Our main objective was a revision of clinical, microbiological and epidemiological results of Clostridium difficile-associated infection in paediatric patients (2010–2015). We compared the diagnoses performed by detection of toxins in faeces and those performed by real-time PCR.
MethodsThis retrospective study included 82 paediatric patients. Detection of toxigenic C. difficile was performed sequentially, in diarrheal faeces and under clinical request.
ResultsA total of 39% of the patients were attended at Haematology–Oncology Unit and >50% of them had previously received cephalosporins. Fever associated with diarrhoea was more frequent in the group of toxin detection, whereas not receiving specific antibiotic treatment was more frequent in the group of positive PCR, without statistically significant differences.
ConclusionsWe highlight the presence of C. difficile infection in children under 2 years old. A diagnostic testing in selected paediatric patients would be advisable when there is clinical suspicion of infection.
Nuestro objetivo principal fue revisar los aspectos clínicos, microbiológicos y epidemiológicos de la infección asociada a Clostridium difficile en pediatría (2010-2015), comparando los diagnósticos realizados por detección de toxinas en heces y por PCR a tiempo real del gen de la toxina B.
MétodosEste estudio retrospectivo incluyó a 82 pacientes pediátricos. La detección de C. difficile toxigénico se realizó de manera secuencial, en heces diarreicas y bajo solicitud expresa.
ResultadosEl 39% de los pacientes procedían de Hemato-Oncología y >50% recibió previamente cefalosporinas. La presencia de fiebre asociada a diarrea fue más frecuente en el grupo de detección positiva de toxinas y no recibir antibioterapia específica fue más frecuente en el grupo con PCR positiva, sin diferencias estadísticamente significativas.
ConclusiónDestacamos la presencia de infecciones en niños menores de 2 años. Sería recomendable realizar un diagnóstico de infección asociada a C. difficile en pacientes pediátricos, siempre que la sospecha clínica lo requiera.
Clostridium difficile is a Gram-positive, obligate anaerobe, spore-forming, toxin A/B-producing bacillus. These characteristics confer its virulence.1 Currently, it is one of the main microorganisms responsible for nosocomial infection and colitis associated with broad-spectrum antibiotic therapy. In the infant population, the epidemiology has changed in the last decade, with an increase in nosocomial and community-acquired cases.2 Zilberberg et al. reported that the annual paediatric hospitalisation rate due to C. difficile infection (CDI) in the USA increased to between 7.24 and 12.8/10,000 hospitalisations (1997–2006), according to data from more than 3700 hospitals,3 especially in children between one and four-years old. Other authors have associated CDI in children with prolonged hospitalisation and risk of death.4 Furthermore, the issue of the clinical significance of isolation of toxigenic C. difficile is maintained, especially in children under two years of age, due to the high presence of asymptomatic carriers of viral infections causing diarrhoea. A colonisation rate of 14% in infants aged between six and 12 months-old, which falls to 0–3% in three-year-old children, has been published.5 In Spain, there are few studies regarding the diagnosis of C. difficile infection in paediatric patients. Our main objective is to review the clinical, microbiological and epidemiological aspects of CDI cases in paediatrics. In addition, we will analyse the possible differences between the diagnoses made using detection of toxins in stools and detection of the toxin B gene using real-time PCR.
Material and methodsA descriptive and retrospective study of toxigenic C. difficile detection was carried out in stool samples from paediatric patients treated by the Microbiology Department (May 2010–May 2015). Our tertiary hospital centre serves a total population of 519,300 inhabitants and has 1153 available beds, 216 of which are paediatric.
Detection of toxigenic C. difficile was performed sequentially in diarrhoea stools.6 It was performed in the same way throughout the study period and only by express request. The presence of solid stools was a rejection criterion. Screening was performed with simultaneous detection of glutamate dehydrogenase (GDH) antigen and toxins A/B using an enzyme immunoassay (Techlab C. diff Quik Chek Complete). With a positive antigen but detection of negative toxins, PCR of the toxin B gene was performed (Cepheid Xpert C. difficile), which included detection of hypervirulent ribotype 027 strains. The interpretation and issuance of the results to the clinic, and the division of study groups is specified below: GDH−/Tox−: negative, GDH+/Tox+: positive (group 1) and GDH+/Tox−/PCRXpert+: positive (group 2). The search for bacterial enteropathogens (Salmonella enterica spp., Campylobacter spp., Shigella spp., Yersinia enterocolitica, Aeromonas spp., Plesiomonas shigelloides) was performed in 71 samples using culture in selective and differential media. Detection of rotavirus and adenovirus was performed in 72 samples and parasite testing was conducted in 12 samples. Screening for Cryptosporidium/Giardia was performed on all stools (<14 years of age) or by rapid immunochromatographic test in immunosuppressed patients, according to routine care.
Definitions:
- I.
Diarrhoea episode: presence of at least three soft/liquid stools within 24h.
- II.
Healthcare-associated CDI: diarrhoea within 48 h after admission until discharge, or during readmission within four weeks after discharge.
- III.
Community-acquired CDI: diarrhoea which occurs in the community or in the first 48h of hospitalisation, with no previous hospitalisation in a period greater than 12 weeks.
- IV.
Undetermined CDI: diarrhoea which occurs in the community between four and 12 weeks from a previous hospitalisation.
To obtain clinical data, medical histories in physical and electronic format were reviewed. The statistical analysis of data was performed using SPSS v. 22. The qualitative variables were analysed using the chi-square test or Fisher's exact test and the quantitative variables were analysed using the Mann–Whitney U test, using medians (Me) and interquartile ranges (IQR, 75th percentile–25th percentile) as dispersion measures. The level of statistical significance was p<0.05.
This study obtained the approval of the Hospital Universitario La Paz Independent Ethics Committee, with code PI-2682.
ResultsEight-two (82) patients were included in the study: seven of them were ruled out as they had incomplete data. The distribution was two cases in 2010 (May–December), 10 cases in 2011, 12 cases in 2012, 19 cases in 2013, 26 cases in 2014 and 13 cases in 2015 (January–May).
Out of the total of 75 patients, healthcare-associated CDI was the most common with 62 cases (82.7%) distributed equally between the two groups. The annual incidence of this type of infection was 5.75 episodes/10,000 paediatric stays (2011), 10.50 episodes/10,000 paediatric stays (2012), 21.75 episodes/10,000 paediatric stays (2013) and 20.19 episodes/10,000 paediatric stays (2014). There were 10 cases (13.3% of the total) of community-acquired CDI distributed among the two groups and three cases (4%) of undetermined CDI.
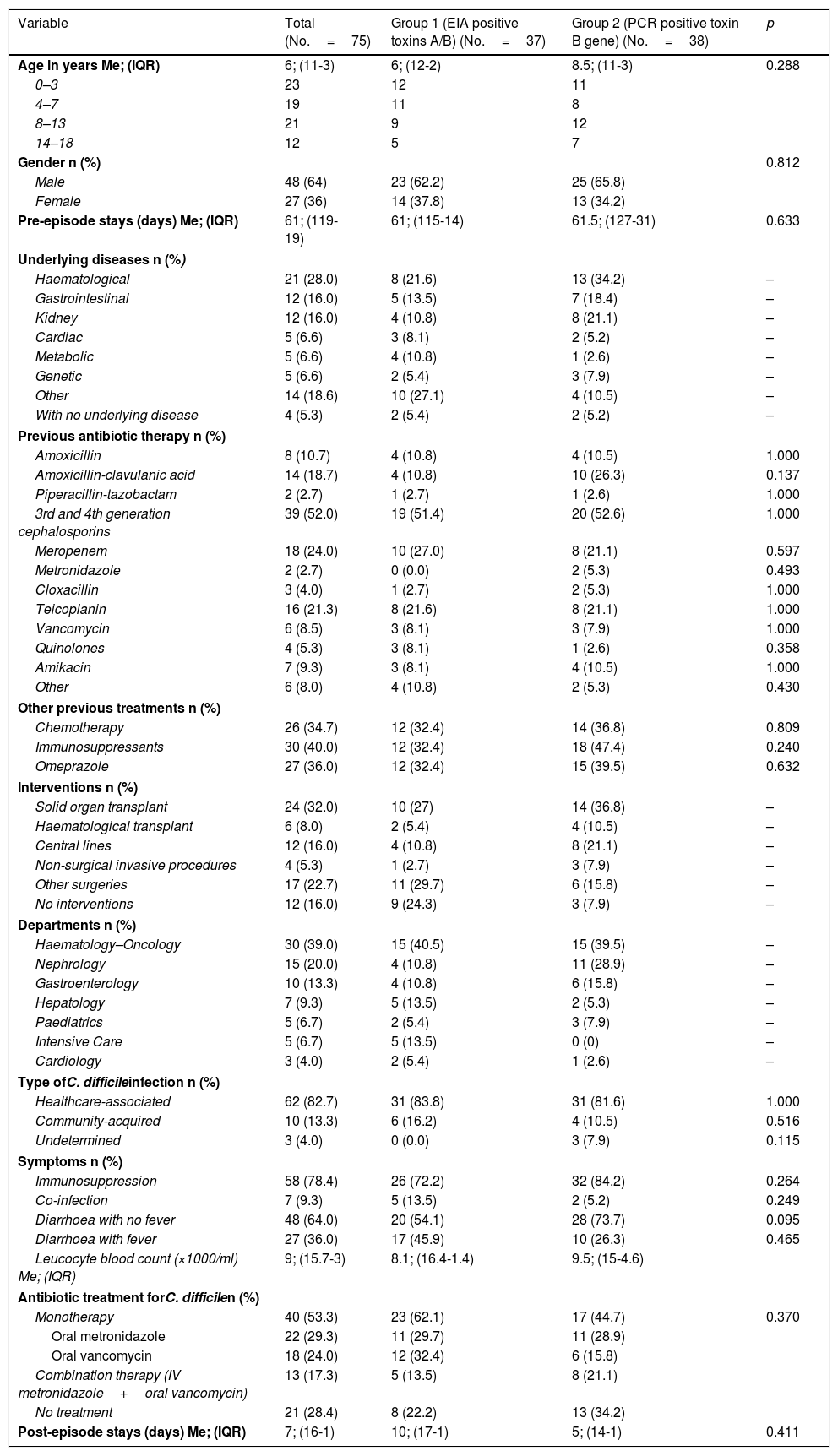
The variables analysed are shown in Table 1. In terms of outcome variables, there were nine recurrences, two admissions to the ICU, nine deaths (one in the 30 days after diagnosis), but none attributable to CDI. There was no paralytic ileus and no cases of pseudomembranous colitis.
Qualitative and quantitative variables of the patients included in the study.
| Variable | Total (No.=75) | Group 1 (EIA positive toxins A/B) (No.=37) | Group 2 (PCR positive toxin B gene) (No.=38) | p |
|---|---|---|---|---|
| Age in years Me; (IQR) | 6; (11-3) | 6; (12-2) | 8.5; (11-3) | 0.288 |
| 0–3 | 23 | 12 | 11 | |
| 4–7 | 19 | 11 | 8 | |
| 8–13 | 21 | 9 | 12 | |
| 14–18 | 12 | 5 | 7 | |
| Gender n (%) | 0.812 | |||
| Male | 48 (64) | 23 (62.2) | 25 (65.8) | |
| Female | 27 (36) | 14 (37.8) | 13 (34.2) | |
| Pre-episode stays (days) Me; (IQR) | 61; (119-19) | 61; (115-14) | 61.5; (127-31) | 0.633 |
| Underlying diseases n (%) | ||||
| Haematological | 21 (28.0) | 8 (21.6) | 13 (34.2) | – |
| Gastrointestinal | 12 (16.0) | 5 (13.5) | 7 (18.4) | – |
| Kidney | 12 (16.0) | 4 (10.8) | 8 (21.1) | – |
| Cardiac | 5 (6.6) | 3 (8.1) | 2 (5.2) | – |
| Metabolic | 5 (6.6) | 4 (10.8) | 1 (2.6) | – |
| Genetic | 5 (6.6) | 2 (5.4) | 3 (7.9) | – |
| Other | 14 (18.6) | 10 (27.1) | 4 (10.5) | – |
| With no underlying disease | 4 (5.3) | 2 (5.4) | 2 (5.2) | – |
| Previous antibiotic therapy n (%) | ||||
| Amoxicillin | 8 (10.7) | 4 (10.8) | 4 (10.5) | 1.000 |
| Amoxicillin-clavulanic acid | 14 (18.7) | 4 (10.8) | 10 (26.3) | 0.137 |
| Piperacillin-tazobactam | 2 (2.7) | 1 (2.7) | 1 (2.6) | 1.000 |
| 3rd and 4th generation cephalosporins | 39 (52.0) | 19 (51.4) | 20 (52.6) | 1.000 |
| Meropenem | 18 (24.0) | 10 (27.0) | 8 (21.1) | 0.597 |
| Metronidazole | 2 (2.7) | 0 (0.0) | 2 (5.3) | 0.493 |
| Cloxacillin | 3 (4.0) | 1 (2.7) | 2 (5.3) | 1.000 |
| Teicoplanin | 16 (21.3) | 8 (21.6) | 8 (21.1) | 1.000 |
| Vancomycin | 6 (8.5) | 3 (8.1) | 3 (7.9) | 1.000 |
| Quinolones | 4 (5.3) | 3 (8.1) | 1 (2.6) | 0.358 |
| Amikacin | 7 (9.3) | 3 (8.1) | 4 (10.5) | 1.000 |
| Other | 6 (8.0) | 4 (10.8) | 2 (5.3) | 0.430 |
| Other previous treatments n (%) | ||||
| Chemotherapy | 26 (34.7) | 12 (32.4) | 14 (36.8) | 0.809 |
| Immunosuppressants | 30 (40.0) | 12 (32.4) | 18 (47.4) | 0.240 |
| Omeprazole | 27 (36.0) | 12 (32.4) | 15 (39.5) | 0.632 |
| Interventions n (%) | ||||
| Solid organ transplant | 24 (32.0) | 10 (27) | 14 (36.8) | – |
| Haematological transplant | 6 (8.0) | 2 (5.4) | 4 (10.5) | – |
| Central lines | 12 (16.0) | 4 (10.8) | 8 (21.1) | – |
| Non-surgical invasive procedures | 4 (5.3) | 1 (2.7) | 3 (7.9) | – |
| Other surgeries | 17 (22.7) | 11 (29.7) | 6 (15.8) | – |
| No interventions | 12 (16.0) | 9 (24.3) | 3 (7.9) | – |
| Departments n (%) | ||||
| Haematology–Oncology | 30 (39.0) | 15 (40.5) | 15 (39.5) | – |
| Nephrology | 15 (20.0) | 4 (10.8) | 11 (28.9) | – |
| Gastroenterology | 10 (13.3) | 4 (10.8) | 6 (15.8) | – |
| Hepatology | 7 (9.3) | 5 (13.5) | 2 (5.3) | – |
| Paediatrics | 5 (6.7) | 2 (5.4) | 3 (7.9) | – |
| Intensive Care | 5 (6.7) | 5 (13.5) | 0 (0) | – |
| Cardiology | 3 (4.0) | 2 (5.4) | 1 (2.6) | – |
| Type ofC. difficileinfection n (%) | ||||
| Healthcare-associated | 62 (82.7) | 31 (83.8) | 31 (81.6) | 1.000 |
| Community-acquired | 10 (13.3) | 6 (16.2) | 4 (10.5) | 0.516 |
| Undetermined | 3 (4.0) | 0 (0.0) | 3 (7.9) | 0.115 |
| Symptoms n (%) | ||||
| Immunosuppression | 58 (78.4) | 26 (72.2) | 32 (84.2) | 0.264 |
| Co-infection | 7 (9.3) | 5 (13.5) | 2 (5.2) | 0.249 |
| Diarrhoea with no fever | 48 (64.0) | 20 (54.1) | 28 (73.7) | 0.095 |
| Diarrhoea with fever | 27 (36.0) | 17 (45.9) | 10 (26.3) | 0.465 |
| Leucocyte blood count (×1000/ml) Me; (IQR) | 9; (15.7-3) | 8.1; (16.4-1.4) | 9.5; (15-4.6) | |
| Antibiotic treatment forC. difficilen (%) | ||||
| Monotherapy | 40 (53.3) | 23 (62.1) | 17 (44.7) | 0.370 |
| Oral metronidazole | 22 (29.3) | 11 (29.7) | 11 (28.9) | |
| Oral vancomycin | 18 (24.0) | 12 (32.4) | 6 (15.8) | |
| Combination therapy (IV metronidazole+oral vancomycin) | 13 (17.3) | 5 (13.5) | 8 (21.1) | |
| No treatment | 21 (28.4) | 8 (22.2) | 13 (34.2) | |
| Post-episode stays (days) Me; (IQR) | 7; (16-1) | 10; (17-1) | 5; (14-1) | 0.411 |
EIA: enzyme immunoassay; IQR: interquartile range (p75-p25); Me: median.
By diagnostic group, there were no statistically significant differences between the variables analysed. Group 1 patients presented with fever associated with diarrhoea more often than those in group 2 (45.9% vs 26.3%), although with no statistically significant differences. The IQR for the leucocyte blood count (×1000/ml) was 15.7-3, with leukocytopaenia (<4000/ml) in 20 patients (13 vs 7), mainly haematological and transplanted. Regarding the treatment with metronidazole or oral vancomycin in monotherapy or combined with IV metronidazole, eight patients from group 1 and 13 from group 2 were not treated. When the diagnostic groups were analysed by year, there were 37 cases for group 1, distributed as follows: two (May–December 2010), four (2011), five (2012), nine (2013), 10 (2014), seven (January–May 2015) and 38 cases for group 2, distributed as follows: two (2011), five (2012), 10 (2013), 16 (2014), five (January–May 2015). With these data, we found that more cases were not diagnosed by PCR than by detection of toxins in the five years.
There were seven co-infections, five in group 1 (rotavirus, Aeromonas hydrophila, Cryptosporidium parvum and two Campylobacter jejuni) and two in group 2 (rotavirus and Aeromonas caviae). After being analysed using PCR, no hypervirulent ribotype 027 strains were detected in group 2.
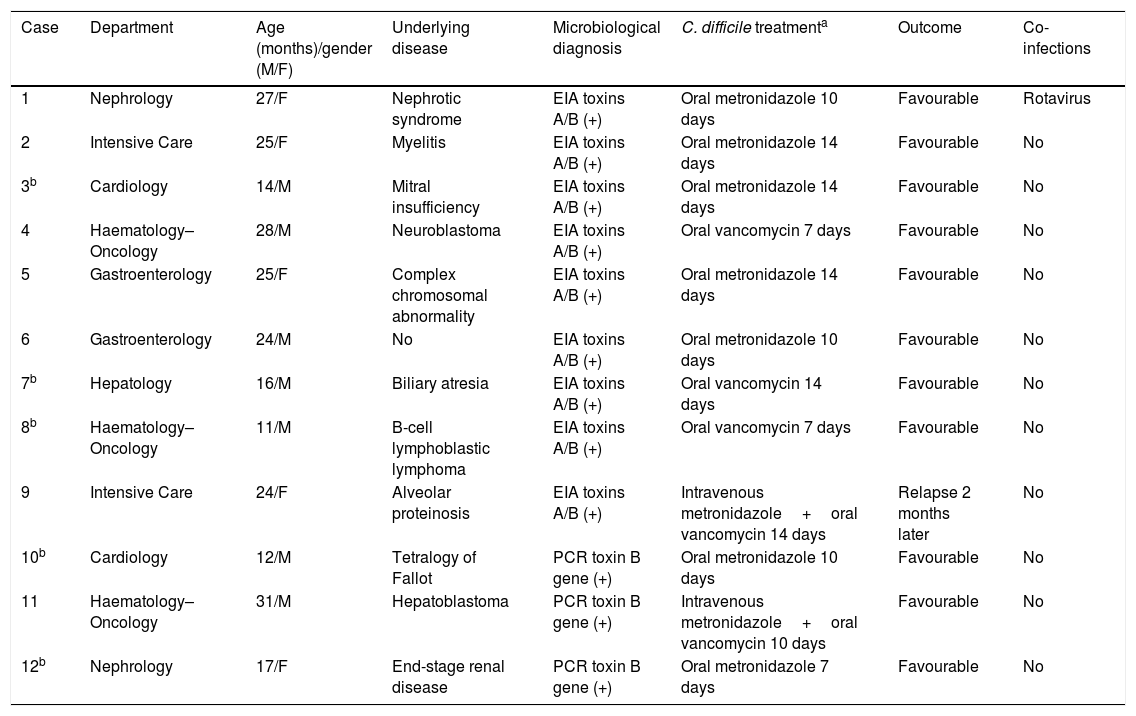
Table 2 shows the 12 cases (of the 17 patients in whom toxigenic C. difficile was detected) in children under three years of age with symptoms and treatment with metronidazole or vancomycin.
Patients under three years of age with C. difficile-associated infection.
| Case | Department | Age (months)/gender (M/F) | Underlying disease | Microbiological diagnosis | C. difficile treatmenta | Outcome | Co-infections |
|---|---|---|---|---|---|---|---|
| 1 | Nephrology | 27/F | Nephrotic syndrome | EIA toxins A/B (+) | Oral metronidazole 10 days | Favourable | Rotavirus |
| 2 | Intensive Care | 25/F | Myelitis | EIA toxins A/B (+) | Oral metronidazole 14 days | Favourable | No |
| 3b | Cardiology | 14/M | Mitral insufficiency | EIA toxins A/B (+) | Oral metronidazole 14 days | Favourable | No |
| 4 | Haematology–Oncology | 28/M | Neuroblastoma | EIA toxins A/B (+) | Oral vancomycin 7 days | Favourable | No |
| 5 | Gastroenterology | 25/F | Complex chromosomal abnormality | EIA toxins A/B (+) | Oral metronidazole 14 days | Favourable | No |
| 6 | Gastroenterology | 24/M | No | EIA toxins A/B (+) | Oral metronidazole 10 days | Favourable | No |
| 7b | Hepatology | 16/M | Biliary atresia | EIA toxins A/B (+) | Oral vancomycin 14 days | Favourable | No |
| 8b | Haematology–Oncology | 11/M | B-cell lymphoblastic lymphoma | EIA toxins A/B (+) | Oral vancomycin 7 days | Favourable | No |
| 9 | Intensive Care | 24/F | Alveolar proteinosis | EIA toxins A/B (+) | Intravenous metronidazole+oral vancomycin 14 days | Relapse 2 months later | No |
| 10b | Cardiology | 12/M | Tetralogy of Fallot | PCR toxin B gene (+) | Oral metronidazole 10 days | Favourable | No |
| 11 | Haematology–Oncology | 31/M | Hepatoblastoma | PCR toxin B gene (+) | Intravenous metronidazole+oral vancomycin 10 days | Favourable | No |
| 12b | Nephrology | 17/F | End-stage renal disease | PCR toxin B gene (+) | Oral metronidazole 7 days | Favourable | No |
EIA: enzyme immunoassay.
Despite the fact that both the number of cases and the severity of CDI continues to increase,7 it is an underestimated cause of diarrhoea in paediatric patients. One of the reasons is the high rate of asymptomatic colonisations in infants less than one year old and the perception of the lack of susceptibility to this infection,8 either due to the immaturity of the enterocytes or of their toxin receptors. In our series of cases, the annual incidence increased throughout the period studied, which could be due to improvements in the diagnostic method and greater clinical suspicion.
Among the risk factors analysed, we highlight a prolonged previous hospitalisation with a high percentage of healthcare-associated CDI. It is worth noting the high number of previous stays (Me=6, IQR=119-19) and underlying conditions, which are mostly haematological. Boyle et al. demonstrated a high risk of CDI in the 100 days after a paediatric bone marrow transplant.9 Broad-spectrum antibiotic therapy, especially 3rd–4th generation cephalosporins (>50%), chemotherapy, immunosuppressants and omeprazole were the most used drugs due to the multiple associated conditions. The main prior interventions were solid organ transplants, followed by bone marrow transplants and abdominal, cardiac and kidney surgical procedures and central line cannulation. Patients were assigned mainly to the Haematology–Oncology (39%), Hepatology, Gastroenterology and Nephrology Departments. Practically all our patients presented with the risk factors described in the literature. Comorbidities, which are mainly haematological, immunosuppression, Hirschsprung's disease and underlying bowel disease are frequently associated with CDI in paediatrics.10
The presence of fever associated with diarrhoea was more common in group 1, and not receiving specific treatment was more common in group 2, but with no significant differences. An increased use of vancomycin in both groups could be explained by the severe underlying conditions in these patients.
The different series describe less severity and mortality in children than in adults, with 88% of paediatric patients with no associated complications and a benign clinical course.11 Of the 17 patients under three years old, five were not treated. Therefore, our study presented 12 cases of CDI in children under three who were treated and all had a favourable response; the youngest patient was 11 months old. There was only one co-infection with rotavirus and one relapse two months later in one patient who was in Intensive Care with alveolar proteinosis and treated with combination therapy.
There are studies which do not recommend performing diagnostic tests systematically in children under two years old. Despite the uncertain interpretation of the results due to high rates of colonisation,12,13 we consider that it would be advisable to perform the diagnosis of C. difficile infection whenever the clinical suspicion so requires, regardless of age.10,14,15 Previous guidelines13 convey the opinion that it is wise to avoid diagnostic tests for CDI in children under one year old, although recent data have shown that 26% of hospitalised children with CDI were under one year old and 5% were neonates. However, there was no possibility of determining if they were true infections or asymptomatic carriers: alternative aetiologies should be sought. It is suggested that a positive result in children who are 2–3 years of age indicates a possible CDI.
Limitations of the study are its retrospective nature and the difficult assessment of the microbiological result using PCR to differentiate colonisation of infection in this population. As advantages, we highlight a large sample size and two representative and homogeneous diagnostic groups.
In conclusion, in our setting we observed a slight increase in CDI cases throughout the period analysed. This is consistent with the literature, which describes CDI as an emerging disease which is on the rise in this type of population. In our case series, we emphasise the presence of infections in children under two years of age and the absence of statistically significant clinical and epidemiological differences between patients diagnosed using enzyme immunoassays of toxins A/B and those diagnosed using PCR of the toxin B gene.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Falces-Romero I, Troyano-Hernáez P, García-Bujalance S, Baquero-Artigao F, Mellado-Peña MJ, García-Rodríguez J. Detección de Clostridium difficile toxigénico en pediatría. Enferm Infecc Microbiol Clin. 2018;36:357–361.