All commercial assays used to measure the presence of Hepatitis C virus (HCV) antibodies set cut-off points to categorise the results, but the problem of false positive results in screening hepatitis C sera is well known.
The aim of this study was to evaluate the results obtained by two chemiluminescent assays in selected sera, and compare these results with the detection of viral RNA in the specimens studied.
Material and methodsTwo hundred reactive sera (positive) were selected, although with a low signal to cut-off ratio (S/CO), were selected, using two chemiluminescent assays and were then subjected to genome amplification.
Results and discussionViral RNA could be only be detected in 8 (4%) of the selected specimens. Taking these results into account, we believe that the design of the current chemiluminescent assays do not provide sufficient specificity when they are used as the only tests for the diagnosis of hepatitis C.
Los ensayos comerciales utilizados para demostrar la presencia de anticuerpos anti-VHC establecen, cada uno de ellos, puntos de corte determinados que categorizan los resultados en función de los mismos, aunque el problema de los resultados falsos positivos en el cribado de sueros de hepatitis C sea bien conocido.
El objetivo de este trabajo ha sido valorar los resultados obtenidos por 2 ensayos quimioluminiscentes en una serie de sueros seleccionados, contrastando estos resultados con la detección de ARN viral en las muestras estudiadas.
Material y métodosSe seleccionaron 200 sueros considerados como reactivos (positivos), aunque con un bajo índice S/CO, utilizando 2 ensayos de quimioluminiscencia y posteriormente fueron sometidos a amplificación genómica.
Resultados y discusiónSolamente en 8 (4%) de las muestras seleccionadas pudo detectarse ARN viral. A la vista de estos resultados, consideramos que el diseño de los ensayos de quimioluminiscencia empleados no ofrecen una especificidad suficiente utilizados como pruebas únicas para el diagnóstico de la hepatitis C.
Hepatitis C virus (HCV) infection is a significant global public health problem. It is estimated that more than 175 million people in the world may be infected by this agent, generating between 3 and 4 million new cases per year.1 In western countries, the prevalence of infection is below 3%, and is around 2% in Spain. However, in other geographical areas such as North Africa, the prevalence reaches 15%, with the main risk factor being the use of contaminated material for the administration of injectables. In these risk groups the prevalence can reach 70%.2
Although the acute form of the infection is asymptomatic in most cases, up to 70–80% of those infected remain as chronic carriers of the virus and approximately 20% of them, in the absence of treatment, have a considerable risk of developing liver cirrhosis. In addition, after 20 years of evolution, about 5% end in a hepatocarcinoma. Currently, in our environment, HCV is the main cause of liver transplantation.3
The first link in the microbiological diagnosis of HCV infection consists of the detection of antibodies against the virus, complemented later with the confirmation by Recombinant ImmunoBlot Assay (RIBA) or the quantification of the viral RNA that allows, besides confirming the infection, for differentiating stages in its evolution.4
Positive anti-HCV results, but weakly reactive ones (low values of the S/CO index), regardless of the system used, have a low positive predictive value of current HCV infection and correspond more frequently to false reactivities or to infections already resolved in the past.5
The objective of this study was to learn the correlation between the weakly reactive results of 2 latest-generation screening tests and how many of these sera with low reactivity correspond to current infection or are past infections that have been resolved or are false positives.
Material and methods100 samples were collected whose anti-HCV S/CO index was between ≥1.00 and ≤3.00, using a LIAISON® XL MUREX HCVAb chemiluminescent immunoassay (CLIA) (DiaSorin) that uses the NS4 and NS3 biotinylated core viral antigens and another 100 samples with the same S/CO index according to the CMIA ARCHITECT Anti-HCV® (Abbott) assay that uses the HCr43 and c100-3 viral antigens. Both tests were conducted and interpreted following the instructions of the manufacturers.
The results obtained with both systems were categorised as “reactive” or “non-reactive”, according to the manufacturer's instructions: the samples with signal-to-cut-off ratio below 1.00 were classified as non-reactive for anti-HCV antibodies, and the samples with signal-to-limit ratio equal to or greater than 1.00 were classified as reactive for anti-HCV antibodies.
Subsequently, a quantification of HCV RNA was carried out in all samples by real-time PCR using the COBAS® Ampliprep/COBAS® Taqman® HCV Quantitative v2.0 (ROCHE) test whose lower limit of detection is 15IU/ml.
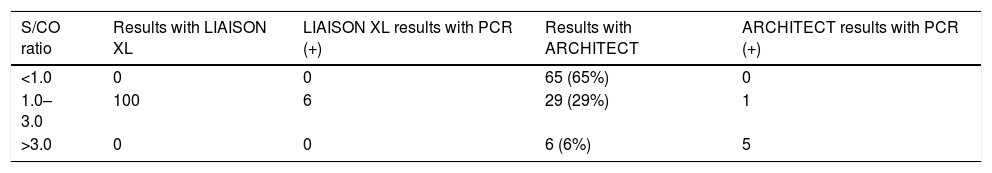
ResultsOf the 100 samples initially evaluated with LIAISON with an anti-HCV S/CO ratio between ≥1.00 and ≤3.00, when tested with ARCHITECT, only 29 were positive, showing the results that appear in Table 1.
Results of samples with S/CO ≥1.00 and ≤3.00 with DiaSorin and their correlation with ARCHITECT and real-time PCR.
| S/CO ratio | Results with LIAISON XL | LIAISON XL results with PCR (+) | Results with ARCHITECT | ARCHITECT results with PCR (+) |
|---|---|---|---|---|
| <1.0 | 0 | 0 | 65 (65%) | 0 |
| 1.0–3.0 | 100 | 6 | 29 (29%) | 1 |
| >3.0 | 0 | 0 | 6 (6%) | 5 |
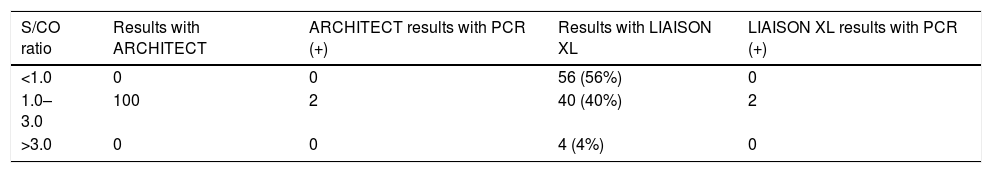
The results of the 100 samples tested by the Abbott reagent, and then by the DiaSorin assay, appear in Table 2: only 40 showed a positive result with the second assay.
Viral RNA was only detected in 8 (4%) of all of the selected samples.
DiscussionAlthough the CDC recommends that in order for a patient to present diagnostic evidence of hepatitis C, a confirmation with a second test equal or more specific than the one used – such as RIBA or a positive nucleic acid amplification of the virus – is necessary to avoid false positive diagnoses,6 especially in populations with low prevalence of the disease, in reality, numerous clinical laboratories simply offer a single positive result when establishing a presumptive diagnosis of hepatitis C, based on a single serological test.7 In most of these situations, the serological test used is usually a chemiluminescence test (CLIA or CMIA) designed to be used as screening in a low-risk population, such as health examinations or job evaluations, where, due to its high sensitivity, very low S/CO ratios can be detected, but, given their lower specificity, they are not sufficient to establish the clinical diagnosis of the disease.
Although with these techniques the presence of a high S/CO, clearly above the limit, presents a high possibility of defining the corresponding serum as carrier of anti-HCV antibodies, the same does not occur with those that have a low S/CO ratio, close to the limit of reactivity, which for this equipment would be between ≥1 and ≤3, and which, even serving as alarms, do not guarantee in any way the individual diagnosis and need to be confirmed preferably with the detection of circulating virus.
The problem of false positives in hepatitis C screening is also relatively well known and may be due to the increase in gammaglobulins, autoimmune diseases, liver diseases or other viral or parasitic infections.8
In our study, when the 200 sera considered as reactive (positive) by some of the assays were subjected to the detection of viral RNA by genomic amplification – considered as a highly reliable diagnostic test – viral RNA was only detected in 8 (4%) of them, while on the other hand, in 10 sera, in which some of the tests had high S/CO ratios of between 3.1 and 6.0, 5 (50%) harboured circulating viruses.
Therefore, the samples that were negative in either of the 2 assays presented a negative PCR, which suggests the irrelevance of performing confirmatory tests in this group, which accounted for more than 60% of the samples. On the other hand, we consider that the design of the chemiluminescence assays employed does not offer sufficient specificity when used as a single test for the individual diagnosis of hepatitis C, requiring, when they are positive, the endorsement of a second confirmatory test.
Although it is known that the design of the chemiluminescence assays, including the 2 tested, are intended primarily for use as screening tests and, therefore, establish low cut points trying to enhance their sensitivity at the expense of limiting their specificity, their use in numerous clinical laboratories with diagnostic criteria, sometimes as a single determination, makes it advisable for manufacturers to consider the values in which the S/CO ratios are low as included in a “grey area” of categorisation, which absolutely require confirmation with another test of greater specificity.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: López-Fabal MF, Pérez-Rivilla A, Gómez-Garcés JL. Valoración de sueros con bajo índice S/CO utilizando 2 sistemas de quimioluminiscencia para la detección de anticuerpos frente al virus de la hepatitis C y su correlación con la detección de ARN viral. Enferm Infecc Microbiol Clin. 2018;36:222–224.