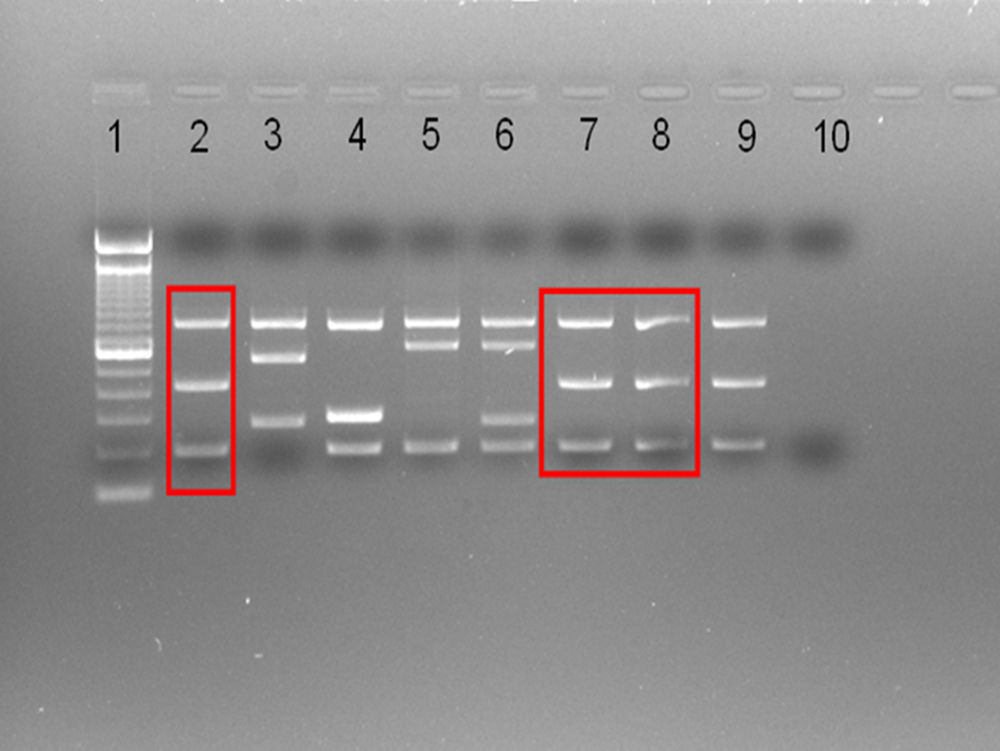
A Brucella spp strain was isolated in the case blood culture, further identified as B. suis by the Reference Laboratory. Using conventional typing and multiplex PCR (Fig. 1) the CITA laboratory identified the strain unequivocally as B. suis biovar 1, never reported previously in Spain. Further MLVA analysis, a method that can discriminate Brucella at strain level,12 showed a pattern identical to that of the reference B. suis biovar 1 strain 1330 (not shown), originally isolated from swine in Minnesota in 1950.13
Multiplex PCR (Suis-ladder11) differentiating B. suis biovars. Lane1: ladder (100base pairs). Lanes 2–6: B. suis reference (control) strains: B. suis biovar 1 – 1330 – (2), B. suis biovar 2-Thomsem – (3), B. suis biovar 3 – 686 – (4), B. suis biovar 4 – 40 – (5), B. suis biovar 5 – 513 – (6). Lanes 7–8: case strain. Lane 9: B. suis biovar 1 reference 1330 control strain (repeated to facilitate comparisons). Lane 10: negative control.
This finding simplified the investigation with the waste suppliers. The plant receives medical waste from a monthly mean of 5000 suppliers throughout Spain. Identification of B. suis biovar 1 strain 1330 focused the investigation in research laboratories exclusively. Among these, only one confirmed its use and had sent waste to the plant regularly. The nearest shipment was three days before the accident.
DiscussionTo the best of our knowledge this represents the first human brucellosis case due to B. suis biovar 1 strain 1330 described worldwide.
The comprehensive microbiological investigation was decisive, since the strain isolated ruled out any animal origin and excluded all biohazardous waste suppliers, except research laboratories.
Human brucellosis by inoculation in laboratory and veterinary staff has been reported, especially when handling vaccine strains,14 however this transmission mechanism has not been reported elsewhere.
Aerosols are commonly implicated in human brucellosis through inhalation or mucocutaneous exposures.14 This mechanism could have been involved also here, but seems unlikely. First, aerosols essentially occur at the end of autoclave cycles, once sterilized. Second, workers wear face shield and masks while performing this task. Finally, the Rose Bengal test was negative in the co-workers sharing the same potentially infected environment.
The absence of other expositions or risk factors strengthens the hypothesis of transmission through the needle fallen from either a broken or non-properly closed container with contaminated waste. Besides, the puncture occurred during the theoretical patient exposure calculated from the date of onset of symptoms. B. suis biovar 1 is highly pathogenic,5,7 and furthermore the worker's immune deficiency could have contributed to the disease acquisition, even with a low infective dose. Personal protective equipment was not enough to prevent transmission. Human brucellosis due to accidental needle inoculation has been identified, especially when handling vaccine strains,14 but never reported previously for B. suis.
To prevent these accidents, sanitary waste treatment companies should follow strict protocols and reject not properly closed containers at the collection place. Likewise, providers of medical waste should include the necessary controls to ensure that contaminated material containers are tightly sealed.
FundingNone.
Conflict of interestThe authors declare no conflict of interest.