Our objectives were to describe the incidence, clinical characteristics, and risk factors for Clostridium difficile infection (CDI) in critically ill patients and to determine C. difficile PCR-ribotypes.
MethodsProspective, observational study in 26 Spanish ICUs. Patients with diarrhea meeting ESCMID criteria for CDI were included. Molecular characterization of isolates was performed using PCR ribotyping.
ResultsOf 4258 patients admitted to the ICUs, 190 (4.5%) developed diarrhea. Only 16 patients (8.4%) were diagnosed with CDI. Ribotype 078/126 (25.0%) was the most frequently identified. The mortality rate was similar in patients with ICD compared to patients with diarrhea not caused by C. difficile (p=0.115). Chronic renal insufficiency was identified as the only factor independently associated with the development of CDI (OR 5.87, 95% CI 1.24–27.83; p=0.026).
ConclusionsThe incidence of CDI in Spanish ICUs is low. Only chronic renal insufficiency was observed to be a risk factor for CDI development.
Pretendemos describir la incidencia, las características clínicas y los factores de riesgo de la infección por Clostridium difficile (ICD) en pacientes ingresados en unidades de cuidados intensivos, así como los ribotipos identificados.
MétodosEstudio observacional, prospectivo, realizado en 26 unidades de cuidados intensivos de España. Se incluyeron pacientes con diarrea y criterios clínicos de la ESCMID por sospecha de ICD. La caracterización molecular se realizó mediante PCR.
ResultadosDe 4.258 pacientes ingresados, 190 (4,5%) presentaron diarrea; en 16 causada por ICD. El ribotipo más frecuentemente aislado fue 078/126 (25%). La tasa de mortalidad cruda fue similar en pacientes con ICD y en pacientes con diarrea no causada por Clostridium difficile (p=0,115). La insuficiencia renal crónica fue identificada como factor independientemente asociado a desarrollo de ICD (OR: 5,87; IC 95%: 1,24-27,83; p=0,026).
ConclusionesLa incidencia de ICD en las unidades de cuidados intensivos españolas es baja. La insuficiencia renal crónica es el único factor identificado para desarrollo de ICD.
Clostridium difficile is a Gram-positive, spore-forming anaerobic bacillus and, the leading cause of healthcare-associated diarrhea.1Clostridium difficile infection (CDI) is a major public health concern in developed countries.1 Some patients remain asymptomatic after exposure to C. difficile, while in others, disease symptoms can vary from mild diarrhea to fulminant colitis.2 Clinicians sometimes fail to suspect CDI in patients with diarrhea, and many microbiology laboratories apply suboptimal diagnostic techniques.3
Another problem is the emergence of the “hypervirulent” 027 ribotype strain of C. difficile that may cause fulminant cases with high mortality rates.4 To date, C. difficile 027 ribotype has only been identified in a limited number of episodes in Spain.5 The vast majority of our knowledge about CDI in the critical care setting is derived from retrospective studies without a predefined diagnostic workup or from studies carried out during outbreaks.6 The main objective of the study was to assess the incidence of CDI in critically ill patients with diarrhea fulfilling European Society of Clinical Microbiology and Infectious Diseases (ESCMID) clinical criteria for CDI suspicion.7 Secondary objectives were to describe clinical presentation, risk factors for development of CDI and the most prevalent C. difficile ribotypes in Spanish intensive care units (ICUs).
MethodsThis multicenter, prospective, observational, non-interventional admitted to 26 ICUs in Spain between February 3 and April 3, 2014. The Institutional Review Board of the Virgen del Rocío Hospital (Seville, Spain) approved the protocol and each patient's informed consent (or the next of kin) was mandatory before enrolment.
Patients who subsequently developed diarrhea (defined as ≥3 unformed stools within 24h7) during their ICU stay were included. For every patient different clinical and analytical variables were recorded. Information was also collected on CDI recurrence, the prevalence of complications, and mortality either in hospital or within 60 days from CDI diagnosis for all patients analyzed.
DefinitionsImmunosuppression was considered in those who had undergone organ transplantation, active malignancy, immunodeficiency due to any primary disease, HIV or treatment with radiation or cytotoxic or corticosteroid drugs use or haematologic disease. The rest of factors were defined as currently accepted. The severity of diarrhea was defined as: mild disease, 4–5 unformed stools per day or white blood cell (WBC) count ≤12,000/μL; moderate disease, 6–9 unformed stools per day or WBC count of 12,000–15,000/μL; severe disease, ≥10 unformed stools per day or WBC count ≥15,000/μL.8 Recurrence was defined as the return of symptoms and a positive stool sample separated from the former by between 15 and 60 days after recovery from a previous episode (at least 3 days without diarrhea and clinical improvement).9
Microbiological diagnosisParticipating ICUs provided cases and incidence data, and local laboratories sent all unformed stool specimens received on a single day to a central reference laboratory regardless with patient age/origin (inpatients and outpatients), the diagnosis requested by the clinician, or the transport medium (except when this contained sporicides, e.g. formaldehyde).
A microbiological diagnosis of CDI was made initially at the local laboratory. At weekly intervals, samples were sent to the reference laboratory located in the Microbiology Department of Hospital General Universitario Gregorio Marañón (Madrid, Spain) and the results were reported 2–5 days later.
Statistical analysisDiscrete variables were expressed as counts (percentage) and continuous variables as means±standard deviation. The chi-squared test or Fisher exact test was used for categorical variables, and the Mann–Whitney U test or Kruskal–Wallis test was used for continuous variables. To identify independent variables associated with CDI development, we performed a multivariate analysis using a binomial logistic regression.
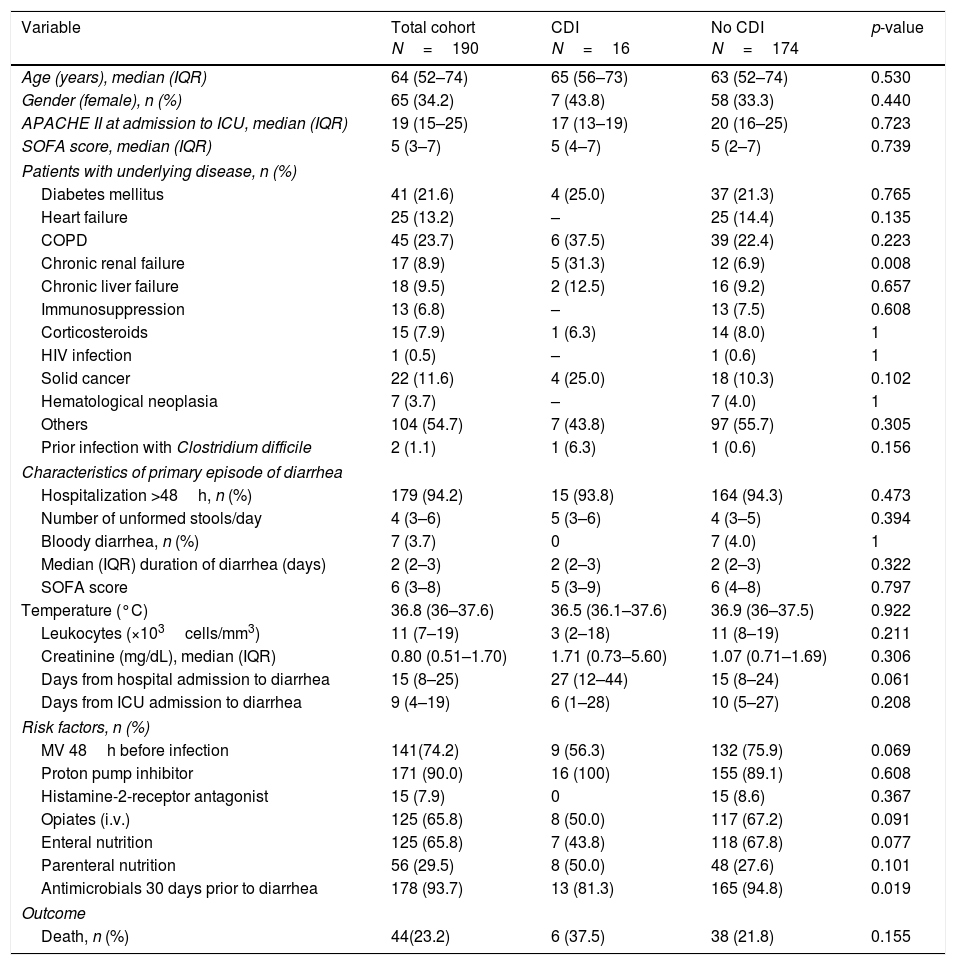
ResultsDuring the study period, 4258 patients were admitted to the participant ICUs. One-hundred and ninety patients (4.5%) developed diarrhea and met the study inclusion criteria. Only 11 out of the 26 participant centers provided positive CDI cases. Sixteen patients were diagnosed with CDI, representing an accumulated incidence of 0.37%. The baseline characteristics are described in Table 1.
Baseline characteristics of patients with and without Clostridium difficile infection.
| Variable | Total cohort N=190 | CDI N=16 | No CDI N=174 | p-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 64 (52–74) | 65 (56–73) | 63 (52–74) | 0.530 |
| Gender (female), n (%) | 65 (34.2) | 7 (43.8) | 58 (33.3) | 0.440 |
| APACHE II at admission to ICU, median (IQR) | 19 (15–25) | 17 (13–19) | 20 (16–25) | 0.723 |
| SOFA score, median (IQR) | 5 (3–7) | 5 (4–7) | 5 (2–7) | 0.739 |
| Patients with underlying disease, n (%) | ||||
| Diabetes mellitus | 41 (21.6) | 4 (25.0) | 37 (21.3) | 0.765 |
| Heart failure | 25 (13.2) | – | 25 (14.4) | 0.135 |
| COPD | 45 (23.7) | 6 (37.5) | 39 (22.4) | 0.223 |
| Chronic renal failure | 17 (8.9) | 5 (31.3) | 12 (6.9) | 0.008 |
| Chronic liver failure | 18 (9.5) | 2 (12.5) | 16 (9.2) | 0.657 |
| Immunosuppression | 13 (6.8) | – | 13 (7.5) | 0.608 |
| Corticosteroids | 15 (7.9) | 1 (6.3) | 14 (8.0) | 1 |
| HIV infection | 1 (0.5) | – | 1 (0.6) | 1 |
| Solid cancer | 22 (11.6) | 4 (25.0) | 18 (10.3) | 0.102 |
| Hematological neoplasia | 7 (3.7) | – | 7 (4.0) | 1 |
| Others | 104 (54.7) | 7 (43.8) | 97 (55.7) | 0.305 |
| Prior infection with Clostridium difficile | 2 (1.1) | 1 (6.3) | 1 (0.6) | 0.156 |
| Characteristics of primary episode of diarrhea | ||||
| Hospitalization >48h, n (%) | 179 (94.2) | 15 (93.8) | 164 (94.3) | 0.473 |
| Number of unformed stools/day | 4 (3–6) | 5 (3–6) | 4 (3–5) | 0.394 |
| Bloody diarrhea, n (%) | 7 (3.7) | 0 | 7 (4.0) | 1 |
| Median (IQR) duration of diarrhea (days) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.322 |
| SOFA score | 6 (3–8) | 5 (3–9) | 6 (4–8) | 0.797 |
| Temperature (°C) | 36.8 (36–37.6) | 36.5 (36.1–37.6) | 36.9 (36–37.5) | 0.922 |
| Leukocytes (×103cells/mm3) | 11 (7–19) | 3 (2–18) | 11 (8–19) | 0.211 |
| Creatinine (mg/dL), median (IQR) | 0.80 (0.51–1.70) | 1.71 (0.73–5.60) | 1.07 (0.71–1.69) | 0.306 |
| Days from hospital admission to diarrhea | 15 (8–25) | 27 (12–44) | 15 (8–24) | 0.061 |
| Days from ICU admission to diarrhea | 9 (4–19) | 6 (1–28) | 10 (5–27) | 0.208 |
| Risk factors, n (%) | ||||
| MV 48h before infection | 141(74.2) | 9 (56.3) | 132 (75.9) | 0.069 |
| Proton pump inhibitor | 171 (90.0) | 16 (100) | 155 (89.1) | 0.608 |
| Histamine-2-receptor antagonist | 15 (7.9) | 0 | 15 (8.6) | 0.367 |
| Opiates (i.v.) | 125 (65.8) | 8 (50.0) | 117 (67.2) | 0.091 |
| Enteral nutrition | 125 (65.8) | 7 (43.8) | 118 (67.8) | 0.077 |
| Parenteral nutrition | 56 (29.5) | 8 (50.0) | 48 (27.6) | 0.101 |
| Antimicrobials 30 days prior to diarrhea | 178 (93.7) | 13 (81.3) | 165 (94.8) | 0.019 |
| Outcome | ||||
| Death, n (%) | 44(23.2) | 6 (37.5) | 38 (21.8) | 0.155 |
APACHE II=Acute Physiology and Chronic Health Evaluation II; COPD=chronic obstructive pulmonary disease; ICU=intensive care unit; IQR=inter-quartile range; SOFA=Sequential Organ Failure Assessment.
When patients with confirmed CDI were compared with those without CDI 1only chronic renal failure had higher prevalence in those with CDI compared with those without CDI (31.3% vs. 6.9%). The median number of unformed stools in patients diagnosed with a CDI per day was 5 (3–6) with a median duration of diarrhea of 2 days (range 2–3). Three patients with CDI (18.8%) presented with paralytic ileus. In addition, two cases of pseudomembranous colitis were reported. The infection was considered severe in seven cases (43.7%). The median length of stay in the ICU, measured in infected survivor patients, was 5 days (3–38). Only two patients (12.5%) suffered a recurrence of CDI, one infected by ribotype 027 and the other one by 014/020. The crude in-ICU mortality was 37.5% (6/16) in patients with CDI compared to 21.8% (38/174) in patients without CDI (p=0.155). One death was reported to be attributable to CDI.
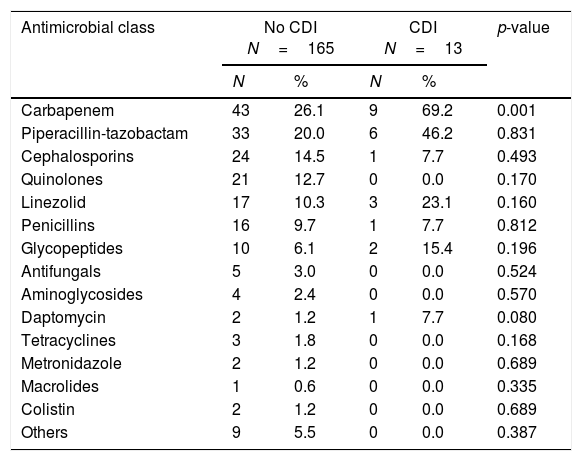
The antibiotics prescribed before the episode of diarrhea are shown in Table 2. Following multivariate statistical analysis, adjusting by age, gender, APACHE II score, and previous antimicrobial therapy, only chronic renal insufficiency was identified in the final model as a factor independently associated with development of CDI (adjusted odds ratio [OR] 5.45 [95% confidence interval {CI} 1.72–25.42]; p=0.035) in this patient cohort. In a second model adjusting by the same variables and as well as prior carbapenem usage, the factors independently associated with development of CDI were the same as in the first regression model.
Prescription of empiric antimicrobial therapy prior to onset of diarrheaa
| Antimicrobial class | No CDI N=165 | CDI N=13 | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Carbapenem | 43 | 26.1 | 9 | 69.2 | 0.001 |
| Piperacillin-tazobactam | 33 | 20.0 | 6 | 46.2 | 0.831 |
| Cephalosporins | 24 | 14.5 | 1 | 7.7 | 0.493 |
| Quinolones | 21 | 12.7 | 0 | 0.0 | 0.170 |
| Linezolid | 17 | 10.3 | 3 | 23.1 | 0.160 |
| Penicillins | 16 | 9.7 | 1 | 7.7 | 0.812 |
| Glycopeptides | 10 | 6.1 | 2 | 15.4 | 0.196 |
| Antifungals | 5 | 3.0 | 0 | 0.0 | 0.524 |
| Aminoglycosides | 4 | 2.4 | 0 | 0.0 | 0.570 |
| Daptomycin | 2 | 1.2 | 1 | 7.7 | 0.080 |
| Tetracyclines | 3 | 1.8 | 0 | 0.0 | 0.168 |
| Metronidazole | 2 | 1.2 | 0 | 0.0 | 0.689 |
| Macrolides | 1 | 0.6 | 0 | 0.0 | 0.335 |
| Colistin | 2 | 1.2 | 0 | 0.0 | 0.689 |
| Others | 9 | 5.5 | 0 | 0.0 | 0.387 |
The most frequently isolated C. difficile ribotypes were 078/126 (n=4; 25.0%), 001 (n=3; 18.7%), 014/020 (n=2; 12.5%), and 027 (n=2; 12.5%) The rest f them (070, 157, 106, R156 and R64) we identified in one case. The two patients with ribotype 027 were both women with a similar APACHE II score at ICU admission.
DiscussionThis is the first multicenter, prospective study describing the incidence, general characteristics, risk factors and complications of patients infected with C. difficile in Spanish ICUs. The present study demonstrates that real incidence of CDI appears to be low in Spain. Chronic renal failure was the only factor independently associated with CDI development which is similar to previously reported by a recent Mayo Clinic findings.10
Accurate diagnosis of CDI is a prerequisite for obtaining consistent epidemiologic data. To avoid this potential limitation, we centralized all sample analyses to a reference laboratory. Our accumulated incidence of CDI was 0.37%, lower than reported in a retrospective study carried out in a tertiary referral hospital in Barcelona, with 3.6% in 2 years.11 A retrospective analysis of patients included in the Spanish nosocomial infection surveillance registry in 2012 revealed an accumulated incidence of CDI of 0.35%.12 In our cohort, only three patients with CDI presented with some form of associated complication, all with paralytic ileus. Regarding mortality among the patients with diarrhea, we found no differences when comparing patients with and without CDI, which was perhaps related to the low severity of the majority of these infections, with 64.3% classified as mild or moderate. These findings are consistent with previous studies.6
Some limitations of our study should be acknowledged. First, we evaluated follow-up of all patients only until day 60 from CDI diagnosis, so CDI may have occurred beyond this timeframe. Secondly, the small number of cases of infection precluded certain statistical comparisons, and may have led to a type II error. Thirdly, we recognize that incidence density is the most accurate indicator. However, in our multicenter study the number of patients with ICU stays was not available for every participating hospital.
ConclusionsIn summary, the key finding of our study was that CDI incidence was lower than expected. There were no clinical or analytical data that distinguished between CDI and other types of diarrhea. Our study highlighted the potential importance of chronic renal insufficiency as a risk factor for the development of CDI in these patients.
Conflict of interestsThe authors declare that they have no conflicts of interest.
The authors acknowledge the investigators at the following centers:
Andalucía
Mª Carmen Lozano Domínguez (Hospital Universitario Virgen del Rocío), Rafael Rodríguez Jiménez y Enrique Muñoz (Hospital Universitario Virgen Macarena), Ana Loza Vázquez y Ana Isabel Aller García (Hospital Universitario Virgen de Valme), Cesar Aragón y Concepción Mediavilla Gradolph (Hospital General Carlos Haya), Mª Victoria de la Torre Prados, Francisco Cota Delgado, Estefania Cámara y Laura Mora Navas (Hospital Universitario Virgen de la Victoria), Rafael Guerrero Pavón y Francisco Solís Cuesta (Hospital Universitario Reina Sofía)
Madrid
Esther Diaz Rodríguez, Alfredo Bardal Ruiz y Alicia García Blanco (Hospital de la Princesa), Eva Herrero de Lucas, Belén Estébanez Montiel, Silvia García Bujalance y Emilio Maseda (Hospital La Paz), Mercedes Catalán González, Mª Ángeles Orellana Miguel y Concepción Camús Sánchez (Hospital 12 de Octubre), Mercedes Nieto Cabrera y Paloma Merino Amador (Hospital Clínico San Carlos), Sara Alcántara Carmona, Rocío Martínez Ruiz y Margarita Sánchez Castilla (Hospital Puerta de Hierro), Alexis Jaspe Codecido y Ana Mª Lajara Montell (Hospital Gregorio Marañón), Josefa Aurora Liétor Villanos y José Romero Vivas (Hospital Ramón y Cajal), Montserrat Rodríguez Aguirregabiria, Domingo Díaz Díaz y Carolina Campelo (Hospital Infanta Leonor).
Valencia
Concepción Gimeno y Juan Carlos Valía (Hospital General de Valencia), Juan Bonastre, Jose Luis López Hontangas, Mª Dolores Gómez Ruiz, Mª José Giménez Martí, Ignacio Moreno Puigdolers (Hospital La Fe de Valencia), Javier Buesa Gómez, Gerardo Aguilar y Nieves Carbonell Monleón (Hospital Clínico de Valencia), José Canovas Robles, Adelina Gimeno Gascón y Silverio Salvador (Hospital General de Alicante), Rafael Zaragoza Crespo, José Miguel Nogueira y Benedicta Sánchez Casado (Hospital Peset).
Cataluña
Jordi Rello Condomines, Jaume Valdría, Jesús Caballero, Rosa Alcaraz y Virginia Rodríguez Garrido (Hospital Vall D’Hebron), Javier Fernández Gómez y Miriam Álvarez (Hospital Clinic), Francisco Alvarez Lerma, Andrés Villasboa Vargas y Virginia Plasencia Miguel (Hospital del Mar), Rosa Granada Vicente y Jordi Niubó (Hospital de Bellvitge), Ricard Ferrer, Ana Parera y Josefa Pérez Jove (Hospital Mutua Terrassa), Jordi Vallés Dafnis y Isabel Sanfeliu Sala (Hospital Parc Taulí), Ignacio Javier Catalán Gómez y Montserrat Morta Pili (ALTHAIA Xarxa Assistencial Universitària de Manresa).
Euskadi
Ramón Cisterna y Unai Bengoetxea Uriarte (Hospital de Basurto), Iratxe Seijas Betolaza, Ildefonso Perales Palacios y Alberto Martinez Ruiz (Hospital de Cruces), Pedro María Olaechea Astigarraga y Patricia Martinez de la Fuente (Hospital Galdakao).
Cantabria
Camilo González Fernández, José Luis Teja Barbero y Mª Pia Roiz Mesones (Hospital Marqués de Valdecilla).