In the last years, an increase of severe cases of invasive disease (ID) due to Streptococcus pyogenes or streptococcus b-hemolytic group A (SGA) had been detected. The aim of this study was to analyse the epidemiology and the clinical features of ID due to SGA in a tertiary paediatric hospital.
Material and methodsThis was a retrospective study of all in-patients with final diagnosis of ID due to SGA during 6 years (2009–2014) in a paediatric hospital.
To consider ID, SGA had to be isolated in sterile samples, in patients with fasciitis necroticans in skin samples or in any sample in patients with the diagnosis of Streptococcal Toxic Shock Syndrome (STSS). The SSTS was defined as hypotension and at least 2 of the following criteria: renal failure, hepatic failure, acute respiratory distress, tissue necrosis or desquamative erythematous rash.
Demographic data, type of infection, risk factors, clinical presentation, analytical data at admission, treatment, need for admission to a pediatric intensive care unit, microbiological data, hospital stay and evolution were collected.
ResultsFifty-two (52) cases were included (12/10,000 of all inpatients); 3 years old was the medium age (p25-75: 1.4–6.9 years) and 28 (53.8%) were boys. Fourteen patients (26.9%) had risk factors. Fever was the major symptom (51 patients, 98.1%). The skin lesions were the most frequent clinical manifestations found (21; 40.4%). In 50 (96%) cases, SGA was isolated in at least one sterile sample. Skin and soft tissue infections were diagnosed in 14 patients (26.9%), 14 (26.9%) pneumonias, 12 (23.1%) bones and joints infections, 10 (19.2%) SSTS, 6 (11.5%) occult bacteremia, 4 (7.7%) meningitis and 2 (3.8%) sepsis.
Surgery was required in 18 cases (34.6%) and 17 patients (32.7%) needed intensive care. The medium hospital stay was 9.5 days (p25-75: 8–15 days). Three patients presented sequels and one patient died.
ConclusionThe ID due to SGA was a rare but serious reason for hospital admission. Skin and soft tissue infections and pleuroneumonia were the most common forms of ID. The mortality of our sample was low despite the serious clinical manifestations.
En los últimos años se ha detectado un incremento de los casos graves de enfermedad invasiva (EI) por Streptococcus pyogenes o estreptococo beta-hemolítico del grupo A (SGA). El objetivo del estudio fue determinar la epidemiología y las características clínicas de las EI por SGA en un hospital pediátrico de tercer nivel.
Material y métodosEstudio retrospectivo realizado en un hospital urbano materno-infantil de tercer nivel. Se incluyeron los pacientes ingresados con diagnóstico final de EI por SGA durante 6 años (2009-2014).
Se consideró EI cuando SGA se aisló en muestras estériles, en pacientes con fascitis necrosante cuando se aisló en muestras de la zona de la lesión y en pacientes con síndrome shock tóxico estreptocócico (SSTS) cuando se aisló en cualquier muestra.
Se recogieron datos demográficos, tipo de infección, factores de riesgo, presentación clínica, datos analíticos al ingreso, tratamiento, necesidad de ingreso en unidad de cuidados intensivos pediátricos (UCIP), datos microbiológicos, estancia hospitalaria y evolución.
ResultadosSe incluyeron 52 casos (12/10.000 ingresos); edad mediana de 3 años (p25-75: 1,4-6,9 años); 28 (53,8%) eran varones. Presentaban factores de riesgo 14 (26,9%) casos. El motivo de consulta incluía fiebre en 51 (98,1%); la clínica acompañante más frecuente fue la cutánea (21; 40,4%). En 50 (96%) casos se aisló SGA en al menos un medio estéril. Se diagnosticaron 14 (26,9%) infecciones de piel y partes blandas, 14 (26,9%) neumonías, 12 (23,1%) infecciones osteoarticulares, 10 (19,2%) SSTS, 6 (11,5%) bacteriemias ocultas, 4 (7,7%) meningitis y 2 (3,8%) sepsis.
Requirieron cirugía 18 (34,6%) casos y 17 (32,7%) ingreso en unidad de cuidados intensivos. La mediana de estancia hospitalaria fue de 9,5 días (p25-75: 8-15 días). Presentaron secuelas 3 pacientes y hubo un fallecimiento.
ConclusiónLa EI por SGA fue un motivo poco frecuente aunque grave de hospitalización. Las infecciones de piel y partes blandas, y las pleuroneumonías fueron las formas de EI más habituales. A pesar de la gravedad, la mortalidad en la serie fue baja.
Invasive disease (ID) due to Streptococcus pyogenes (GAS) is uncommon in paediatrics, with an estimated incidence of two–three cases for every 100,000 inhabitants and year.1,2 It is a serious disease, which mainly affects previously healthy patients and which presents a significant mortality (around 5–20%3–6), especially in relation to the presence of necrotising fasciitis, streptococcal toxic shock syndrome (STSS) or an underlying disease. Between 1982 and 2002, the surveillance programme endorsed by the European Union, Euro-STREP, detected a progressive increase in cases.7 The cause of this increase was unknown but several hypotheses were considered, such as the increase in the infective power of the bacterium, alterations to the immune system and cyclical patterns in the incidence of focal infection.8–11 This programme includes patients of all ages (Spain is not part of the programme). Furthermore, publications on paediatrics regarding ID due to GAS are scarce and most are based on series with few cases.5,12 The objective of this study was to describe the epidemiology and clinical characteristics of IDs due to GAS in a tertiary paediatric hospital.
MethodsDesignA retrospective study conducted in a tertiary maternity and children's hospital with 275 paediatric beds, which serves an area of 1,800,000 inhabitants (300,000 under 18 years of age) and which annually receives around 100,000 paediatric visits.
Study populationPatients aged from one month to 18 years who were admitted to the hospital with a final diagnosis of ID due to GAS over a six-year period (January 2009–December 2014) were included.
DefinitionsID:
- •
Patients with isolation of GAS in normally sterile samples (blood, urine, cerebrospinal fluid [CSF], etc.).
- •
Patients with necrotising fasciitis when it is isolated from samples taken from the lesion.
- •
Patients with STSS when GAS is isolated from any sample (pharyngeal smear, wound, skin, ear discharge, etc.).
STSS: presence of hypotension and at least two of the following: kidney failure, clotting or liver abnormalities; acute respiratory distress syndrome, tissue necrosis or erythematous rash with peeling.13
Sepsis: systemic inflammatory response syndrome (SIRS) in the presence, or as a result, of suspected or confirmed infection.14
Hidden bacteraemia: hidden bacteraemia is defined as the presence of bacteria in the blood of a febrile child with good general condition, in the absence of an identifiable source of infection.15
For the identification of cases, on the one hand, all patients with any sterile culture medium positive for GAS were selected from the hospital laboratory's database and, on the other hand, patients with a diagnosis at discharge of necrotising fasciitis and STSS were selected from the hospital discharge database (ICD M72.6 and A48.3). Their medical records were reviewed and those that met the inclusion criteria were selected.
Biological samples were processed in the microbiology laboratory using Gram stain and culturing in conventional media. Bacterial identification was obtained using routine biochemical and enzyme tests (API 20 STREP. bioMérieux; Marcy l’Etoile, France) and was confirmed by latex agglutination specific for the GAS antigen (Strep Kit. Diamondial; Langenhagen, Germany).
Study variablesFor each case, demographic data, type of infection, risk factors, clinical presentation, analytical data on admission, treatment, need for admission to the paediatric intensive care unit (PICU), microbiological data, hospital stay and outcome were collected.
Statistical analysisThe data extracted were stored and processed in a specific Microsoft Access database. Quantitative and categorical variables were tabulated. Subsequently, they were analysed with the SPSS v 21.0 statistics program for Windows. The descriptive statistics were displayed using medians and interquartile range for the quantitative variables and absolute numbers and percentages for the categorical variables.
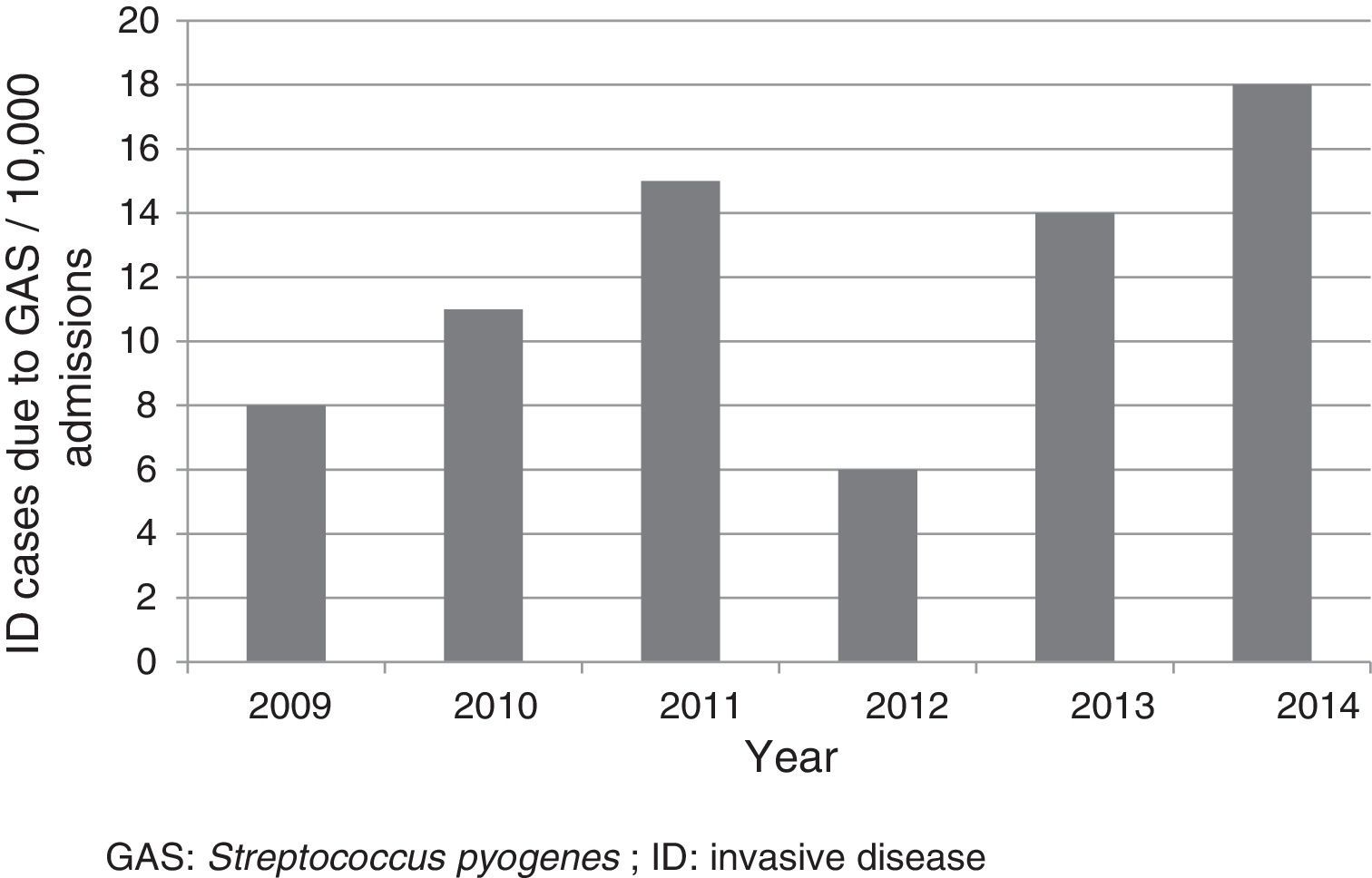
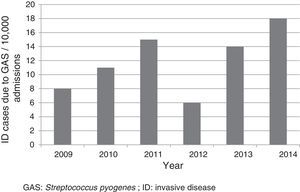
ResultsFifty-two cases were included, which corresponded to a mean annual rate of 12 cases for every 10,000 admissions (Fig. 1). The median age of patients was three years (p25-75: 1.4–6.9 years); 33 (63.5%) were less than five years old; 28 (53.8%) were male. Nineteen (36.5%) were referred from other centres for assessment and admission, of whom 12 came from hospitals and seven from primary care centres. Six patients were undergoing previous treatment with amoxicillin, three for tonsillopharyngitis and three for otitis.
Fourteen patients (26.9%) presented with risk factors:
- •
Twelve had a skin condition: eight had acute skin diseases (four wounds, two dermatitis and two chickenpox); and four had chronic skin diseases (two patients with epidermolysis, one with cutis aplasia and another with atopic dermatitis), three of whom had an acute outbreak of their disease.
- •
Two were undergoing treatment with immunosuppressants; corticosteroids and tacrolimus.
No patient had a history of surgery or burn injuries.
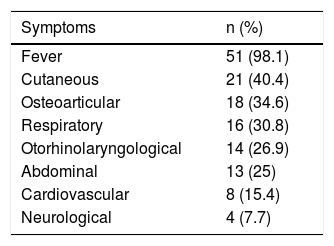
The most common symptoms are listed in Table 1. The reason for consultation included fever in 51 patients (98.1%), with onset 1–7 days before consultation, and with a median maximum recorded temperature of 39.4°C (p25-75: 38.7–40°C); this was the only symptom in five cases. There was one case without fever, which corresponded to a patient with skin abscesses and a positive blood culture.
Symptoms presented in the emergency department by patients with invasive disease due to Streptococcus pyogenes (n=52).
| Symptoms | n (%) |
|---|---|
| Fever | 51 (98.1) |
| Cutaneous | 21 (40.4) |
| Osteoarticular | 18 (34.6) |
| Respiratory | 16 (30.8) |
| Otorhinolaryngological | 14 (26.9) |
| Abdominal | 13 (25) |
| Cardiovascular | 8 (15.4) |
| Neurological | 4 (7.7) |
Several patients presented with more than one symptom.
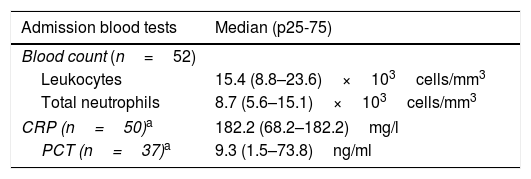
Table 2 shows the results of the admission blood tests and of the microbiological tests: in 50 (96%), GAS was isolated in at least one sterile medium, and the two remaining cases corresponded to children with necrotising fasciitis and a culture from a wound which was positive for GAS. A pharyngeal test was performed in 48 patients, which was positive in 12.
Results of the admission blood tests and cultures performed on patients with invasive disease due to Streptococcus pyogenes.
| Admission blood tests | Median (p25-75) |
|---|---|
| Blood count (n=52) | |
| Leukocytes | 15.4 (8.8–23.6)×103cells/mm3 |
| Total neutrophils | 8.7 (5.6–15.1)×103cells/mm3 |
| CRP (n=50)a | 182.2 (68.2–182.2)mg/l |
| PCT (n=37)a | 9.3 (1.5–73.8)ng/ml |
| Sterile cultures | Positive (%) |
|---|---|
| Blood culture (n=51) | 33 (64.7) |
| Pleural fluid (n=12) | 11 (91.6) |
| CSF (n=8) | 4 (50) |
| Surgical drainage cultures (n=7) | 4 (57.1) |
| Joint fluid (n=6) | 3 (50) |
| Bone culture (n=1) | 1 (100) |
CRP: C-reactive protein; CSF: cerebrospinal fluid; PCT: procalcitonin.
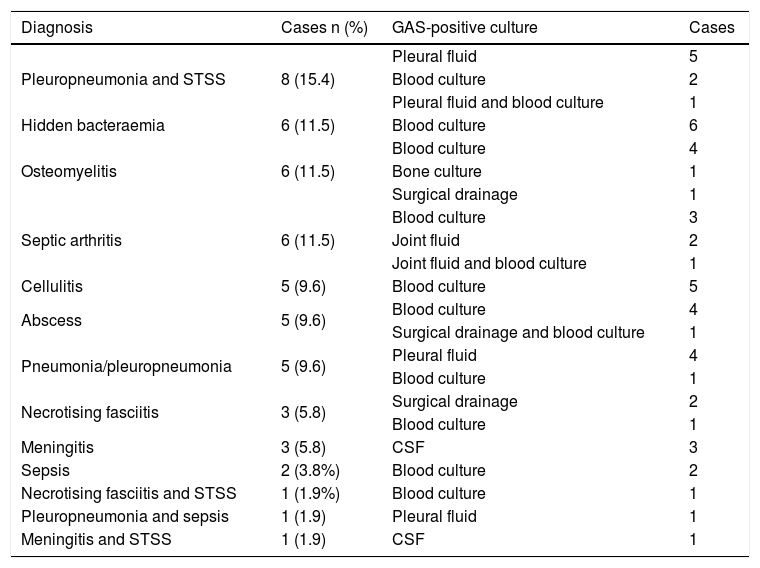
Fourteen cases (26.9%) of skin and soft tissue infection (10 of which had a skin condition as a risk factor), 14 cases (26.9%) of pneumonia/pleuropneumonia, 12 cases (23.1%) of osteoarticular infection, 10 (19.2%) of STSS, six (11.5%) of hidden bacteraemia, four (7.7%) of meningitis and two (3.8%) of sepsis were diagnosed. There were 11 patients with more than one diagnosis (Table 3).
Diagnoses and sterile positive cultures of the 52 patients with invasive disease due to Streptococcus pyogenes.
| Diagnosis | Cases n (%) | GAS-positive culture | Cases |
|---|---|---|---|
| Pleuropneumonia and STSS | 8 (15.4) | Pleural fluid | 5 |
| Blood culture | 2 | ||
| Pleural fluid and blood culture | 1 | ||
| Hidden bacteraemia | 6 (11.5) | Blood culture | 6 |
| Osteomyelitis | 6 (11.5) | Blood culture | 4 |
| Bone culture | 1 | ||
| Surgical drainage | 1 | ||
| Septic arthritis | 6 (11.5) | Blood culture | 3 |
| Joint fluid | 2 | ||
| Joint fluid and blood culture | 1 | ||
| Cellulitis | 5 (9.6) | Blood culture | 5 |
| Abscess | 5 (9.6) | Blood culture | 4 |
| Surgical drainage and blood culture | 1 | ||
| Pneumonia/pleuropneumonia | 5 (9.6) | Pleural fluid | 4 |
| Blood culture | 1 | ||
| Necrotising fasciitis | 3 (5.8) | Surgical drainage | 2 |
| Blood culture | 1 | ||
| Meningitis | 3 (5.8) | CSF | 3 |
| Sepsis | 2 (3.8%) | Blood culture | 2 |
| Necrotising fasciitis and STSS | 1 (1.9%) | Blood culture | 1 |
| Pleuropneumonia and sepsis | 1 (1.9) | Pleural fluid | 1 |
| Meningitis and STSS | 1 (1.9) | CSF | 1 |
CSF: cerebrospinal fluid; GAS: Streptococcus pyogenes; STSS: streptococcal toxic shock syndrome.
Initially, all patients were treated with third-generation cephalosporins or penicillins. Clindamycin was added during admission in 16 patients. Eighteen patients (34.6%) required surgical procedures: 11 pleural drainages, five skin debridements (four fasciitis and one abscess) and two osteoarticular procedures. No patient received treatment with non-specific gamma globulin.
Seventeen patients (32.7%) were admitted to the PICU: all those with STSS (eight had pleuropneumonia, one meningitis and another necrotising fasciitis), three with meningitis, three with pleuropneumonia and one with cellulitis and an active outbreak of epidermolysis bullosa. Compared to those who did not require admission to the PICU, these patients had higher acute-phase reactant levels in the admission blood tests (median CRP: 298 vs 139mg/l, p=0.006 and median PCT: 17.9 vs 3.7ng/ml, p=0.04; respectively). The median stay in the PICU was seven days (p25-75: 3–12 days). Eleven (21.2%) required mechanical ventilation, 12 (23.1%) required inotropic support with vasoactive drugs and one patient required circulation with extracorporeal membrane.
Three cases presented with co-infections; one patient had bronchiolitis caused by respiratory syncytial virus and two patients had associated bacteraemia (Streptococcus pneumoniae and Escherichia coli).
The median hospital stay was 9.5 days (p25-75: 8–15 days). The outcome was good in 48 patients (92.3%) and three had sequelae: one critical patient had neuropathy and maintained renal failure; another had hearing loss; and the remaining patient had VI cranial nerve palsy. The first case was a patient with congenital nephrotic syndrome and a kidney transplant with STSS who died a few months later. The latter two cases were of children with meningitis.
DiscussionThis study confirmed the low frequency of admissions as a result of ID due to GAS. However, the rate was higher than that reported in the literature (rate of 4.6–5.6 for every 10,000).6,16 The differences could partly be due to the particular characteristics of each centre. In our case, as it was a study conducted in a reference paediatric hospital, the number of cases referred to complete the diagnosis and treatment was high. It is worth noting the upward trend of cases reported in various articles, especially those conducted in the general population, and in different geographic locations.17–21 The reasons for this increase worldwide are not known, but it may be due to the emergence of more virulent clones.8,10
The young age of patients seems to facilitate the acquisition of this type of infection, as they are common in children under five years,12,22 probably due to the immaturity of the immune system. Most patients are previously healthy.
Among the patients with risk factors, the most common were skin conditions. Typically, chickenpox has been described as a predisposing factor for ID due to GAS, especially in children under four years of age, in whom the risk of suffering from the disease can be 20 times higher.2 In our study, only 3.8% of cases presented with chickenpox, with the data being highly variable in other published series, depending on the rate of chickenpox vaccination of the population studied (0–45%).6,16,23–25 During the study period, in our setting the chickenpox vaccine was not universally administered in childhood. The vaccine rate is therefore not known, although it is estimated that between 10 and 14 years of age, 20.1% of adolescents were not immunised.26 Since 2016, the vaccine has been funded by the Spanish national health system, administering two doses at 12–15 months old and at 2–3 years old, which will make it possible to assess the impact of its administration in the future.
In terms of the initial clinical presentation, almost all patients reported fever accompanied by non-specific symptoms, normally skin, osteoarticular and/or respiratory symptoms, a characteristic which hinders the differential diagnosis with other mild paediatric processes,5 delaying the diagnosis and the start of treatment. In light of the suspicion of ID due to GAS, having basic analytical data available was useful, as in most patients there was a marked increase in acute-phase reactants, especially in those who required admission to the PICU due to increased severity. Likewise, the role of the rapid diagnostic test for GAS as a diagnostic tool should be emphasised. This is an accessible and rapid technique which can be very useful.
The most common diagnoses were skin and soft tissue infections, followed by pneumonia with or without effusion and osteoarticular infections. These data are consistent with the most common types of infection associated with ID due to GAS described in the literature.5,6,8,12 The rate of bacteraemia observed, 64.7%, was within the described intervals, which range from 56% to 85%.7,8,17,22 STSS, a serious condition with high mortality, is more common in adults.25,27 Studies published on the general population show an STSS prevalence of 8–14%, which falls to 7–8% in the paediatric population.16 However, in the series that we present, 10 patients (19.2%) met the criteria for this condition. This accumulation of cases could be due to the characteristics of our hospital, which facilitated the concentration of serious cases, although it may also have influenced the virulence of the bacteria and the susceptibility of the hosts involved.
GAS is susceptible to β-lactams, as, to date, no resistant strains have been reported, which is why these antibiotics are maintained as first-line treatment.8 All patients in this study received IV β-lactams empirically as soon as the diagnosis was suspected. In patients with a more unfavourable outcome, clindamycin was added with the aim of improving the performance of monotherapy.28
Admission of patients to the PICU and/or surgical treatments are common in this type of disease.5 In our series, one-third of patients required admission to the PICU and almost 40% required surgical treatment. The hospital stay was long, extending to more than one week in 75% of cases. The outcome was good in most cases and there was only one death (1.9%), which is below the rate published in the literature (3–10%).3,5,6 This was perhaps related to the types of ID included in our study and to the early diagnosis performed.
The limitations of this study were mainly those inherent to its retrospective nature. Certain aspects, mainly related to the presentation and symptomatology, could not be reflected adequately and completely in the medical records. This could mean that the real frequency of the various symptoms differed to a certain extent from that finally determined. Furthermore, the fact that it was a single-centre study made it difficult to compare the admission rate and the type of ID due to GAS with other studies. As our hospital is a referral centre, it is possible that the most serious cases were over-represented. This fact has been mentioned previously.
In conclusion, ID due to GAS was an extremely rare reason for hospitalisation. The most common forms of presentation were skin and soft tissue infection and pneumonia. Due to its severity and high morbidity and mortality, it may be considered a public health problem. We believe that it would be important to establish epidemiological surveillance measures which make it possible to determine the real status of this disease. The option of including it in the list of diseases which must be declared at state level could be considered, as knowledge of the distribution of GAS would facilitate advances in the development and evaluation of new vaccines, as well as improvements in diagnosis and early treatment.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Arias-Constantí V, Trenchs-Sainz de la Maza V, Sanz-Marcos NE, Guitart-Pardellans C, Gené-Giralt A, Luaces-Cubells C. Enfermedad invasiva por Streptococcus pyogenes: ingresos durante 6 años. Enferm Infecc Microbiol Clin. 2018;36:352–356.
This paper was presented, partially as an oral presentation, at the 64th Congress of the Asociación Española de Pediatría [Spanish Paediatric Association] (Valencia, 2016).