To assess the impact of 18F-FDG-PET/CT on the diagnosis and management of patients with Staphylococcus aureus bacteraemia (SAB).
MethodsPost hoc analysis of a prospective cohort of consecutive adult patients diagnosed with SAB (January 2013–December 2017). Patients who underwent 18F-FDG-PET/CT at the discretion of the attending physician were included. Endpoints were the identification of previously unknown infectious foci and changes in clinical management, defined as changes in the duration or class of antibiotic therapy, a surgical procedure on the source of infection or a change in the decision to remove or retain an implantable device.
ResultsWe included 39 patients (median age: 69 years, IQR:60–79). Fifteen (39%) patients did not have an infectious focus identified before 18F-FDG-PET/CT). Thirty new infectious foci were detected in 22/39 (56%) patients. In 11/15 (73%) patients without an identified focus at least one infectious focus was detected by 18F-FDG-PET/CT. In 22/26 (85%) patients with implantable devices, 18F-FDG-PET/CT confirmed or ruled out infection or detected local complications. Out of 13 device infections, 10 were detected by 18F-FDG-PET/CT (7/10 for the first time). In 19/39 (49%) patients 18F-FDG-PET/CT results led to changes in clinical management (15 changes in antibiotic therapy, 2 device removals, 2 surgical procedures, 1 avoidance of a surgical procedure).
Conclusions18F-FDG-PET/CT may be a useful asset in the management of selected SAB cases, allowing the identification of previously undetected infectious foci and optimization of therapy, particularly in patients with endovascular devices. Indication should be made on a case-by-case basis.
Evaluar el impacto de la 18F-FDG-PET/TC en el diagnóstico y manejo de pacientes con bacteriemia por Staphylococcus aureus (BSA).
MétodosAnálisis post hoc de una cohorte prospectiva de pacientes adultos consecutivos con BSA (enero 2013–diciembre 2017). Se incluyeron aquellos pacientes en los que se realizó una 18F-FDG-PET/TC a criterio del médico tratante. Los criterios de valoración fueron la identificación de nuevos focos infecciosos y los cambios en el manejo clínico (definidos como modificaciones en la duración o clase del tratamiento antibiótico, intervención quirúrgica sobre el foco infeccioso o cambios en la decisión de retirar o mantener un dispositivo implantable).
ResultadosSe incluyeron 39 pacientes (edad mediana:69 años, RIC:60-79). En 15 (39%) pacientes no se había identificado un foco infeccioso antes de la 18F-FDG-PET/TC. Se identificaron 30 nuevos focos infecciosos en 22/39 (56%) pacientes. En 11/15 (73%) pacientes sin un foco infeccioso identificado la 18F-FDG-PET/TC detectó al menos un foco infeccioso. En 22/26 (85%) pacientes con dispositivos implantables la 18F-FDG-PET/TC permitió confirmar/descartar infección del dispositivo o detectar complicaciones locales. Diez de 13 infecciones de dispositivos fueron detectadas por 18F-FDG-PET/TC (7/10 desconocidas previamente). En 19/39 (49%) pacientes los hallazgos en la 18F-FDG-PET/TC conllevaron cambios en el manejo clínico (15 modificaciones de tratamiento antibiótico, 2 retiradas de dispositivo, 2 intervenciones quirúrgicas, 1 procedimiento quirúrgico evitado).
ConclusionesLa 18F-FDG-PET/TC puede ser de utilidad en la BSA, ya que permite identificar nuevos focos infecciosos y modificar el manejo clínico, sobre todo en pacientes con dispositivos endovasculares. La indicación ha de individualizarse en cada paciente.
Staphylococcus aureus is an important human pathogen and one of the major causes of bloodstream infections.1S. aureus bacteraemia (SAB) causes significant morbidity, with mortality ranging from 20 to 50% according to different studies.2–6 The persistence of SAB and the source and extension of the infection are key for defining the duration of antibiotic therapy and determining prognosis in each case.2 Approximately one-third of patients with SAB develop metastatic complications7,8 and only 40–60% of these metastatic foci present with localizing signs or symptoms that may guide the use of diagnostic tests.9,10 A focus of infection is not found in approximately 25% of patients with SAB.11 The ability to adequately control the source of infection in patients with SAB is associated with better outcomes.2,3,12,13
Although the performance of an echocardiogram is well established for patients with complicated SAB or predisposing conditions for infective endocarditis (IE),5 other imaging techniques might be of use for identifying other focal infections and guiding the clinical management in patients with SAB. 18F fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) has proven to be a useful diagnostic tool in patients with suspected prosthetic valve endocarditis and intracardiac device infections.14 It has also been proposed as an alternative imaging modality to diagnose infectious foci in patients with bacteraemia caused by S. aureus or other Gram-positive cocci.15–19 However, there is still a lack of agreement on when and in which cases should 18F-FDG-PET/CT be performed in patients with SAB. Moreover, previous studies have not evaluated the performance of 18F-FDG-PET/CT in patients with SAB and implantable devices.
In the present study, we aimed to assess the impact of 18F-FDG-PET/CT on the diagnosis of infectious foci and the clinical management of patients with SAB.
Patients and methodsStudy design and participantsThis study is a post hoc analysis of data collected within a prospective, observational, single-center cohort study of consecutive patients with SAB that was conducted between January 2013 and December 2017 at Hospital Universitari Vall d’Hebron,20 a 1000-bed tertiary university hospital in Barcelona (Spain). All cases were prospectively evaluated by an infectious diseases specialist, but decisions on clinical management and antimicrobial therapy were made by the attending physician. All patients 18 years of age or older diagnosed with monomicrobial SAB were included. In each patient, only the first episode of SAB during the study period was included. Patients were followed for 90 days after completing SAB treatment or until death. If clinical monitoring concluded within 90 days after treatment, follow-up was completed by telephone interview. We also checked primary care records and other regional hospital registries if needed.
We retrospectively reviewed those patients in the cohort who underwent a 18F-FDG-PET/CT according to the attending physician's medical opinion.
Study variables and data collectionDemographic, clinical and microbiological data were prospectively collected for the original cohort. Follow-up blood cultures were drawn at the attending physician's discretion. Data regarding 18F-FDG-PET/CT were retrospectively collected through an electronic chart review and entered in a database specifically designed for the study.
DefinitionsBacteraemia duration was defined as the number of days between the first and the last positive blood culture for S. aureus. Persistent SAB was defined as the isolation of S. aureus in blood cultures after 72 h of active antimicrobial therapy according to susceptibility testing. Persistent fever was defined as at least one determination of axillary temperature above 37.5°C after 72 h of active antimicrobial therapy. Complicated SAB was defined as the persistence of positive blood cultures after ≥3 days of active treatment, development of septic thrombophlebitis, infective endocarditis, infected arterial aneurysm, endovascular graft infection, or other metastatic distant foci; and any device-related infection where the device could not be removed in the first 3 days. SAB-definite therapy was defined as the main antibiotic administered during therapy.
The source of bacteraemia was established according to the Centers for Disease Control criteria.21 SAB was considered catheter-related if the Infectious Diseases Society of America guidelines’ criteria for a definite diagnosis of catheter-related bloodstream infection were met22 or if there were clinical signs of phlebitis or purulence at the catheter insertion site without any other plausible primary source of the bacteraemia. When a source of infection could not be identified, it was classified as an unknown source. Definite IE was defined according to the 2015 European Society of Cardiology guidelines,23 cases before August 2015 were retrospectively reviewed. Endovascular device infections (e.g. pacemaker infections) and IE were considered different entities. Appropriate source control was defined as the removal of all intravascular catheters (confirmed or suspected as a source of SAB) present at least 24 h before the first positive blood culture, drainage of an abscess (if present) or removal of infected devices (including prosthetic heart valves). In the absence of any of these factors, we considered the source as appropriately controlled.
Relapse was defined as a new episode of SAB with the same susceptibility pattern as the index case within 90 days of finishing SAB treatment.
Changes in clinical management were defined as: (1) changes in the duration or class of antibiotic therapy; (2) the performance or avoidance of a surgical procedure on the source of infection; or, (3) a change in the decision to remove or retain an implantable device or a long-term central venous catheter (excluding procedures that required open surgery).
The usefulness of 18F-FDG-PET/CT (detecting local complications, leading to changes in clinical management or confirming or ruling out infection) was established after individually discussing each case with two infectious diseases specialists.
For additional methods information see Supplementary Material.
EndpointsEndpoints were the number and location of previously unknown infectious foci identified by 18F-FDG-PET/CT and the proportion of patients in which 18F-FDG-PET/CT results entailed changes in clinical management, as defined above.
Diagnostic workupAn echocardiogram was recommended for all patients with persistent bacteraemia or persistent fever if the following criteria were met: absence of an uncontrolled known infectious focus; community-acquired SAB; SAB of unknown source; presence of metastatic distant foci; or predisposing conditions for endocarditis. Transthoracic echocardiography (TTE) was performed as a first-line technique, followed by transoesophageal echocardiography (TOE) in patients with a negative TTE, high index of suspicion for IE, and no contraindications for TOE. Other diagnostic imaging tests (e.g., ultrasound, computed tomography, magnetic resonance, scintigraphy) were performed according to the presence of guiding signs and symptoms at the discretion of the attending physician.
In case an endovascular infection was suspected, a cardiospecific 18F-FDG-PET/CT angiography (18F-FDG-PET/CTA) was performed.14,24 Images were reviewed by a group of experts on cardiac imaging, including a radiologist, a nuclear medicine physician and a cardiologist.
Microbiological studiesBlood cultures were performed with the BacT/ALERT 3D system (bioMérieux, Marcy l’Étoile, France) and isolate's identification was performed using the VITEK 2 or VITEK MS systems (bioMérieux) or by commercial molecular tests (Cepheid Xpert MRSA/SA BC, Sunnyvale, California). Antimicrobial susceptibility testing was performed in accordance with the European Committee on Antimicrobial Susceptibility Testing guidelines by use of disk diffusion techniques.
Statistical analysisQualitative variables were described by absolute count and relative percentage, and continuous variables were described as median and interquartile range. Statistical analyses were performed using SPSS software, version 21.
Ethics approvalThis study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the hospital ethics committee. Written informed consent was obtained from all patients, except those unable to consent due to the severity of their clinical condition, in which case the local ethics committee waived the need to obtain written informed consent.
ResultsPatients included in the studyWe identified 476 patients with at least one episode of SAB during the study period. Thirty-nine (8%) of these patients had a 18F-FDG-PET/CT performed during hospitalization and were included in this study. Among these 39 patients, 11 (28%) were methicillin-resistant S. aureus strains. The demographic and clinical characteristics of the patients who had a 18F-FDG-PET/CT performed are shown in Table 1.
Demographic and clinical characteristics of the 39 patients with Staphylococcus aureus bacteraemia who had a 18F-FDG-PET/CT performed.
| Variables | Patients (n = 39) |
|---|---|
| Age, years, median [IQR] | 69 [60–79] |
| Female sex | 9 (23) |
| MRSA strain | 11 (28) |
| Pre-existing conditions | |
| Charlson comorbidity index, median [IQR] | 3 [1–4] |
| Charlson comorbidity index (age adjusted), median [IQR] | 6 [3–8] |
| Valvular heart disease | 22 (56) |
| Diabetes mellitus | 17 (44) |
| Chronic kidney disease | 14 (36) |
| Malignancy | 10 (26) |
| Immunosuppression | 8 (21) |
| Renal replacement therapy | 6 (15) |
| Liver cirrhosis | 4 (10) |
| Implantable devices | 26 (67) |
| Endovascular devices (other than vascular catheters) | 17 (44) |
| Osteosynthesis | 9 (23) |
| Prosthetic heart valves | 8 (21) |
| Other | 1 (3) |
| Vascular cathetera | 13 (33) |
| Acquisition of Staphylococcus aureus bacteraemia | |
| Community-acquired | 13 (33) |
| Non-nosocomial healthcare-related | 18 (46) |
| Nosocomial | 8 (21) |
| Presumed portal of entry | |
| Catheter-related | 7 (18) |
| Cutaneous | 7 (18) |
| Surgical wound infection | 2 (5) |
| Urinary tract infection | 1 (3) |
| Pneumonia | 1 (3) |
| Intraabdominal | 1 (3) |
| Unknown | 20 (51) |
| Clinical data | |
| Persistent bacteraemia | 22 (56) |
| Persistent fever | 3 (8) |
| Complicated S. aureus bacteraemia | 31 (79) |
| Intensive care unit stay | 5 (13) |
| Vasopressor support | 3 (8) |
| Time from onset to start of active antimicrobial therapy, days, median [IQR] | 0 [0–1] |
| Duration of antibiotic treatment, days, median [IQR] | 41 [24–68] |
| Definite antimicrobial therapy | |
| MSSA bacteraemia | 28 (72) |
| Cloxacillin | 15 (54) |
| Cefazolin | 6 (21) |
| Levofloxacin + rifampinb | 4 (14) |
| Trimethoprim/sulfamethoxazoleb | 2 (7) |
| Daptomycin | 1 (4) |
| MRSA bacteraemia | 11 (28) |
| Daptomycin | 5 (46) |
| Ceftaroline | 3 (27) |
| Daptomycin + cloxacillin | 2 (18) |
| Trimethoprim/sulfamethoxazole + rifampinb | 1 (9) |
Data are presented as N (%) unless otherwise indicated. 18F-FDG-PET/CT: 18F fluorodeoxyglucose positron emission tomography/computed tomography, IQR: interquartile range, MRSA: Methicillin-resistant Staphylococcus aureus, MSSA: Methicillin-sensitive Staphylococcus aureus.
An implantable device infection was diagnosed in 13 (33%) patients. Follow-up blood cultures were obtained from all 39 (100%) patients. Bacteraemia lasted for a median of 3 days (IQR: 0–8) from the start of active antibiotic therapy to the last positive blood culture. Echocardiography was performed in 38 (97%) patients, TOE was performed in 30 (77%) cases. One single patient—who had a prosthetic heart valve and a high level of suspicion for IE—could not have an echocardiogram performed because of respiratory failure and intolerance to undergo a TOE. Instead, a 18F-FDG-PET/CT was directly performed without further image testing.
A potentially controllable infectious focus was found in 24 (62%) patients, but source control was only possible in 13 cases. In the remaining 11 cases, source control could not be achieved due to extreme fragility of the patient or unacceptable high risk of the surgical procedure (e.g. removal of endovascular prosthesis).
An implantable device infection was diagnosed in 13 (33%) patients. Eleven (28%) patients were diagnosed with definite IE (6 prosthetic valve endocarditis and 5 native valve endocarditis). In 9 (82%) cases, the diagnosis of endocarditis was done before performing the 18F-FDG-PET/CT. Abnormal valvular or perivalvular uptake was present in 5/6 cases of prosthetic valve endocarditis and 2/5 cases of native valve endocarditis. One patient with a previous normal TTE was diagnosed with prosthetic valve endocarditis by 18F-FDG-PET/CT (TOE could not be performed due to respiratory failure). Another patient with SAB of unknown origin and a previous normal TOE was diagnosed with prosthetic valve endocarditis, vascular graft infection and septic pulmonary embolisms by 18F-FDG-PET/CT. Finally, there was a patient in which prosthetic valve endocarditis was diagnosed by TTE and 18F-FDG-PET/CT was performed to assess if a pacemaker was also infected. 18F-FDG-PET/CT ruled out pacemaker infection but found a paravalvular abscess that required surgery.
All-cause mortality was 26% (10/36) at 30 days and 33% (13/39) at 90 days. Bacteraemia relapsed in 5 (13%) cases during follow-up. One patient was diagnosed with a perivalvular abscess that could not be surgically managed and after relapsing a TOE showed persistence of the abscess; in another case a prosthetic joint infection not previously detected by 18F-FDG-PET/CT was diagnosed. Another patient had the 18F-FDG-PET/CT performed after the relapse: he underwent a TTE and an abdominal CT scan during the initial evaluation of SAB and, as no infectious foci were found, received antibiotic therapy for 16 days. After the relapse of SAB, vertebral osteomyelitis was identified by 18F-FDG-PET/CT. No infectious foci were detected in the remaining 2 patients with relapsed bacteraemia.
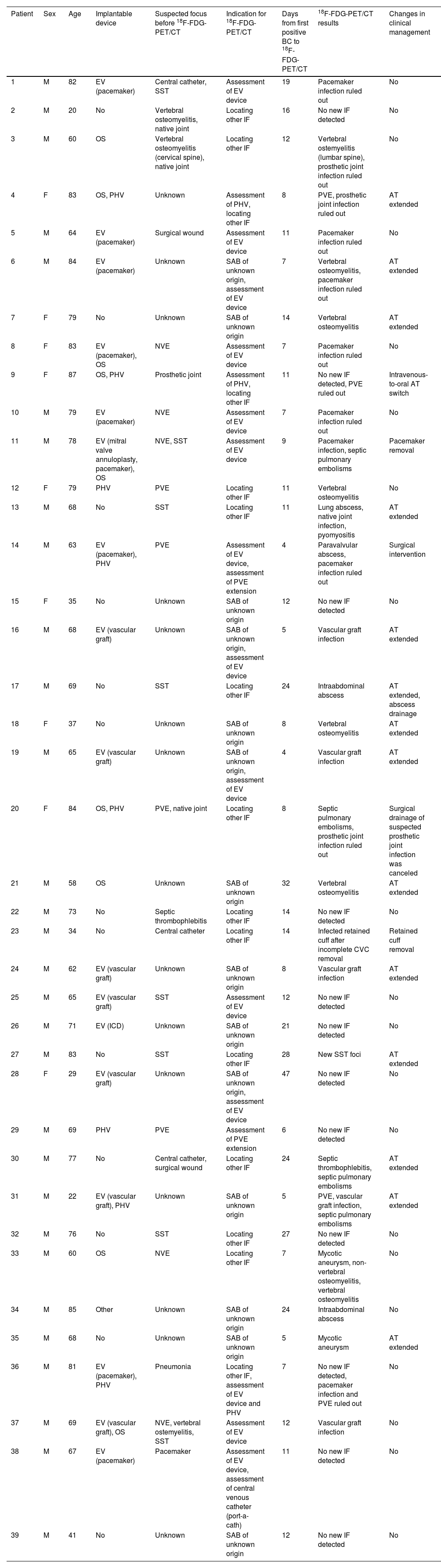
18F-FDG-PET/CT findingsThe median interval from the first positive blood culture to 18F-FDG-PET/CT was 11 days (IQR: 7–16). Before performing 18F-FDG-PET/CT, 15 (38.4%) patients did not have an infectious focus identified. Thirty-two (82.1%) patients had at least one infectious focus detected by 18F-FDG-PET/CT. Two-thirds of the patients with a positive 18F-FDG-PET/CT had a single infectious focus detected by 18F-FDG-PET/CT, as opposed to one-third of the patients in whom multiple infectious foci were identified. 18F-FDG-PET/CT results, timing and changes in clinical management for each patient are detailed in Table 2.
Findings and changes in clinical management of patients with Staphylococcus aureus bacteraemia after performing a 18F-FDG-PET/CT.
| Patient | Sex | Age | Implantable device | Suspected focus before 18F-FDG-PET/CT | Indication for 18F-FDG-PET/CT | Days from first positive BC to 18F-FDG-PET/CT | 18F-FDG-PET/CT results | Changes in clinical management |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 82 | EV (pacemaker) | Central catheter, SST | Assessment of EV device | 19 | Pacemaker infection ruled out | No |
| 2 | M | 20 | No | Vertebral osteomyelitis, native joint | Locating other IF | 16 | No new IF detected | No |
| 3 | M | 60 | OS | Vertebral osteomyelitis (cervical spine), native joint | Locating other IF | 12 | Vertebral ostemyelitis (lumbar spine), prosthetic joint infection ruled out | No |
| 4 | F | 83 | OS, PHV | Unknown | Assessment of PHV, locating other IF | 8 | PVE, prosthetic joint infection ruled out | AT extended |
| 5 | M | 64 | EV (pacemaker) | Surgical wound | Assessment of EV device | 11 | Pacemaker infection ruled out | No |
| 6 | M | 84 | EV (pacemaker) | Unknown | SAB of unknown origin, assessment of EV device | 7 | Vertebral osteomyelitis, pacemaker infection ruled out | AT extended |
| 7 | F | 79 | No | Unknown | SAB of unknown origin | 14 | Vertebral osteomyelitis | AT extended |
| 8 | F | 83 | EV (pacemaker), OS | NVE | Assessment of EV device | 7 | Pacemaker infection ruled out | No |
| 9 | F | 87 | OS, PHV | Prosthetic joint | Assessment of PHV, locating other IF | 11 | No new IF detected, PVE ruled out | Intravenous-to-oral AT switch |
| 10 | M | 79 | EV (pacemaker) | NVE | Assessment of EV device | 7 | Pacemaker infection ruled out | No |
| 11 | M | 78 | EV (mitral valve annuloplasty, pacemaker), OS | NVE, SST | Assessment of EV device | 9 | Pacemaker infection, septic pulmonary embolisms | Pacemaker removal |
| 12 | F | 79 | PHV | PVE | Locating other IF | 11 | Vertebral osteomyelitis | No |
| 13 | M | 68 | No | SST | Locating other IF | 11 | Lung abscess, native joint infection, pyomyositis | AT extended |
| 14 | M | 63 | EV (pacemaker), PHV | PVE | Assessment of EV device, assessment of PVE extension | 4 | Paravalvular abscess, pacemaker infection ruled out | Surgical intervention |
| 15 | F | 35 | No | Unknown | SAB of unknown origin | 12 | No new IF detected | No |
| 16 | M | 68 | EV (vascular graft) | Unknown | SAB of unknown origin, assessment of EV device | 5 | Vascular graft infection | AT extended |
| 17 | M | 69 | No | SST | Locating other IF | 24 | Intraabdominal abscess | AT extended, abscess drainage |
| 18 | F | 37 | No | Unknown | SAB of unknown origin | 8 | Vertebral osteomyelitis | AT extended |
| 19 | M | 65 | EV (vascular graft) | Unknown | SAB of unknown origin, assessment of EV device | 4 | Vascular graft infection | AT extended |
| 20 | F | 84 | OS, PHV | PVE, native joint | Locating other IF | 8 | Septic pulmonary embolisms, prosthetic joint infection ruled out | Surgical drainage of suspected prosthetic joint infection was canceled |
| 21 | M | 58 | OS | Unknown | SAB of unknown origin | 32 | Vertebral osteomyelitis | AT extended |
| 22 | M | 73 | No | Septic thrombophlebitis | Locating other IF | 14 | No new IF detected | No |
| 23 | M | 34 | No | Central catheter | Locating other IF | 14 | Infected retained cuff after incomplete CVC removal | Retained cuff removal |
| 24 | M | 62 | EV (vascular graft) | Unknown | SAB of unknown origin | 8 | Vascular graft infection | AT extended |
| 25 | M | 65 | EV (vascular graft) | SST | Assessment of EV device | 12 | No new IF detected | No |
| 26 | M | 71 | EV (ICD) | Unknown | SAB of unknown origin | 21 | No new IF detected | No |
| 27 | M | 83 | No | SST | Locating other IF | 28 | New SST foci | AT extended |
| 28 | F | 29 | EV (vascular graft) | Unknown | SAB of unknown origin, assessment of EV device | 47 | No new IF detected | No |
| 29 | M | 69 | PHV | PVE | Assessment of PVE extension | 6 | No new IF detected | No |
| 30 | M | 77 | No | Central catheter, surgical wound | Locating other IF | 24 | Septic thrombophlebitis, septic pulmonary embolisms | AT extended |
| 31 | M | 22 | EV (vascular graft), PHV | Unknown | SAB of unknown origin | 5 | PVE, vascular graft infection, septic pulmonary embolisms | AT extended |
| 32 | M | 76 | No | SST | Locating other IF | 27 | No new IF detected | No |
| 33 | M | 60 | OS | NVE | Locating other IF | 7 | Mycotic aneurysm, non-vertebral osteomyelitis, vertebral osteomyelitis | No |
| 34 | M | 85 | Other | Unknown | SAB of unknown origin | 24 | Intraabdominal abscess | No |
| 35 | M | 68 | No | Unknown | SAB of unknown origin | 5 | Mycotic aneurysm | AT extended |
| 36 | M | 81 | EV (pacemaker), PHV | Pneumonia | Locating other IF, assessment of EV device and PHV | 7 | No new IF detected, pacemaker infection and PVE ruled out | No |
| 37 | M | 69 | EV (vascular graft), OS | NVE, vertebral ostemyelitis, SST | Assessment of EV device | 12 | Vascular graft infection | No |
| 38 | M | 67 | EV (pacemaker) | Pacemaker | Assessment of EV device, assessment of central venous catheter (port-a-cath) | 11 | No new IF detected | No |
| 39 | M | 41 | No | Unknown | SAB of unknown origin | 12 | No new IF detected | No |
SAB: Staphylococcus aureus bacteraemia, 18F-FDG-PET/CT: 18F fluorodeoxyglucose positron emission tomography/computed tomography, BC: blood culture, M: male, F: female, EV: endovascular, OS: osteosynthesis, PHV: prosthetic heart valve, ICD: Implantable cardioverter-defibrillator, SST: skin and soft tissue, NVE: native valve endocarditis, PVE: prosthetic valve endocarditis, IF: infectious foci, AT: antibiotic therapy.
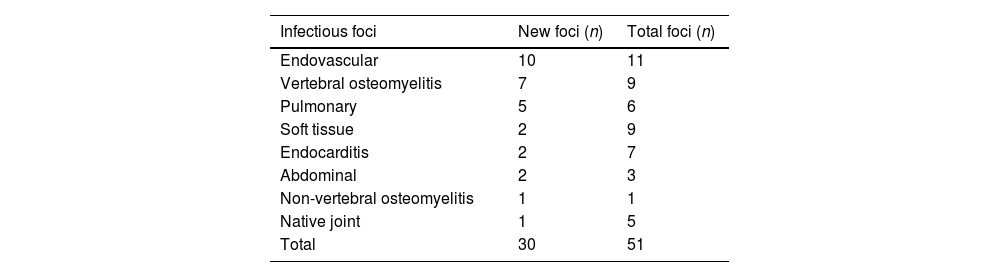
Overall, 30 new infectious foci were detected in 22 (56%) patients, as shown in Table 3. One or more new infectious foci were detected by 18F-FDG-PET/CT in 11 out of 15 (73%) patients without an identified focus and 12 out of 22 (55%) patients with persistent bacteraemia. In patients with a known infectious focus, new foci were detected in 11/24 (46%) cases. In 22 out of 26 (85%) patients with implantable devices, 18F-FDG-PET/CT confirmed or ruled out infection or detected local complications. In 9/10 patients with an implantable device and no known infectious focus, at least one infectious focus was detected. Ten out of thirteen (77%) device infections were detected by 18F-FDG-PET/CT, 7 of these device infections were diagnosed exclusively according to the 18F-FDG-PET/CT findings (4 endovascular devices, 2 prosthetic heart valves and 1 biliary drainage catheter).
Localization of infectious foci identified by 18F-FDG-PET/CT.
| Infectious foci | New foci (n) | Total foci (n) |
|---|---|---|
| Endovascular | 10 | 11 |
| Vertebral osteomyelitis | 7 | 9 |
| Pulmonary | 5 | 6 |
| Soft tissue | 2 | 9 |
| Endocarditis | 2 | 7 |
| Abdominal | 2 | 3 |
| Non-vertebral osteomyelitis | 1 | 1 |
| Native joint | 1 | 5 |
| Total | 30 | 51 |
18F-FDG-PET/CT: 18F fluorodeoxyglucose positron emission tomography/computed tomography.
In 20 (51%) patients 18F-FDG-PET/CT results led to changes in clinical management (Table 2). This proportion was higher (67%) in the 15 patients without an identified infectious focus, particularly in those with an implantable device (7/10), compared to 9/24 patients with a known infectious focus (38%). There were 15 changes in antibiotic therapy: in 14 cases treatment was extended and in 1 case treatment was switched to oral antibiotic therapy (IE was ruled out in a patient with prosthetic joint infection and a high index of suspicion in which TOE could not be performed). Two endovascular devices were removed: one pacemaker and one catheter cuff that had inadvertently remained in site after removing an infected central venous line. There were 2 surgical procedures derived from 18F-FDG-PET/CT results: one prosthetic valve replacement after diagnosing a perivalvular abscess in a patient with prosthetic valve endocarditis and one abdominal abscess that required surgical drainage. Finally, surgical debridement of periprosthetic joint fluid collections was not performed in one case after a negative 18F-FDG-PET/CT result.
DiscussionOur results show that the 18F-FDG-PET/CT identified new infectious foci in 56% of the patients, and this percentage was higher (73%) in patients without a previously identified infectious focus. Furthermore, it led to changes in clinical management in approximately half of the patients. Considering only patients with implantable devices, 18F-FDG-PET/CT provided useful information in 85% of cases (detecting local complications and confirming or ruling out infection) and was able to identify 77% of device infections.
These findings are in line with results from previous studies that have evaluated the performance of 18F-FDG-PET/CT in Gram-positive bacteraemia and indicate that it can identify previously unknown infectious foci in more than 50% of patients.18,25 However, it is still unclear which subgroups of patients with Gram-positive bacteraemia could benefit the most from 18F-FDG-PET/CT. In our study, we found the performance of 18F-FDG-PET/CT to be higher in patients without a previously identified infectious focus. Furthermore, although some studies have outlined in the baseline characteristics the presence of implantable devices,15,18,26 this is, to the best of our knowledge, the first study to assess the performance of 18F-FDG-PET/CT in SAB in this subgroup of patients.
Even though the optimal timing of 18F-FDG-PET/CT in SAB is yet to be defined, preceding studies have described a median time of 7–14 days between first positive blood culture and 18F-FDG-PET/CT performance,18,26 which coincides with our results. As one of the expected benefits of 18F-FDG-PET/CT would be achieving an early diagnosis of infectious foci and/or complications, the consensus in previous publications seems to be to have it performed as soon as possible (preferably in the first 7–14 days after the onset of bacteraemia).17,18 Nonetheless, Brøndserud et al. found no significant difference in the ability of 18F-FDG-PET/CT to detect sites of infection in relation to the duration of bacteraemia in patients with Gram-positive bacteraemia.25
As reported in previous studies, endovascular, vertebral osteomyelitis and pulmonary foci were the most frequently newly detected foci by 18F-FDG-PET/CT,17 probably because localizing symptoms are less common in endovascular and pulmonary locations. Although our study was not designed to assess the performance of 18F-FDG-PET/CT in prosthetic or native valve endocarditis, the fact that only two cases of IE were diagnosed by 18F-FDG-PET/CT stands out. This is probably due to the expertise of the multidisciplinary endocarditis team in our center. We do not perform 18F-FDG-PET/CT routinely when prosthetic valve IE is suspected, but rather in selected cases in which further information apart from the TTE or TOE is required. Even though the usefulness of 18F-FDG-PET/CT in prosthetic valve endocarditis has already been established, its diagnostic value in native valve endocarditis remains to be defined.27,28
In our study, we found a 90-day mortality rate of 33% in patients with SAB who had a 18F-FDG-PET/CT performed, higher than reported by previous studies.18 This variability in mortality might be explained by differences in the study population, as our subjects were older (median age 69 years) and probably at higher risk for complications (56% had persistent SAB and 79% complicated bacteraemia).
Although 18F-FDG-PET/CT is costly, a previous study by Vos et al. conducted in the Netherlands suggests that performing a 18F-FDG-PET/CT in patients with high-risk Gram-positive bacteraemia might be cost-effective.29 However, and while more data on cost-effectiveness are needed, it could be further improved by accurately selecting patients with high-risk SAB that might benefit most from this technique.
There are several limitations that should be taken into account when interpreting these results. First, this is a retrospective analysis of a prospective cohort and, as such, the original cohort was not designed to analyze and interpret the data hereby presented. The evaluation of the 18F-FDG-PET/CT indication and changes in clinical management were performed retrospectively. However, we aimed to reduce bias by assessing the changes in clinical management in consensus by two infectious diseases specialists. Second, as a single-center study, the sample size is relatively small. Third, there is no comparative group and, therefore, we could not assess the impact of not performing 18F-FDG-PET/CT in a similar group of patients. Notwithstanding that our conclusions might be restricted by these limitations, we believe that sharing our real-world experience can contribute to a deeper understanding of the utility of 18F-FDG-PET/CT in the management of patients with SAB. The results of this exploratory study could help design future prospective studies that might cast light on which patients with SAB will benefit from a 18F-FDG-PET/CT.
In conclusion, our results suggest that 18F-FDG-PET/CT can be a useful tool in the management of selected SAB cases. Performing a 18F-FDG-PET/CT can identify previously undetected infectious foci and, accordingly, help optimize clinical management. This strategy might be particularly useful in patients with endovascular devices and SAB without previously known infectious foci. However, until more data are available regarding the optimal timing and subgroups of patients that might benefit from this intervention, we believe that indication should be made on a case-by-case basis.
Authors’ contributionsPS, RW, NFH and BA designed the study. PS, RW and MPA were responsible for data collection. MPA was responsible for obtaining hospital ethics committee approval. PS, RW, MPA, MNP, AR, MB, MS, DRP, MNL, NFH and BA contributed to data analysis and interpretation. PS and RW drafted the manuscript, which was critically revised and amended by MPA, MNP, AR, MB, MS, DRP, MNL, NFH and BA. PS and RW contributed equally to this work. All authors read and approved the final version of the manuscript.
FundingSupported by the Plan Nacional de I + D + I 2013–2016, Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, and the Spanish Network for Research in Infectious Diseases [REIPI RD16/0016/0003]. REIPI is co-financed by the European Development Regional Fund ‘A way to achieve Europe’ and the Operative program Intelligent Growth 2014–2020.
Conflicts of interestAll the authors declare no conflicts of interest.
The authors would like to thank Dr. Carles Pigrau for his helpful advice and guidance.