Clostridioides difficile infection (CDI) is a disease that is potentially preventable by vaccination. A good knowledge of its epidemiology, which can change over time, is warranted for prevention purposes and to help decision-making on the use of vaccines in public health programs. The objective of the research was to determine the epidemiology of healthcare-associated CDI (HA-CDI) and community-associated CDI (CA-CDI) in hospitalized patients in Spain using point prevalence data.
MethodsPoint prevalence survey data on infections of hospitalized patients for years 2012–2019 were analyzed. HA-CDI and CA-CDI prevalence rates were calculated. Both HA-CDI and CA-CDI, as well as age group prevalence rates, were examined for trends. Patient comorbidities were tested for association to CDI.
ResultsThe prevalence of CDI in Spanish hospitals has grown exponentially from 14.1% in 2012 to 35.9% in 2019 (cases/10.000 hospitalized patients). Almost two thirds of the cases are of nosocomial onset. This increase was observed for HA-CDI and CA-CDI at an annual rate of 1.11% (CI 95% 1.08–1.15) and 1.09% (CI 95% 1.04–1.13), respectively. Patients 50 years old or older represent 87% of the total number of cases. Patients suffering from neoplasm (OR 1.39), immunodeficiency (OR 3.26), neutropenia (OR 3.70), cirrhosis (OR 1.92) and chronic renal failure (OR 1.91) have a significant increased risk of developing CDI, after adjusting for age.
ConclusionIn Spain, the prevalence rate of both HA-CDI and CA-CDI have been increasing. Burden of CDI as well as clinical and epidemiological characteristics of CDI patients will help to support public health decision-making.
La infección por Clostridioides difficile (ICD) es una enfermedad potencialmente prevenible mediante vacunación. Es necesario conocer adecuadamente su epidemiología para ayudar a la toma de decisiones sobre su prevención y el uso de vacunas en programas de salud pública. El objetivo de esta investigación es determinar la epidemiología de ICD relacionada con la asistencia sanitaria (IRAS-CD) e ICD asociada a la comunidad (IAC-CD) en pacientes hospitalizados en España.
MétodosAnalizamos los datos de encuestas de prevalencia puntual en pacientes hospitalizados durante los años 2012-2019. Calculamos las tasas de prevalencia de IRAS-CD e IAC-CD, y por grupos de edad, examinando sus tendencias. Evaluamos la asociación de ciertas comorbilidades con la ICD.
ResultadosLa prevalencia de ICD en hospitales españoles ha crecido exponencialmente desde el 14,1% en 2012 al 35,9% en 2019 (casos/10.000 pacientes hospitalizados). Casi 2/3 de los casos son de inicio nosocomial. Este aumento se ha observado en IRAS-CD (1,11%; IC 95%: 1,08-1,15) e IAC-CD (1,09%; IC 95%: 1,04-1,13). Los pacientes de 50 años o más representan el 87% del total de casos. Los pacientes con neoplasia (OR: 1,39), inmunodeficiencia (OR: 3,26), neutropenia (OR: 3,70), cirrosis (OR: 1,92) e insuficiencia renal crónica (OR: 1,91) tienen un riesgo significativamente mayor de desarrollar ICD tras ajustar por edad.
ConclusiónEn España la tasa de prevalencia de IRAS-CD e IAC-CD ha ido en aumento. Conocer la carga de la ICD y las características clínicas y epidemiológicas de los pacientes con ICD ayudará a la toma de decisiones en salud pública.
Clostridioides difficile is the most frequent cause of healthcare-associated infectious diarrhea in Spain and other industrialized countries.1 The spectrum of C. difficile infection (CDI) ranges from mild diarrhea to severe life-threatening colon infection and sepsis due to megacolon. In Europe, it is estimated that it causes 152,905 healthcare-associated CDI (HA-CDI) cases annually.2C. difficile is the 8th most frequently detected microorganism among healthcare-associated infections (HAIs) across Europe and the number of deaths occurring as a direct consequence of HA-CDI can be estimated at 3700 per year in the European Union and European Economic Area (EU/EEA).3
On January 2016, the ECDC (European Centre for Disease Prevention and Control) launched a hospital-based surveillance system of CDI in EU/EEA member states, using a common European protocol to estimate the incidence, describe the epidemiology, and assess the burden and adverse outcomes of CDIs by providing participating hospitals with a standardized protocol in order to obtain homogeneous measurement and comparability among hospitals.4
The Estudio de Prevalencia de las Infecciones Nosocomiales (EPINE) is a series of annual prevalence studies of infections—both healthcare-associated and community-onset—in hospitalized patients developed in Spain since 1990. In 2012 the protocol was updated to share the same characteristics regarding HAIs as the European ECDC Point Prevalence Survey. The EPINE database is a comprehensive and reliable source of information, following a standardized methodology compatible with infection surveillance definitions, able to identify trends and characteristics of infections in hospitalized population in Spain.
Three C. difficile vaccines are currently in phase III trials worldwide.5–7 To enable decision-making on the potential use of these vaccines in public health programs, the national epidemiology of the disease is a critical factor.
The objectives of this study were to determine the prevalence of both HA-CDI and community-associated CDI (CA-CDI) in hospitalized patients in Spain, as well as to identify seasonal trends and associated characteristic of the patients during the 2012–2019 period.
MethodsData were extracted from the EPINE database. EPINE is a point prevalence survey (PPS) that collects information on infections and antimicrobial use on hospitalized patients in Spain. The EPINE working group is organized by the Sociedad Española de Medicina Preventiva Salud Pública e Higiene, and it is currently recognized by the Ministry of Health as an official surveillance system for infections in hospitalized patients. Since 2012, it is based on the PPS conducted by the ECDC every 5 years.8 Additionally, EPINE includes information on community infections and additional risk factors. In brief, acute care hospitals adhere to surveillance on a voluntary or compulsory basis, depending on the autonomous health authorities. All inpatients in a single day are recruited by trained investigators. Data collection includes hospital, ward, patient, use of antimicrobials, and infections data. These data are sent to a coordinating center for processing and analysis, and the generated reports are sent to hospitals and National Health authorities.
All patients recorded on the survey during the 2012–2019 period were included (465,620 patients, with an average of 58,203 patients per year). A total of 380 hospitals participated in the survey, with an average of 292 hospitals per year. CDI diagnosis relies on ECDC case-definitions, which include: (a) diarrheal stools or toxic megacolon and a positive laboratory assay for C. difficile toxin A and/or B in stools, or a toxin-producing C. difficile organism detected in stool via culture or other means e.g. a positive PCR result; or (b) pseudomembranous colitis revealed by lower gastro-intestinal endoscopy; or (c) colonic histopathology characteristic of C. difficile infection (with or without diarrhea) on a specimen obtained during endoscopy, colectomy or autopsy. HA-CDI included infections with onset of signs and symptoms on day 3 or later following admission; or onset in the community within four weeks of discharge from a healthcare facility. CA-CDI included infections that did not meet HA-CDI criteria, and those of unknown origin. The methodology of the survey was similar to that stated by ECDC for point prevalence surveys (PPS).8,9 Hospitals were not randomly chosen, they participated on a voluntary basis and they represented above 50% of the Spanish acute care hospitalized patients on any given day.
In addition to HAI, the patient form included community-onset CDI, as well as demographic data (age and gender), information on underlying clinical conditions such as diabetes mellitus, renal failure, malignant neoplasm, chronic lung disease, immunosuppression, neutropenia, chronic wounds, cirrhosis and hypoalbuminemia, and co-morbidity score (McCabe-Jackson). In addition, hospital characteristics such as type (primary, secondary and tertiary) and size, according to the number of beds (small: less than 200 beds; medium: 200–500 beds; large: more than 500 beds) are reported. Hospital and patient forms were recorded electronically in a web-based system (www.epine.es) and sent to an independent central analysis unit for further validation and analysis. A hospital report was sent back to every participating hospital to avoid possible disagreements before final integration of the collected results in a centralized database.
Prevalence rates were expressed as the number of patients with CDI per 10,000 hospitalized patients. We focused our analysis on the 2012–2019 period, for which secular trends were evaluated by logistic regression. However, for a broader description of the annual prevalence, historical available data on CDI prevalence of further past years (1999–2011), as well as trend and exponential adjustment, were also included.
We described the main characteristics of the patients with C. difficile infection, according to type of facilities, clinical conditions, exposures, and demographic features. These characteristics were compared in the patients with CDI versus the rest of the patients. To study the association of the clinical-demographic variables with the prevalence of CDI, the prevalence odds ratio (OR), both crude and adjusted by age, and their 95% intervals, were estimated using logistic regression.
Differences in mean age were compared using Student's t-test. The chi-square test was used to compare the proportions of use of invasive devices.
The incidence of the disease (Incidence density ID, number of cases for patient-days of hospitalization) in the hospitalized population was estimated from prevalence data by the relationship ID=P/d10; P being prevalence of CDI, and d the estimated duration of CDI infection in Spain.11,12
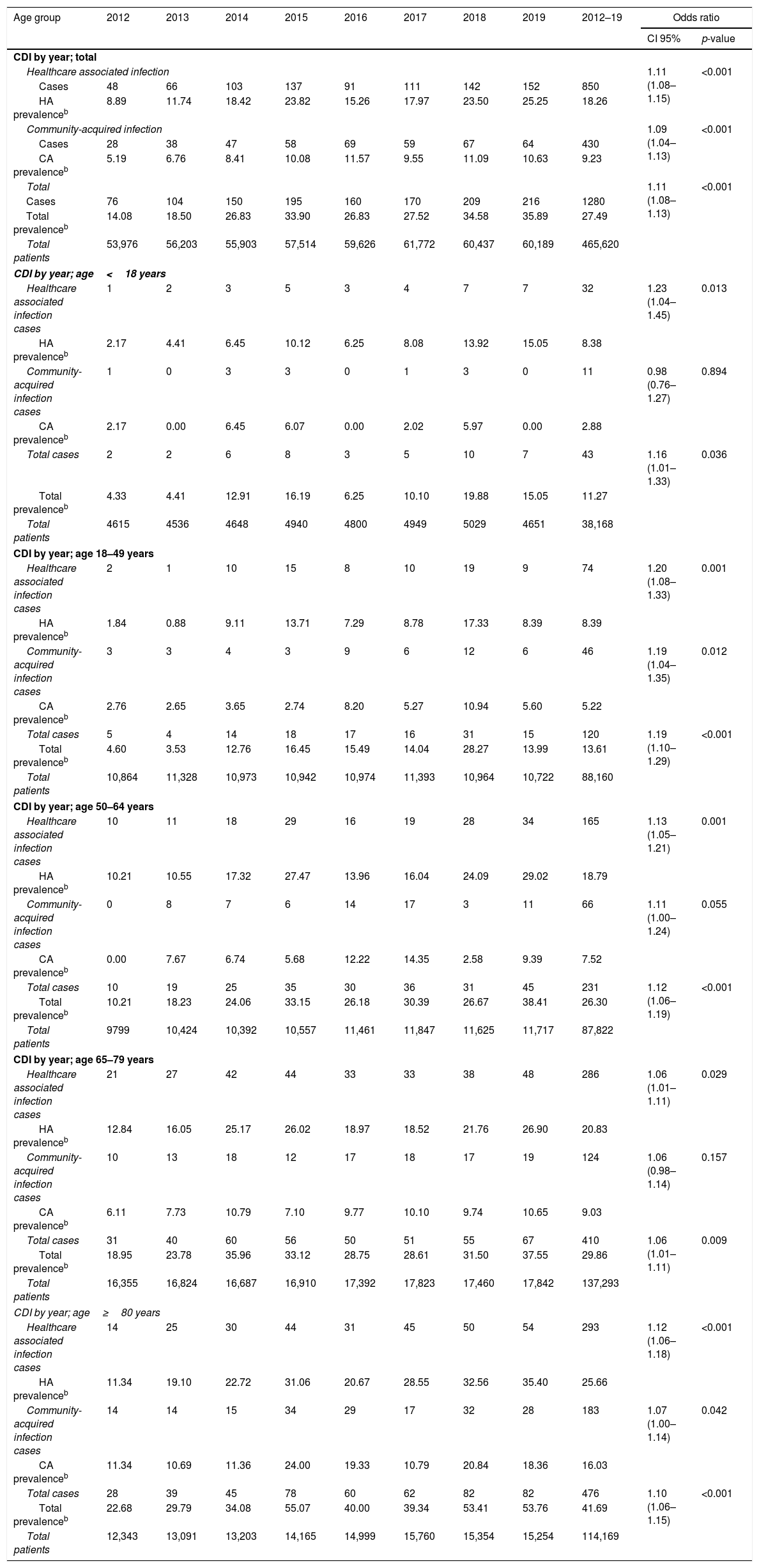
ResultsFrom 2012 to 2019, the CDI prevalence (cases per 10.000 hospitalized patients) increased at an annual rate of 11% (OR 1.11; CI 95% 1.08–1.13) and the significant increase on CDI prevalence was also seen for all age groups [Table 1].
| Age group | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2012–19 | Odds ratio | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI 95% | p-value | ||||||||||
| CDI by year; total | |||||||||||
| Healthcare associated infection | 1.11 (1.08–1.15) | <0.001 | |||||||||
| Cases | 48 | 66 | 103 | 137 | 91 | 111 | 142 | 152 | 850 | ||
| HA prevalenceb | 8.89 | 11.74 | 18.42 | 23.82 | 15.26 | 17.97 | 23.50 | 25.25 | 18.26 | ||
| Community-acquired infection | 1.09 (1.04–1.13) | <0.001 | |||||||||
| Cases | 28 | 38 | 47 | 58 | 69 | 59 | 67 | 64 | 430 | ||
| CA prevalenceb | 5.19 | 6.76 | 8.41 | 10.08 | 11.57 | 9.55 | 11.09 | 10.63 | 9.23 | ||
| Total | 1.11 (1.08–1.13) | <0.001 | |||||||||
| Cases | 76 | 104 | 150 | 195 | 160 | 170 | 209 | 216 | 1280 | ||
| Total prevalenceb | 14.08 | 18.50 | 26.83 | 33.90 | 26.83 | 27.52 | 34.58 | 35.89 | 27.49 | ||
| Total patients | 53,976 | 56,203 | 55,903 | 57,514 | 59,626 | 61,772 | 60,437 | 60,189 | 465,620 | ||
| CDI by year; age<18 years | |||||||||||
| Healthcare associated infection cases | 1 | 2 | 3 | 5 | 3 | 4 | 7 | 7 | 32 | 1.23 (1.04–1.45) | 0.013 |
| HA prevalenceb | 2.17 | 4.41 | 6.45 | 10.12 | 6.25 | 8.08 | 13.92 | 15.05 | 8.38 | ||
| Community-acquired infection cases | 1 | 0 | 3 | 3 | 0 | 1 | 3 | 0 | 11 | 0.98 (0.76–1.27) | 0.894 |
| CA prevalenceb | 2.17 | 0.00 | 6.45 | 6.07 | 0.00 | 2.02 | 5.97 | 0.00 | 2.88 | ||
| Total cases | 2 | 2 | 6 | 8 | 3 | 5 | 10 | 7 | 43 | 1.16 (1.01–1.33) | 0.036 |
| Total prevalenceb | 4.33 | 4.41 | 12.91 | 16.19 | 6.25 | 10.10 | 19.88 | 15.05 | 11.27 | ||
| Total patients | 4615 | 4536 | 4648 | 4940 | 4800 | 4949 | 5029 | 4651 | 38,168 | ||
| CDI by year; age 18–49 years | |||||||||||
| Healthcare associated infection cases | 2 | 1 | 10 | 15 | 8 | 10 | 19 | 9 | 74 | 1.20 (1.08–1.33) | 0.001 |
| HA prevalenceb | 1.84 | 0.88 | 9.11 | 13.71 | 7.29 | 8.78 | 17.33 | 8.39 | 8.39 | ||
| Community-acquired infection cases | 3 | 3 | 4 | 3 | 9 | 6 | 12 | 6 | 46 | 1.19 (1.04–1.35) | 0.012 |
| CA prevalenceb | 2.76 | 2.65 | 3.65 | 2.74 | 8.20 | 5.27 | 10.94 | 5.60 | 5.22 | ||
| Total cases | 5 | 4 | 14 | 18 | 17 | 16 | 31 | 15 | 120 | 1.19 (1.10–1.29) | <0.001 |
| Total prevalenceb | 4.60 | 3.53 | 12.76 | 16.45 | 15.49 | 14.04 | 28.27 | 13.99 | 13.61 | ||
| Total patients | 10,864 | 11,328 | 10,973 | 10,942 | 10,974 | 11,393 | 10,964 | 10,722 | 88,160 | ||
| CDI by year; age 50–64 years | |||||||||||
| Healthcare associated infection cases | 10 | 11 | 18 | 29 | 16 | 19 | 28 | 34 | 165 | 1.13 (1.05–1.21) | 0.001 |
| HA prevalenceb | 10.21 | 10.55 | 17.32 | 27.47 | 13.96 | 16.04 | 24.09 | 29.02 | 18.79 | ||
| Community-acquired infection cases | 0 | 8 | 7 | 6 | 14 | 17 | 3 | 11 | 66 | 1.11 (1.00–1.24) | 0.055 |
| CA prevalenceb | 0.00 | 7.67 | 6.74 | 5.68 | 12.22 | 14.35 | 2.58 | 9.39 | 7.52 | ||
| Total cases | 10 | 19 | 25 | 35 | 30 | 36 | 31 | 45 | 231 | 1.12 (1.06–1.19) | <0.001 |
| Total prevalenceb | 10.21 | 18.23 | 24.06 | 33.15 | 26.18 | 30.39 | 26.67 | 38.41 | 26.30 | ||
| Total patients | 9799 | 10,424 | 10,392 | 10,557 | 11,461 | 11,847 | 11,625 | 11,717 | 87,822 | ||
| CDI by year; age 65–79 years | |||||||||||
| Healthcare associated infection cases | 21 | 27 | 42 | 44 | 33 | 33 | 38 | 48 | 286 | 1.06 (1.01–1.11) | 0.029 |
| HA prevalenceb | 12.84 | 16.05 | 25.17 | 26.02 | 18.97 | 18.52 | 21.76 | 26.90 | 20.83 | ||
| Community-acquired infection cases | 10 | 13 | 18 | 12 | 17 | 18 | 17 | 19 | 124 | 1.06 (0.98–1.14) | 0.157 |
| CA prevalenceb | 6.11 | 7.73 | 10.79 | 7.10 | 9.77 | 10.10 | 9.74 | 10.65 | 9.03 | ||
| Total cases | 31 | 40 | 60 | 56 | 50 | 51 | 55 | 67 | 410 | 1.06 (1.01–1.11) | 0.009 |
| Total prevalenceb | 18.95 | 23.78 | 35.96 | 33.12 | 28.75 | 28.61 | 31.50 | 37.55 | 29.86 | ||
| Total patients | 16,355 | 16,824 | 16,687 | 16,910 | 17,392 | 17,823 | 17,460 | 17,842 | 137,293 | ||
| CDI by year; age≥80 years | |||||||||||
| Healthcare associated infection cases | 14 | 25 | 30 | 44 | 31 | 45 | 50 | 54 | 293 | 1.12 (1.06–1.18) | <0.001 |
| HA prevalenceb | 11.34 | 19.10 | 22.72 | 31.06 | 20.67 | 28.55 | 32.56 | 35.40 | 25.66 | ||
| Community-acquired infection cases | 14 | 14 | 15 | 34 | 29 | 17 | 32 | 28 | 183 | 1.07 (1.00–1.14) | 0.042 |
| CA prevalenceb | 11.34 | 10.69 | 11.36 | 24.00 | 19.33 | 10.79 | 20.84 | 18.36 | 16.03 | ||
| Total cases | 28 | 39 | 45 | 78 | 60 | 62 | 82 | 82 | 476 | 1.10 (1.06–1.15) | <0.001 |
| Total prevalenceb | 22.68 | 29.79 | 34.08 | 55.07 | 40.00 | 39.34 | 53.41 | 53.76 | 41.69 | ||
| Total patients | 12,343 | 13,091 | 13,203 | 14,165 | 14,999 | 15,760 | 15,354 | 15,254 | 114,169 | ||
Source: EPINE 2012–2019.
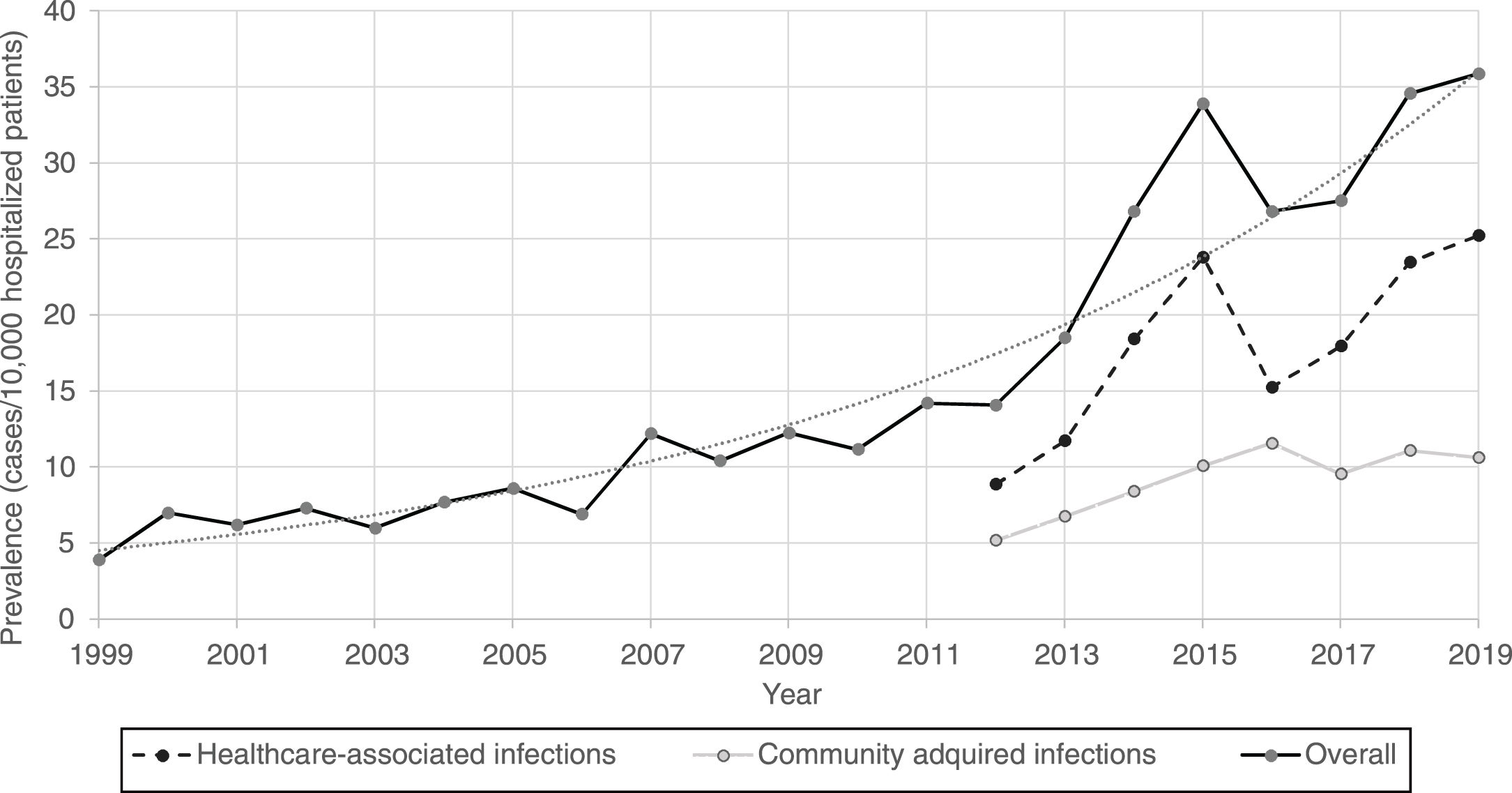
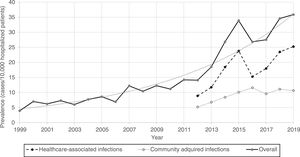
Furthermore, CDI prevalence also increased exponentially in hospitalized patients in Spain from 1999 (3.9%) to 2019 (35.9%) [Fig. 1].
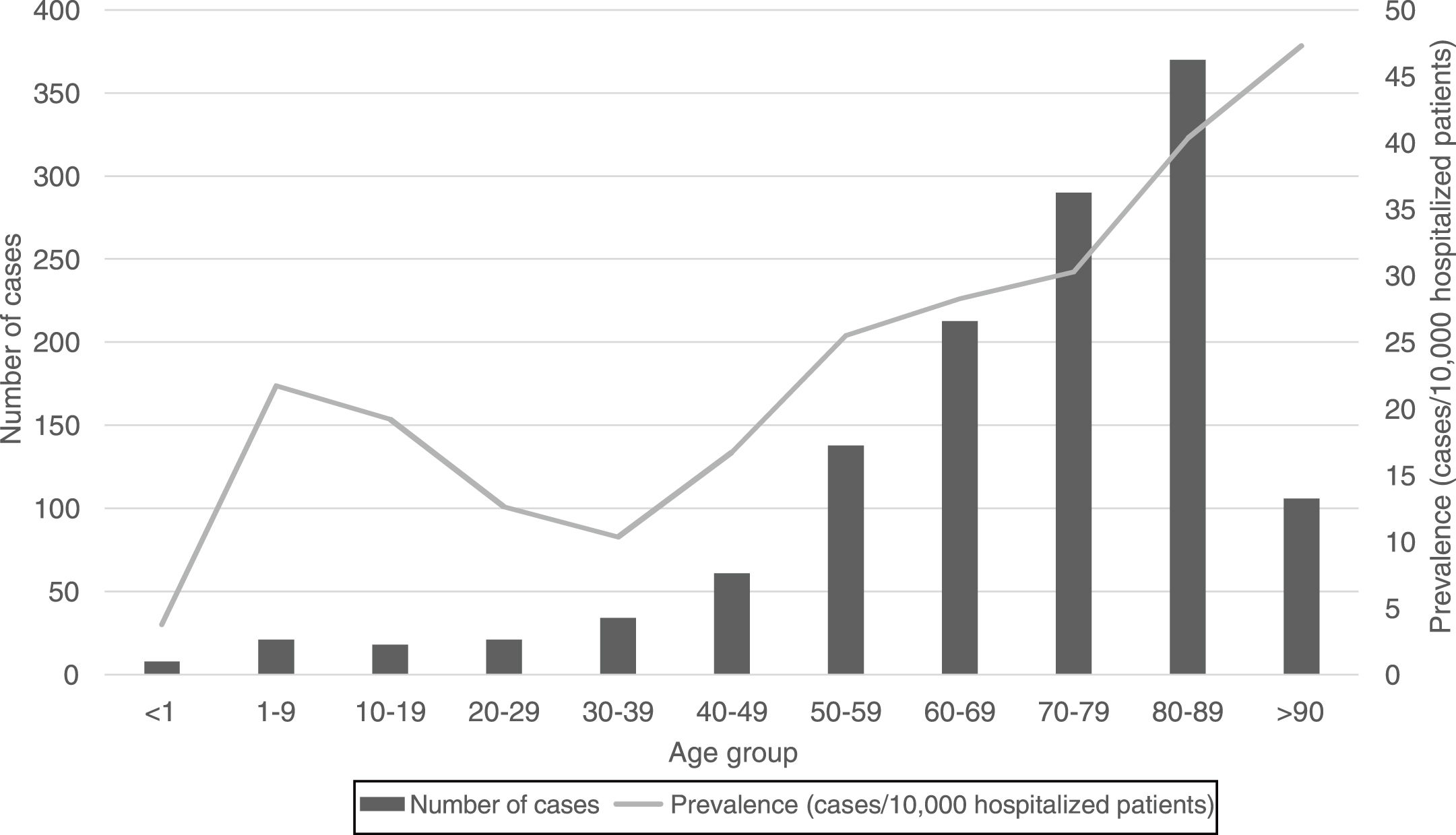
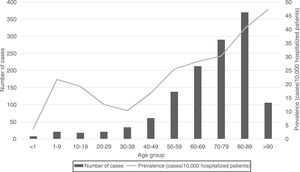
Regarding the origin of CDI, both HA-CDI (OR 1.11; CI 95% 1.08–1.15), and CA-CDI (OR 1.09; CI 95% 1.04–1.13) increased during the period (2012–2019). This statistically significant increase was observed for all age groups in HA-CDI, but it was only observed for CA-CDI in the 18–49-years (p-value 0.012) and ≥80-years (p-value 0.042) groups [Table 1]. Overall, CDI prevalence rate by age, excluding neonates, depicts a classical J-type curve, being the lowest for the 30–39 years-old group (10.4%) and increasing to 47.3% for the group of more than 85-years old. Most of the cases (87%) were in patients of 50-years old and older [Fig. 2].
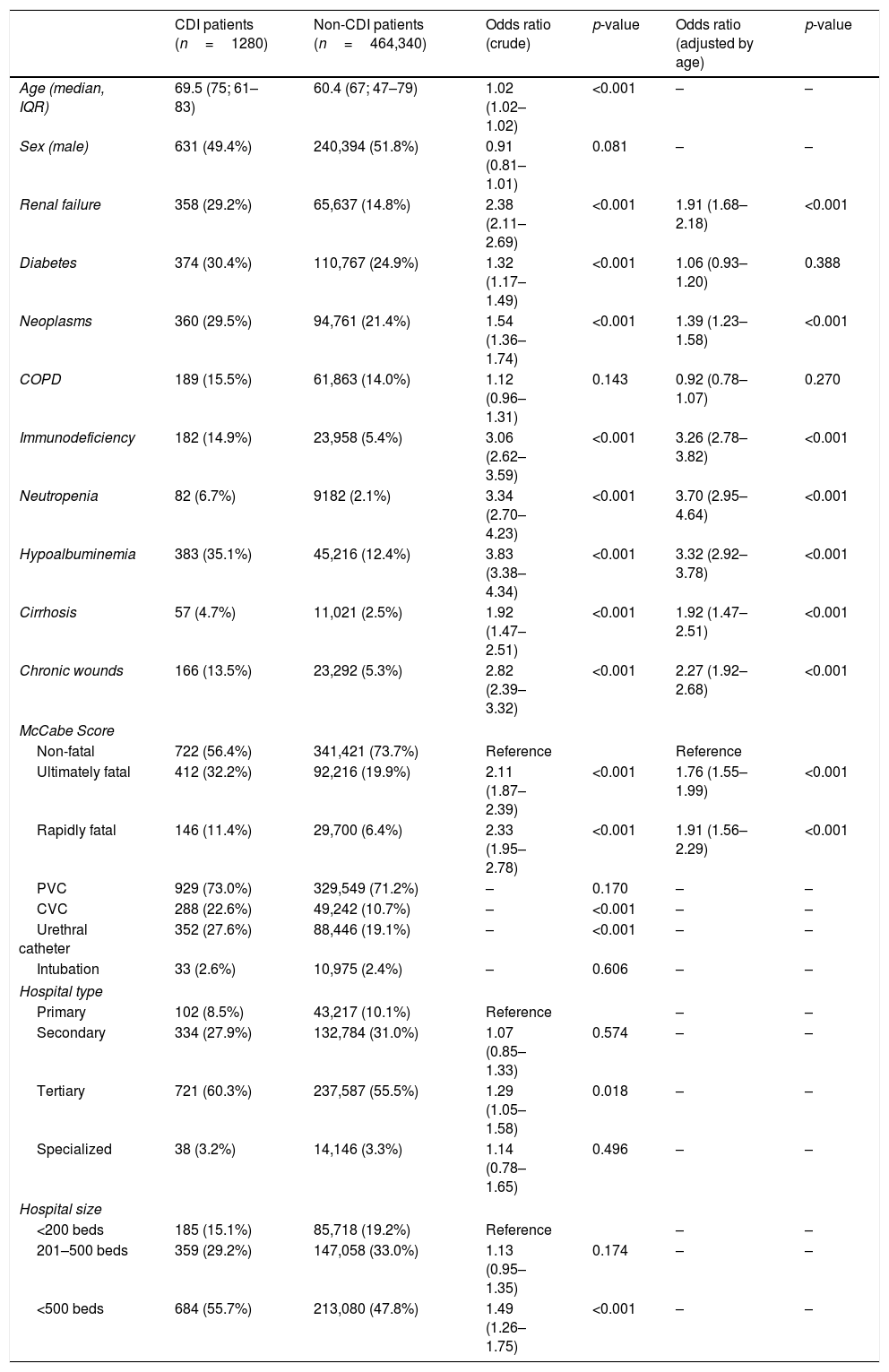
We found significant differences in relation to the age of patients with CDI (69.5; median 75; IQR 61–83) and patients without CDI (60.4; median 67; IQR 47–79). No differences were found in relation to sex. Many clinical characteristics of the patients were associated to CDI: comorbidities such as renal failure (OR 2.4), diabetes mellitus (OR 1.3), neoplasms (OR 1.5), immunodeficiency (OR 3.1), cirrhosis (1.9) and chronic wounds (OR 2.8); as well as clinical parameters like neutropenia (OR 3.3) and hypoalbuminemia (OR 3.8). For patients with COPD, no statistically significant association was found, the OR being 1.12 (p=0.143). Ultimately (OR 2.1), and rapidly fatal (OR 2.3) basal prognosis of the patient, measured by McCabe-Jackson score, was also associated to CDI. When adjusting by age, no statistically significant results were obtained for patients with diabetes (OR 1.06; p=0.388) and COPD (OR 0.92; p=0.270). For the rest of the comorbidities and characteristics studied, significant results were obtained (p<0.001). Hospitals with more than 500 beds (OR 1.5), as well as tertiary care hospitals (OR 1.3) were statistically associated to a higher CDI prevalence of CDI, but no age adjustment analysis was performed. As for invasive devices, the differences found between the groups with and without infection were analyzed, these being statistically significant in the case of central venous catheter and urethral catheter (p<0.001); but not in the case of the peripheral venous catheter (p=0.170) and intubation (0.606) [Table 2].
Main clinical, and hospital characteristics associated to CDI patients. Period 2012–2019.a
| CDI patients (n=1280) | Non-CDI patients (n=464,340) | Odds ratio (crude) | p-value | Odds ratio (adjusted by age) | p-value | |
|---|---|---|---|---|---|---|
| Age (median, IQR) | 69.5 (75; 61–83) | 60.4 (67; 47–79) | 1.02 (1.02–1.02) | <0.001 | – | – |
| Sex (male) | 631 (49.4%) | 240,394 (51.8%) | 0.91 (0.81–1.01) | 0.081 | – | – |
| Renal failure | 358 (29.2%) | 65,637 (14.8%) | 2.38 (2.11–2.69) | <0.001 | 1.91 (1.68–2.18) | <0.001 |
| Diabetes | 374 (30.4%) | 110,767 (24.9%) | 1.32 (1.17–1.49) | <0.001 | 1.06 (0.93–1.20) | 0.388 |
| Neoplasms | 360 (29.5%) | 94,761 (21.4%) | 1.54 (1.36–1.74) | <0.001 | 1.39 (1.23–1.58) | <0.001 |
| COPD | 189 (15.5%) | 61,863 (14.0%) | 1.12 (0.96–1.31) | 0.143 | 0.92 (0.78–1.07) | 0.270 |
| Immunodeficiency | 182 (14.9%) | 23,958 (5.4%) | 3.06 (2.62–3.59) | <0.001 | 3.26 (2.78–3.82) | <0.001 |
| Neutropenia | 82 (6.7%) | 9182 (2.1%) | 3.34 (2.70–4.23) | <0.001 | 3.70 (2.95–4.64) | <0.001 |
| Hypoalbuminemia | 383 (35.1%) | 45,216 (12.4%) | 3.83 (3.38–4.34) | <0.001 | 3.32 (2.92–3.78) | <0.001 |
| Cirrhosis | 57 (4.7%) | 11,021 (2.5%) | 1.92 (1.47–2.51) | <0.001 | 1.92 (1.47–2.51) | <0.001 |
| Chronic wounds | 166 (13.5%) | 23,292 (5.3%) | 2.82 (2.39–3.32) | <0.001 | 2.27 (1.92–2.68) | <0.001 |
| McCabe Score | ||||||
| Non-fatal | 722 (56.4%) | 341,421 (73.7%) | Reference | Reference | ||
| Ultimately fatal | 412 (32.2%) | 92,216 (19.9%) | 2.11 (1.87–2.39) | <0.001 | 1.76 (1.55–1.99) | <0.001 |
| Rapidly fatal | 146 (11.4%) | 29,700 (6.4%) | 2.33 (1.95–2.78) | <0.001 | 1.91 (1.56–2.29) | <0.001 |
| PVC | 929 (73.0%) | 329,549 (71.2%) | – | 0.170 | – | – |
| CVC | 288 (22.6%) | 49,242 (10.7%) | – | <0.001 | – | – |
| Urethral catheter | 352 (27.6%) | 88,446 (19.1%) | – | <0.001 | – | – |
| Intubation | 33 (2.6%) | 10,975 (2.4%) | – | 0.606 | – | – |
| Hospital type | ||||||
| Primary | 102 (8.5%) | 43,217 (10.1%) | Reference | – | – | |
| Secondary | 334 (27.9%) | 132,784 (31.0%) | 1.07 (0.85–1.33) | 0.574 | – | – |
| Tertiary | 721 (60.3%) | 237,587 (55.5%) | 1.29 (1.05–1.58) | 0.018 | – | – |
| Specialized | 38 (3.2%) | 14,146 (3.3%) | 1.14 (0.78–1.65) | 0.496 | – | – |
| Hospital size | ||||||
| <200 beds | 185 (15.1%) | 85,718 (19.2%) | Reference | – | – | |
| 201–500 beds | 359 (29.2%) | 147,058 (33.0%) | 1.13 (0.95–1.35) | 0.174 | – | – |
| <500 beds | 684 (55.7%) | 213,080 (47.8%) | 1.49 (1.26–1.75) | <0.001 | – | – |
Source: EPINE 2012–2019.
Length of hospital stay before HA-CDI averaged 26.2 days (median 16 days; IQR 9–33 days).
To calculate the incidence density of the disease in hospitalized patients (as number of cases of CDI per hospital patient-days), we used different estimates of the length of stay attributable to CDI: the interval 7.6–20.0 days includes every estimate utilized.11,12
For the entire period of study, we estimated 1.4–3.6 cases per 10,000 patient-days. In 2019, our estimation peaked at 1.8–4.7 cases per 10,000 patient-days.
DiscussionThis study illustrates a remarkable rise in the prevalence of CDI in Spain in recent years and its association with conditions such as renal failure, cancer, diabetes, immunodeficiency, or liver cirrhosis.
During the last 21 years, the prevalence of CDI cases in Spanish hospitals has grown exponentially, and by 2019 reached a peak (prevalence of 35.9 cases per 10.000 hospitalized patients), with an incidence density of 1.8–4.7 cases per 10,000 patient-days. This increase in both types of infections (HA-CDI and CA-CDI) (OR 1.11, p<0.001) could be explained by the growing use of broad-spectrum antimicrobials in Spanish hospitals, as well as the older age, prevalence of risk factors among the hospitalized population during the period of study,13 and in part by the increase in the diagnosis related to a better awareness of the disease.
We compared our results to other European estimates of CDI. Between 2011 and 2013, Davies et al., in a prospective multicenter European study, described an incidence density of 3.2–3.5 in Spain, which is within our estimates.14 However, they estimated the actual incidence density rate to be much greater, up to 9.8–11 cases per 10,000 patient-days. According to their discussion, they hypothesized that a large number of European hospitals underdiagnose CDI, possibly in up to 40,000 patients per year due to insufficient testing. In their retrospective, observational study using Spanish National Hospital Discharge Database data, Esteban-Vasallo et al. offer estimates of incidence per population which are not directly comparable to our data. However, their reported trends, with the incidence rate increasing from 7.8 cases per 100.000 population in 2010–2012 to 13.0 cases per 100.000 population in 2013–2015, are comparable to our reported increase in prevalence.15 Similarly, Soler et al.16 and Marco-Martínez et al.17 reported an incidence of 4.1 and 22 cases per 10.000 hospital discharges in 1997–2005 and 2005–2010, respectively, which also pose comparison problems. However, both studies support the suspected increase in CDI cases in periods prior to our studies with statistical significance.
An unsteady climbing of prevalence for years 2014 and 2015 is noticeable. This result can be attributed to the use of more sensitive diagnostic tests in all Microbiology laboratories in Spain. Thus, the proportion of Microbiology laboratories that used very sensitive tests ranged from 15.3% in 2008 to 57.3% in 2013.18 In addition, a high increase during the 2014–2015 period was observed in the Madrid region (data not shown). A large hospital in Madrid was experiencing a large outbreak by the 027 ribotype strain.19
Almost two thirds of the cases were of nosocomial onset. These HA-CDI number of cases consistently increased in all age groups during the last 8-year period (11.4% annually).
The prevalence of community-acquired CDI also increased during the period but at a lower annual rate (8.7%). However, this increase was not significant for some age groups, probably due to the scarce number of cases included in these age groups.
In general, the prevalence rate depicts a J-shaped format and CDI prevalence increased almost linearly from 30–39 to >90 years old groups. However, the prevalence of CDI was low in neonates, related to this singular age category of patients, known to be partially protected against CDI.20 CDI cases in patients of 50 years-old or older represented 87% of the total number of cases.
Several comorbidities were found to be associated to higher rates of CDI. In the case of renal failure and chronic wounds, this association has been reported in previous studies.17 Notably, most of these comorbidities associated to higher rates of CDI infection also correlated to poor response to active immunizations. Oncological, immunosuppressed, neutropenic, and cirrhotic patients, known to have in different degrees a poorer response to active immunizations, had a significant increased risk of developing CDI. Patients with chronic wounds were also more prone to develop CDI (OR 2.8) but this condition could also be correlated with other comorbidities already recognized as risk factors. All these factors should be taken into account in order to define risk categories for efficient patient vaccination.
The length of stay was revealed as a prominent feature of HA-CDI cases since average and median length of stay from admission to date of HA-CDI onset amounted 26.2 and 16 days, respectively, much longer than average total length of hospital stay (8.3 days) in Spanish hospitals,21 and comparable to the 21 average days estimated by Esteban-Vasallo et al. in secondary diagnosis cases of CDI.15
Hospital size and level of care were strongly correlated. Largest hospitals and third care institutions tend to attend more complicated patients, with enhanced length of hospitalization and more intensive and broader spectrum use of antimicrobials; characteristics frequently described as risk factors and determinants for CDI development.
There are several limitations to consider. Firstly, the scope of the study is limited to the epidemiology of hospitalized CDI cases. This study does not reveal information from non-hospitalized patients. Nevertheless, non-hospitalized cases account for a minor impact in health and social costs. Furthermore, given the small number of cases for some age categories, we were limited in the analyses that could be performed. Being a cross-sectional series, the incidence was not directly computable. Incidence estimates was obtained by indirect calculations based on attributable length of stay.
The variability of the participating centers each year could represent an important limitation. However, we found that 74% of hospitals participated at least during 5 years of the survey, contributing to 94% of the total of patients in the sample. This indicates that year-to-year variability in participating hospitals occurred mainly in small hospitals. Although the participation of hospitals is not random, the large size of the study population, which represents more than 50% of the population hospitalized in Spain in one day does not seem to seriously compromise the generalization of the results.
Optimally, stratified analyses, such as prevalence rates by age strata, underlying medical conditions and sex, are performed to determine high-risk populations and to provide useful information for cost-effective analyses that could support future CDI vaccine decision-making processes. Unfortunately, these comparisons and analyses could not be performed completely due to the scarce number of cases detected.
It should also be noted that the data retrieved from hospitals did not include laboratory-linked strain data for CDI cases. This is a major limitation and prevents the monitoring of ecological trends over time with varied CDI strain types. Although some potential vaccines are currently undergoing clinical trials, it is not yet known how broadly protective these candidate vaccines will be against the different C. difficile strains and which of them will escape from being detected by traditional strategy.
This research depicts the burden of the disease in Spanish hospitals and the increasing trend in the prevalence of CDI and should help to develop further strategies for the prevention of the disease. Given the importance of age, comorbidities, and length of hospital stay as associated factors to the development of CDI, any vaccination program aiming at the prevention of CDI should incorporate, as it is stated for influenza vaccination, age threshold and/or the presence of comorbidities as characteristics defining target groups for vaccination.
Ethical approvalThe study was approved by the Ethical committee of the Puerta de Hierro-Majadahonda Hospital.
FundingThis research has not received specific aid from agencies of the public sector, commercial sector, or non-profit entities.
Conflict of interestNone declared.